Evaluar la capacidad del endourólogo para evaluar la composición del cálculo mediante la observación de imágenes endoscópicas.
Materiales y métodosUna serie de 20 videoclips de tratamientos endoscópicos de cálculos urinarios que también estaba disponible el resultado de la espectroscopia infrarroja se cargó en un sitio de YouTube accesible solo a miembros del South Eastern Group for Urolithiasis Research (SEGUR) a quienes se les preguntó para identificar la composición de los cálculos.
ResultadosUn total de 32 endourólogos de 9 países diferentes participaron en el estudio. El número promedio de detecciones correctas de participantes fue de 7.81 ± 2.68 (1–12). La precisión general fue del 39% (250 de 640 predicciones). Cálculos de dihidrato de oxalato de calcio se han detectado correctamente en el 69.8%, monohidrato de oxalato de calcio en el 41.8%, ácido úrico en el 33.3%, oxalato de calcio / ácido úrico en el 34.3% y cistina en el 78.1%. Las tasas de precisión para estruvita (15.6%), fosfato de calcio (0%) y oxalato de calcio / fosfato de calcio (9.3%) fueron bastante bajas.
ConclusionesLa observación del cálculo durante el procedimiento endoscópico no fue confiable para identificar la composición de la mayoría de los cálculos, aunque los cálculos de oxalato de calcio dihidrato y cistina pueden identificarse con buena precisión. Sin embargo, se debe alentar la presentación de fotos o videos de cálculo intacto y su estructura interna para implementar los resultados del análisis de cálculo después de la cirugía. Los endourólogos deben mejorar su capacidad de identificación visual de los diferentes tipos de cálculos.
To assess the surgeon's ability to evaluate the composition of the stone by observation of endoscopic images.
Materials and methodsA series of 20 video clips of endoscopic treatments of urinary stones of which was also available the result of infrared spectroscopy was uploaded to a YouTube site accessible only to members of the South Eastern Group for Urolithiasis Research (SEGUR) who were asked to identify the composition of the stones.
ResultsA total of 32 clinicians from 9 different countries participated in the study. The average number of correct detections of participants was 7.81 ± 2.68 (range 1–12). Overall accuracy was 39% (250 out of 640 predictions). Calcium oxalate dihydrate stones have been correctly detected in 69.8%, calcium oxalate monohydrate in 41.8%, uric acid in 33.3%, calcium oxalate/uric acid in 34.3% and cystine in 78.1%. Precision rates for struvite (15.6%), calcium phosphate (0%) and mixed calcium oxalate/calcium phosphate (9.3%) were quite low.
ConclusionsObservation of the stone during the endoscopic procedure was not reliable to identify the composition of most stones although it gave some information allowing to identify with a good sensitivity calcium oxalate dihydrate and cystine stones. Nevertheless, photo or video reporting of the intact stone and its internal structure could should be encouraged to implement results of still mandatory post-operative stone analysis. Endourologists should improve their ability of visual identification of the different types of stones.
The analysis of the stone is a crucial step of the work up of renal stone forming patients as it provides relevant information on the pathogenetic mechanisms of renal stone formation.1 The use of methods able to provide reliable and detailed information on the composition of the stone has been strongly recommended.2 On the other hand, the analysis of the stone can be performed only after the spontaneous expulsion of the stone or its fragments or after its surgical removal. For this reason, many efforts have been made to develop imaging modalities able to reliably diagnose in-vivo the physico-chemical composition of the stone before the procedure of stone removal.3 The endoscopic view of the stone can give real-time information that can be exploited to plan the modality of treatment. Moreover, the magnified vision of the whole stone and of its internal structure can provide information that implements the results of the ex-vivo analysis of the retrieved stone fragments. The purpose of this study was to assess the surgeon's ability to evaluate the composition of the stone by simple observation of endoscopic images.
Materials and methodsA series of video clips related to endoscopic treatments of urinary stones of which the result of the subsequent analysis with Infrared spectroscopy was available was selected from a library of endoscopic images.
In order to contain the time of the survey within 15 min we decided to select a limited number of samples that we feel representative of the common endourological practice. Based on the morpho-constitutional stone classification of Daudon4 we selected a series that would represent the four morphological types of calcium oxalate monohydrate (COM) stones (Ia-d), the three types of calcium oxalate dihydrate (COD) stones (II a–c), and the common morphology of anhydrous uric acid (UA) (III a), struvite (IVc)(ST) and cystine stones (Va-b)(CY). We also considered a sample of a stone composed mainly by carbapatite (CAP) (IVa2) and two mixed stones of calcium oxalate monohydrate and carbapatite (IVa1) (COM + CAP) and of calcium oxalate monohydrate and uric acid (Ia + IIIb)(COM + UA). Instead, we excluded from the investigation less frequent stones such as the stones of uric acid dihydrate uric acid (IIIb), urate stones (IIIc), ammonium urate stones (IIId), brushite (IVd) and protein (VI).
The series included 8 stones of pure COM, 3 stones of COD (>50%), 3 stones of pure UA, 2 mixed stones of COM + CAP, one CAP stone, one mixed stone of COM + UA, one ST and one CY stone.The images were arranged in a playlist and uploaded to a YouTube site accessible only to study participants. The URL of the site access was simultaneously sent to 32 clinicians who had preliminarily accepted to participate in the study. Participants in the study were members of the South Eastern Group for Urolithiasis Research (SEGUR) and residents attending to their institutions. The participants had to try to identify the composition of the stone in each clip and report the results on a specific form. The average number of positive predictions of the composition of the stone was calculated for all participants of the study and separately for clinicians with longer clinical experience and residents. Overall accuracy was calculated by summing the number of correct predictions of stone compositions divided by the total number of predictions. Percentages of correct detections of the composition of the stone for each of the clips have been calculated. Precision (number of positive detections of a specific composition by total number of stones with a specific composition) for each chemical type of stones was also computed. User accuracy was considered the probability that a prediction of a certain class composition was really in that class composition
ResultsA total of 32 clinicians participated in the study, 5 from Bulgaria, 3 from Greece, 6 from Italy, 3 from Northern Macedonia, 1 from Portugal, 2 from Romania, 3 from Serbia, 5 from Spain and 4 from Turkey.
The average number of correct detections was 7.81 ± 2.68 (range 1–12).
Mean number of correct detections was higher, but not significantly, in senior endourologists than in younger urologists and residents (8.35 ± 2.20 vs 7.20 ± 3.09, p = 0.231).
Mean number of correct detections was higher, but not significantly, for endourologists working in institutions where stones were routinely analysed by infrared spectroscopy or X-ray diffractometry than for those from institutions where stones were analysed with wet chemical or not analysed (8.16 ± 2.20 vs 7.20 ± 3.09, p = 0.406).
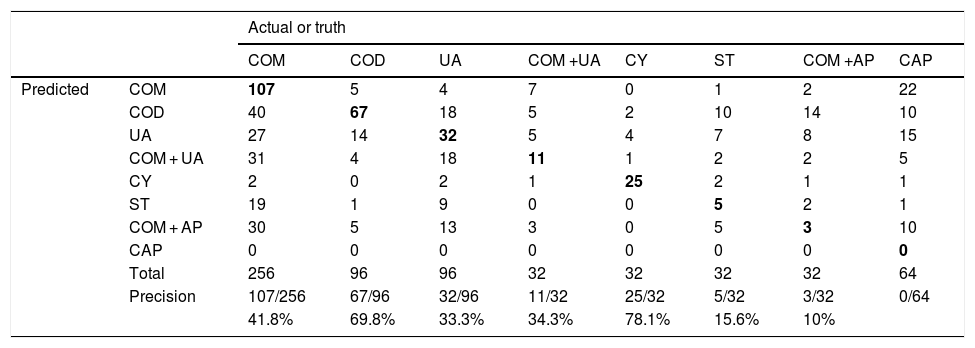
A confusion or error matrix was built, where columns represented true or actual classes of stone composition and rows the observers’ predictions (Table 1). In the matrix the correctly classified compositions (in bold) are located along the upper-left to lower-right diagonal of the confusion matrix.
Confusion or error matrix: a column represents true or actual classes of stone composition and rows the observers’ predictions.
| Actual or truth | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| COM | COD | UA | COM +UA | CY | ST | COM +AP | CAP | ||
| Predicted | COM | 107 | 5 | 4 | 7 | 0 | 1 | 2 | 22 |
| COD | 40 | 67 | 18 | 5 | 2 | 10 | 14 | 10 | |
| UA | 27 | 14 | 32 | 5 | 4 | 7 | 8 | 15 | |
| COM + UA | 31 | 4 | 18 | 11 | 1 | 2 | 2 | 5 | |
| CY | 2 | 0 | 2 | 1 | 25 | 2 | 1 | 1 | |
| ST | 19 | 1 | 9 | 0 | 0 | 5 | 2 | 1 | |
| COM + AP | 30 | 5 | 13 | 3 | 0 | 5 | 3 | 10 | |
| CAP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 256 | 96 | 96 | 32 | 32 | 32 | 32 | 64 | |
| Precision | 107/256 | 67/96 | 32/96 | 11/32 | 25/32 | 5/32 | 3/32 | 0/64 | |
| 41.8% | 69.8% | 33.3% | 34.3% | 78.1% | 15.6% | 10% | |||
Overall accuracy calculated by summing the number of correct predictions of stone compositions and dividing by the total number of predictions, was 39% (250 out of 640 predictions).
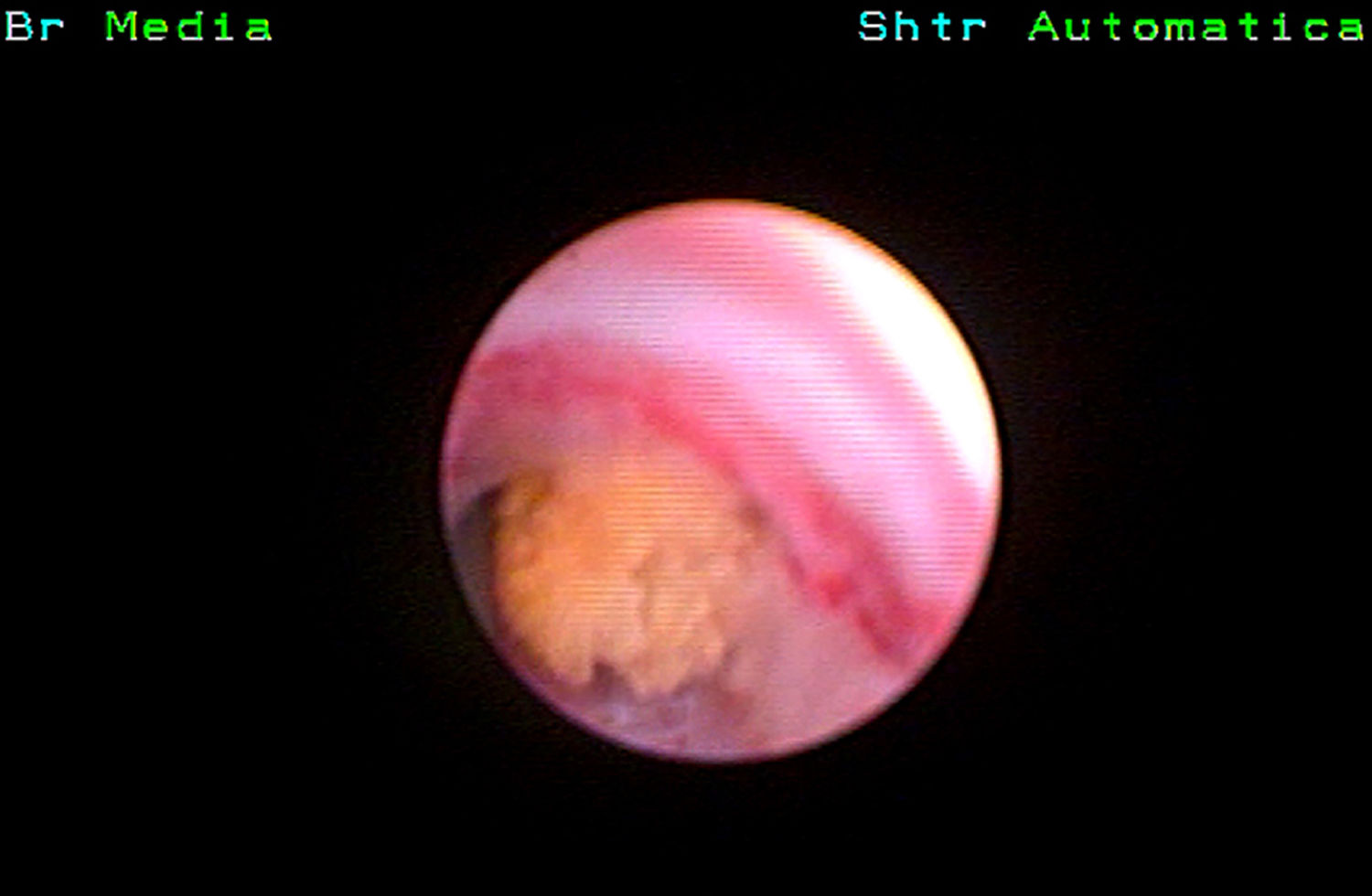
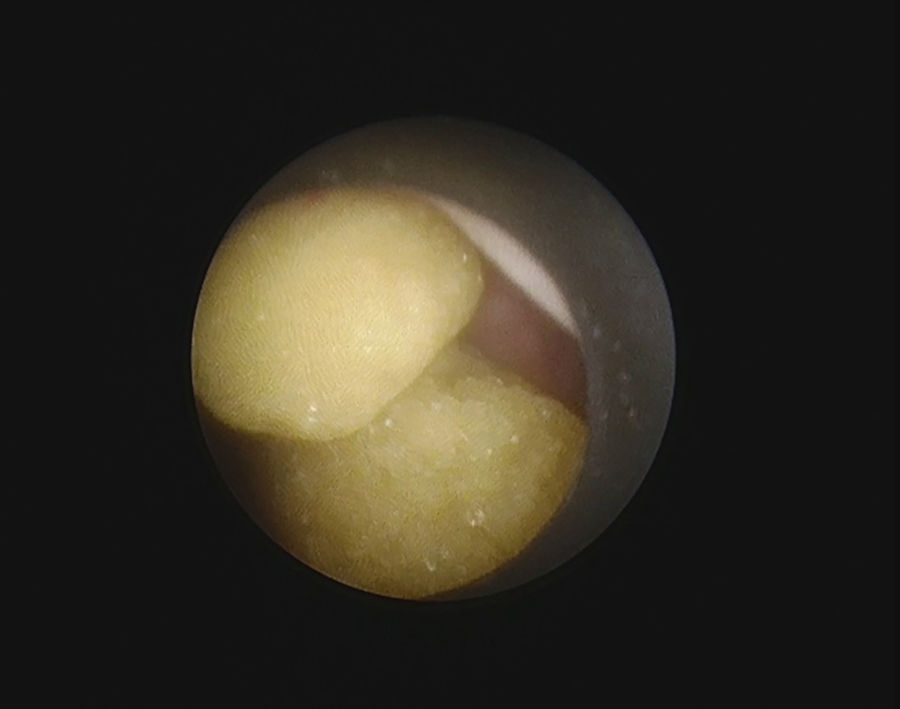
Precision or produced accuracy was computed as the probability that a videoclip was classified correctly in a given class of composition. COD stones (Fig. 1) have been correctly detected in 67/96 (69.8%, range 65.6–78.1%), COM in 107/256 (41.8%, range 6.2–81.2%), UA in 32/96 (33.3%, range 15.6–46.8%), COM + UA in 11/32 (34.3%) and CY (Fig. 2) in 25/32(78.1%).Precision rates for ST (5/32, 15.6%), CAP (0/64) and COM + CAP (3/30, 10%) were quite lower. User accuracy was considered the probability that a prediction of a certain class composition was really in that class composition. COD was falsely diagnosed in 99 cases, COM in 41, UA in 80, COM + UA in 63, CY in 9, ST in 32, COM + AP in 66. A diagnosis of CAP was never made.
Only five times COD was misdiagnosed as COM whereas COM was more frequently misdiagnosed as COD (40 predictions). Uric acid was misdiagnosed as COD in 18/96 (18.7%), as COM in 4/96 (4.1%), as COM + UA in 18/96 (18.7%), as COM + AP in 13/96 (13.5%), as ST in 9/96 (9.3%) and as CY in 2/96 (2.1%).
DiscussionThe knowledge of the physico-chemical composition of the stone is crucial not only to understand the mechanism of stone formation and to choose the measures for the prophylaxis of recurrences after the removal of the stone, but also to plan the strategy of stone treatment. The knowledge of the composition of the stone can modify our therapeutic choices as some stones, such as those of uric acid, can be treated by oral chemolysis, other stones, as brushite stones, are particularly hard and resistant to extracorporeal treatment and others, such as cystine stones, show high recurrence requiring complete clearance of all the fragments which can be more easily obtained with percutaneous treatment. For these reasons the possibility of knowing the composition of the stones before the procedure of stone removal is highly desirable.
A first step of in vivo stone evaluation is the computed tomography (CT) of the stone and the assessment of stone density, which allows a first differentiation based on Hounsfield Units (HU). A great deal of research has been carried out in this direction with the use of different technologies which suggested that HU and their variants are useful for predicting the composition of stones although they do not allow a precise identification of the composition of the stone in the single patient. Particularly, HU characterization showed high efficacy in distinguishing low-density uric acid stones from other types of stones.3 Dual Energy Computed Tomography (DECT) classifies urinary calculi into uric acid and non-uric acid stones with almost 100% accuracy.4 On the contrary, calcium oxalate monohydrate and calcium oxalate dihydrate stones cannot be easily discriminated by DECT, because they have similar chemical compositions and closer atomic numbers. Stone morphological information represents an adjunctive tool to differentiate stones with similar density values. Laboratory ex-vivo morphological evaluation of calcium oxalate stones by binocular stereoscopic microscopy5 or micromorphology using scanning electron microscopy6 have been extensively described. Calcium oxalate dihydrate (COD) stones show a spiculated surface with bipyramidal crystals with sharp angles and blunt edges, whereas calcium oxalate monohydrate (COM) stones are usually smooth although sometimes present a mammillary or mulberry-shaped surface morphology.
Morphology of the surface and the internal structure of the urinary stones have also been studied in vivo with imaging studies. Distinct radiographic patterns of COM and COD stones were demonstrated by plain film X-ray7–9 and high resolution helical computed tomography using bone windows and a narrow slice width to enhance visualization of surface and internal stone structure.10–12 A method for quantitative measurement of some morphological aspects of the stone at 3D CT images has been recently developed by Duan et al.13. The calculated shape index showed high sensitivity and specificity in differentiating COM from COD stones both in an experimental setting by using micro-computerized tomography and in clinical evaluation of CT images. These evidences confirm that kidney stones have unique morphological features that strongly correlate with chemical composition. On the other hand, the observation of the stones with binocular stereoscopic microscope is considered an important preliminary step of the analysis of the urinary stone allowing to identify the stone structure and the specific areas to be taken to be subjected to analysis with spectroscopy or other methods. Nayir14 showed a sensitivity of 93% for binocular stereoscopic microscopy using as gold standard the result of X-ray diffraction in comparison with a much lower sensitivity of 58% obtained by wet chemical analysis. Endoscopic observation of the stone could be comparable to microscopic observation because allows to have a magnified view of the stone both when still intact before lithotripsy and during the fragmentation process shows the inner components of the stone and their stratification. Today it is impossible or quite rare to observe the intact stone in the laboratory, while the analysis is often limited to the evaluation of small fragments of which we usually do not know the precise localization in the overall structure of the stone. For this reason, in the era of stone dusting the examination of the stone and its different components and stratifications in vivo before lithotripsy becomes an important objective. Unfortunately, the results of our video-identification survey of stone composition are unsatisfactory with a total efficacy of only 39%. This result can be explained by the limitations of the method itself or by the insufficient training of endourologists to the visual identification of the stone composition.
However, our study confirmed that endoscopic observation of the stone can give some information on stone composition allowing to identify with a good sensitivity calcium oxalate dihydrate and cystine stones. Specific training of endourologists to recognize the morphological aspects of stones of different composition could increase the effectiveness of the method, although it is undeniable that the method has limitations, particularly in the recognition of the composition of mixed stones. In future, advances in endoscope technology, such as Raman spectroscopy, polarization endoscopy and hyperspectral imaging, and digital image post-processing could further improve the diagnostic efficacy of endoscopic observation by allowing in-vivo analysis with results comparable to those of on bench laboratory evaluation.15 Raman spectroscopy seems to be a promising tool to be integrated into lithotripsy laser systems. Miernik et al.16 demonstrated a 100% sensitivity and specificity in comparison to infrared spectroscopy of a compact portable Raman spectroscopy system for immediate automated postoperative ex vivo analysis of the urinary stone composition. The same Authors coupled their experimental Raman system to common commercial lithotripsy laser fibers (200 and 940 µm) to develop a tool able to be used for real-time stone analysis during endoscopic treatment17. Hopefully, this technology will be further developed for future clinical practice.
A potential bias of our study was the low number of stones included in our survey, that we have voluntarily limited in order to contain the time required to complete the survey preventing a drop of attention of the participants. On the other hand, the aim of the study was to evaluate the potential role of the video-identification of the most common types of stones in the clinical routine. Another possible limitation of our study was the quality of the images that were used for subjective evaluation of stone composition. However, our purpose was a real-life evaluation of possible diagnostic use of this information in daily clinical practice. It is also possible that the material sent to the laboratory was not representative of the whole stone because as usual, only a few fragments were recovered. This condition again represents what normally happens in the clinical practice where minor fragments are left behind and no effort is made to send for analysis separate material from different stratifications of the stones.
ConclusionsIn conclusion, the endoscopic view of the stone under standard conditions provides, by itself, limited information on stone composition, although new technological advances could increase the information that can be obtained through the endoscopic assessment of the stone. Pending the imminent technological advances, the post-operative analysis in the laboratory of the stone fragments expelled or retrieved after the lithotripsy is still mandatory. Our study is the only to assess the possibility of identifying the composition of urinary stone by endoscopic visualization, demonstrating that even experienced surgeons may not be able to recognize the composition of the stone. However, it should be stressed the importance to carefully observe the stone during the endoscopic lithotripsy procedure and to accurately describe it in our report. A video clip or a photo illustrating the intact stone and its internal structure during the fragmentation process should be taken and attached to the report. In parallel, urology residents should receive a specific training on the macroscopic aspect of urinary stones and should be trained in endoscopic observation and recognition of the most frequent types of renal stones.
Please cite this article as: Sampogna G, Basic D, Geavlete P, et al. Identificación endoscópica de la composición de los cálculos urinarios: un estudio del Southeastern Group for Lithiasis Research (SEGUR 2). Actas Urol Esp. 2021;45:154–159.
El resto de componentes del grupo SEGUR se detallán en el Anexo: Chalil Arif (Alexandroupolis, Greece), Murat Bağcioğlu (Kars, Turkey), Alberto Budia-Alba (Valencia, Spain), Juan Pablo Caballero-Romeu (Elche-Alicante, Spain), Elisa De Lorenzis (Milano,Italy), Joao Dores (Lisboa,Portugal), Bilal Eryildirim (Istanbul, Turkey), Ognyan Gatsev (Sofia, Bulgaria), Stilianos Giannakopoulos (Alexandroupolis, Greece), Stefan Hristoforov (Sofia, Bulgaria), Mirko Jovanović (Belgrade, Serbia), Andreas Karagiannis (Athens, Greece), Vladimir Lozanovski (Skopje, Macedonia), Alessandro Maletta (Lecco,Italy), Bloju Marin (Bucharest, Romania), Emanuele Montanari (Milano, Italy), Tomislav Mostrov (Northern Macedonia), Kremena Petkova (Sofia, Bulgaria), Petur Petrov (Sofia, Bulgaria), Carmine Sciorio (Lecco, Italy), Emrah Yuruk (Istanbul, Turkey), Pablo Vargas Andreu (Alicante, Spain), Vladimir Vasic (Serbia), Sara Villarroya Castillo (Valencia,Spain), Bojan Vuckovic (Prokuplje, Serbia), Stefano Paolo Zanetti (Milano, Italy).