The diagnosis and molecular staging of bladder cancer based on the detection of gelatinases mRNA (MMP-2 and MMP-9) in peripheral blood circulating and mononuclear cells have shown promising results. We analyze if the determination of the corresponding protein synthesis products makes it possible to diagnose and characterize patients with bladder cancer.
Materials and methodsQuantification of the serum levels of MMP-2, MMP-9 and TIMP-2 in a series of 42 individuals (31 patients with bladder cancer in different stages and 11 healthy controls) using the ELISA technique was carried out. The determinations were compared between cases and controls (Mann–Whitney U) and between different groups of tumors (Mann–Whitney U or Kruskal–Wallis), according to the clinical–pathological characteristics (age, gender, T category, M category or grade). Diagnostic yield of these markers was evaluated by analysis of the ROC curves.
ResultsThere is a correlation between the determinations of MMP-2 and TIMP-2 (R=0.699; p>0.0001) and MMP-9 and TIMP-2 (R=0.305; p=0.049). Patients with bladder cancer have higher levels of MMP-9 (p<0.0001) and TIMP-2 (p=0.047) than the controls. Furthermore, the MMP-9/TIMP-2 ratio is also superior in cancer patients (p<0.001). Differences were not detected between cancer and controls regarding age (p=0.64) or gender (p=0.64). Differences were also not detected regarding MMP-2 (p=0.35) or MMP-2/TIMP-2 rate (p=0.45). Within the cancer patient population, the MMP-2 and MMP-9 values differ according to T category (p=0.022 and p=0.038, respectively) and those of the TIMP-2 according to M category (p=0.036). ROC curve analysis showed that both MMP-9 and the MMP-9/TIMP-2 ratio discriminate patients with cancer and controls, with equivalent diagnostic accuracy (ABC 0.953) and cut offs of 3.93ng/mL (S 90%; Sp 81%) and 0.053ng/mL (S 96%; Sp 84%), respectively.
ConclusionsThe results obtained suggest that both serum MMP-9 and TIMP-2 would have an application in the prediction of the development and progression of bladder cancer, and a potential utility as clinical markers of the disease. Multicenter, prospective studies that confirm their preliminary results are necessary.
El diagnóstico y la estadificación molecular del cáncer vesical basados en la detección de ARNm de gelatinasas (MMP-2 y MMP-9) en células circulantes y mononucleares de sangre periférica han mostrado resultados prometedores. Analizamos si la determinación de los correspondientes productos de síntesis proteica permite diagnosticar y caracterizar pacientes con neoplasia vesical.
Material y métodoSe ha llevado a cabo la cuantificación de los niveles séricos de MMP-2, MMP-9 y TIMP-2 en una serie de 42 individuos (31 pacientes con cáncer vesical en diversos estadios y 11 controles sanos) mediante técnica de ELISA. Se compararon las determinaciones entre casos y controles (U Mann–Whitney), así como entre diferentes grupos de tumores (U Mann–Whitney o Kruskal–Wallis), según las características clínico-patológicas (edad, sexo, categoría T, categoría M o grado). Se evaluó el rendimiento diagnóstico de estos marcadores mediante análisis de curvas ROC.
ResultadosExiste correlación entre las determinaciones de MMP-2 y TIMP-2 (R=0,699; p>0,0001) y de MMP-9 y TIMP-2 (R=0,305; p=0,049). Los pacientes con cáncer de vejiga presentan niveles más elevados de MMP-9 (p<0,0001) y TIMP-2 (p=0,047) que los controles. Así mismo, el cociente MMP-9/TIMP-2 también es superior en pacientes con cáncer (p<0,001). No se detectan diferencias entre cáncer y control respecto a edad (p=0,64) o sexo (p=0,64). Tampoco se detectan diferencias con respecto a MMP-2 (p=0,35) ni al cociente MMP-2/TIMP-2 (p=0,45). Dentro de la población de pacientes con cáncer los valores de MMP-2 y MMP-9 difieren según categoría T (p=0,022 y p=0,038, respectivamente) y los de TIMP-2 según categoría M (p=0,036). El análisis de curvas ROC mostró que tanto MMP-9 como el cociente MMP-9/TIMP-2 discriminan pacientes con cáncer y controles, con equivalente exactitud diagnóstica (ABC 0,953) y unos puntos de corte de 3,93ng/ml (S 90%; E 81%) y de 0,053ng/ml (S 96%; E 84%), respectivamente.
ConclusionesLos resultados obtenidos sugieren que tanto MMP-9 como TIMP-2séricos podrían tener una aplicación en la predicción del desarrollo y progresión del cáncer vesical, y potencial utilidad como marcadores clínicos de la enfermedad. Se requieren estudios multicéntricos prospectivos que confirmen estos resultados preliminares.
Therapeutic failure in bladder cancer is due, generally, to the development of a hidden metastasis, already present at primary treatment.1 The extent of the disease and the histological grade are the best prognosticators of disease control and survival in patients with bladder cancer.2 However, nowadays and in order to establish some correlation with higher probability of distant spread, many molecular markers are being under thorough investigation: in patients neoplastic tissue, urine or blood.
Despite the high development of different researches on epigenetics, genomics and proteomics,3,4 we must assume that there is lack of molecular markers with clinical utility for bladder cancer. An important aspect of the metastatic cascade is the degradation of extracellular matrix (ECM) by specific proteolytic enzymes. Therefore, the family of matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases that regulate the integrity and composition of the ECM, is one of the best candidates to be considered biomarkers of bladder cancer.5 Their role in the degradation of connective tissue stroma and basement membrane, natural barriers tumoral progression is known, as well as their involvement in modulation of normal cell behavior and cell communication.7
The regulation of the activity of MMP-2 and MMP-9 (also known as gelatinase MMP) occurs at many levels, including transcription, secretion, activation and inhibition by tissue inhibitors of metalloproteinases (TIMP). Overproduction of MMP by tumors interacting with lymphatic and vascular systems could lead to increased levels of MMP and TIMP, not only in tissues but also in other biological fluids, such as blood or urine. Many studies have emphasized the MMP and TIMP overexpression in bladder cancer.8–13 Our group has investigated a new approach to assess the activity of MMP using RT-PCR in peripheral blood mononuclear cells, with special emphasis on gene amplification of MMP-9, MMP-2 and TIMP-2 in healthy controls and patients with bladder cancer.14
Materials and methodsThe study was conducted on 42 subjects, age and gender controls matched healthy individuals (n=11) and patients with bladder cancer (n=31) diagnosed and treated in our center. Histopathology according to WHO criteria was performed in all patients with cancer and the staging of the disease according to the criteria of the AJCC 2006. All patients gave their consent to donate biological material, in accordance with the requirements of the Clinical Research Ethics Committee of the center.
Blood samples from each subject were collected into plastic tubes (tubes BDcat # 366703) under aseptic conditions at the time of anesthesia induction. The blood samples were collected into plastic tubes (tubes BDcat # 366703) without clot activator, to prevent the release of gelatinase during platelet activation and to allow clotting at room temperature. Within 30′ after extraction the tubes were centrifuged at 1600g for at least 15min at 4°C. The supernatant was frozen at −80°C until analyzed. Each aliquot was used only once to prevent enzymatic activation due to freeze/thaw ingprocess.
Serum MMP-2, MMP-9 and TIMP-2 were measured by enzyme-linked immunosorbent assay technique (ELISA) using reagents from the company R & DSystems, Inc., Minneapolis, MN, USA (MMP2: Cat # DMP2F0; MMP9: Cat # DMP900 and TIMP2: Cat # DTM200) following the manufacturer's technical specifications. Each sample group was tested in triplicate on each ELISA plate.
A descriptive analysis of the series was conducted and the homology between groups and controls for age and sex were compared. Medians for the different values between groups were compared using Mann–Whitney U (two groups) and Kruskal Wallis (more than 2 groups). Value at p<0.05 was considered significant. When the variables determined differences between cases and controls, the diagnostic ability to differentiate cancer patients and controls was studied using Receiver Operating Characteristic curve analysis (ROC). Analyses were performed using the integrated package Statistical Analysis System (SAS) SAS Institute Inc.
Results42 subjects (36 men and 6 women) were studied, with average age 64 (95% CI: 59.7–68.3, range 29–90), including healthy subjects for control (n=11) and bladder cancer patients (n=31). The equivalence between cases and controls with respect to sex (Fisher exact test, p=0.64) and age (F of Snedecor, p=0.64) was confirmed. The distribution of patients with neoplasia by T category was: Ta (n=7), T1 (n=8), T2 (n=7), T3 (n=4) and T4 (n=5). All patients received primary transurethral resection of bladder tumor and 10 of them underwent also cystectomy. The preoperative evaluation revealed metastatic spread (M1) in 5 cases (16%) and 2 tumors histopathologically defined grade 1 (6.5%), 6 grade 2 (19.4%) and 23 grade 3 (74.2%).
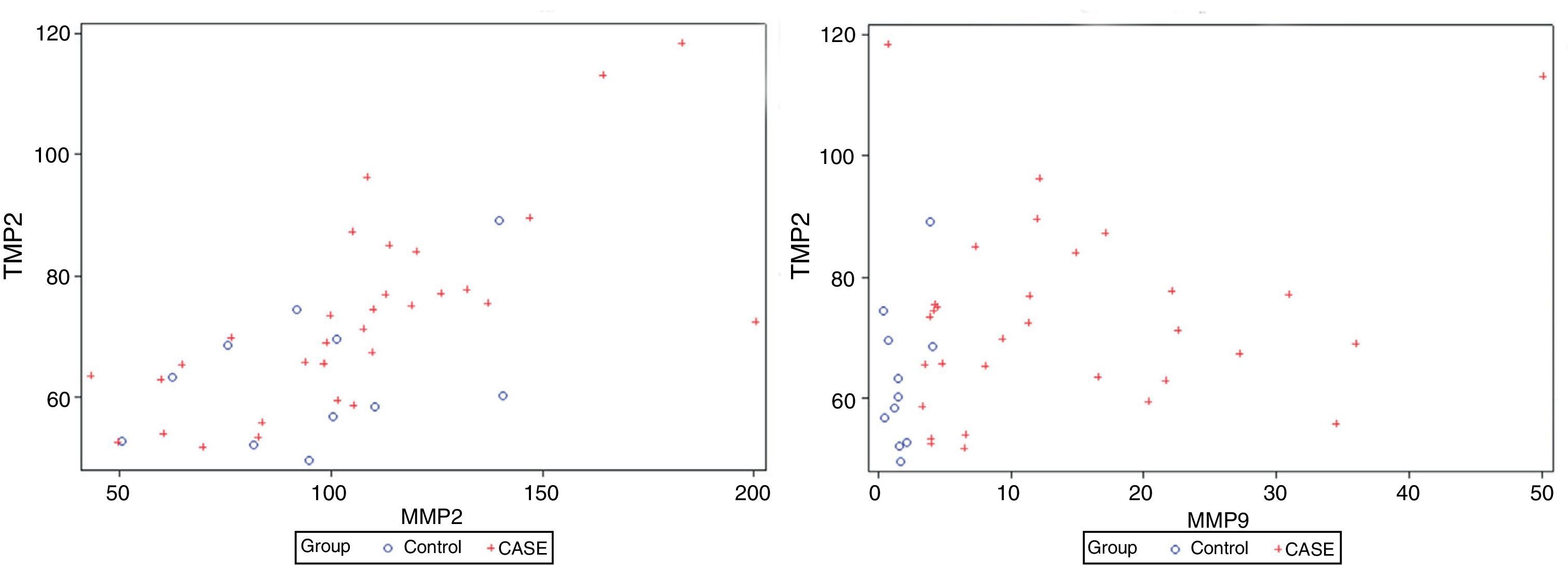
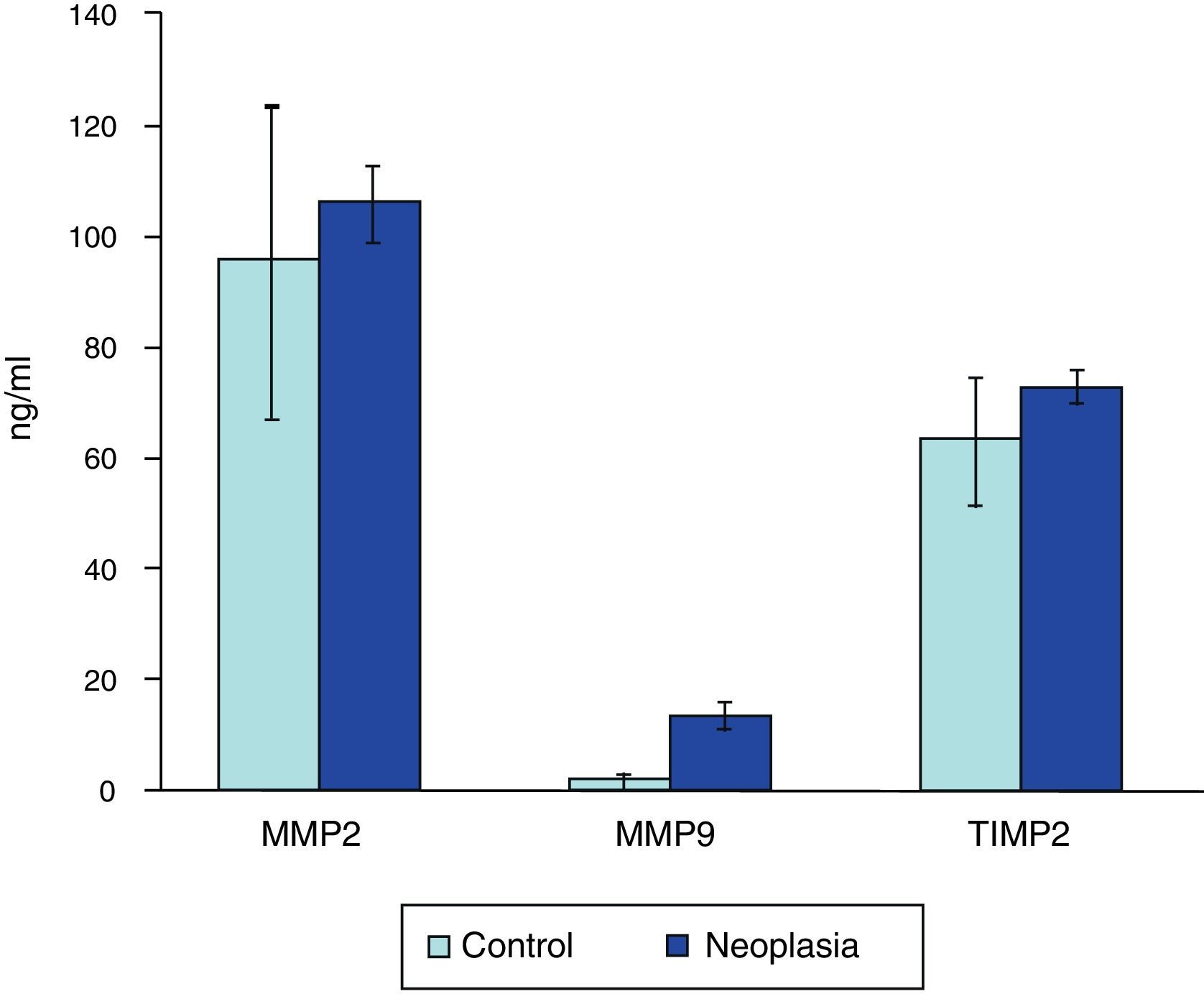
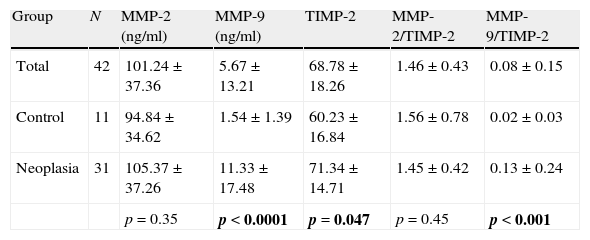
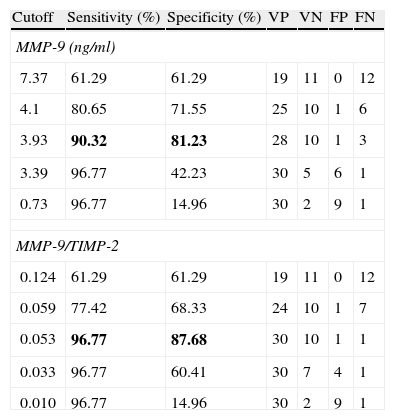
There is correlation between the measurements of MMP-2 and TIMP-2 (R=0.699, p>0.0001), and between MMP-9 and TIMP-2 (R=0.305, p=0.049) (Fig. 1). No correlation between the levels of MMP-2 and MMP-9 (R=0.126, p=0.43) was found. Serum levels of MMP-9 were significantly higher in cancer patients than in the control group (Mann–Whitney U, p<0.0001). Levels of TIMP-2 were also significantly higher in cancer patients (p=0.047), whereas levels of MMP-2 were not (p=0.35) (Fig. 2). There were no differences either in value of the ratio of MMP-2/TIMP-2 (p=0.45), but differences in the value of the ratio MMP-9/TIMP-2 (p<0.001) were observed (Table 1).
Serum values of MMP-9, MMP-2 and TIMP-2 and their respective ratios.
| Group | N | MMP-2 (ng/ml) | MMP-9 (ng/ml) | TIMP-2 | MMP-2/TIMP-2 | MMP-9/TIMP-2 |
| Total | 42 | 101.24±37.36 | 5.67±13.21 | 68.78±18.26 | 1.46±0.43 | 0.08±0.15 |
| Control | 11 | 94.84±34.62 | 1.54±1.39 | 60.23±16.84 | 1.56±0.78 | 0.02±0.03 |
| Neoplasia | 31 | 105.37±37.26 | 11.33±17.48 | 71.34±14.71 | 1.45±0.42 | 0.13±0.24 |
| p=0.35 | p<0.0001 | p=0.047 | p=0.45 | p<0.001 |
Values are expressed as medians±interquartile range. The p-value corresponds to the U Mann–Whitney test. Bold values reach statistical significance.
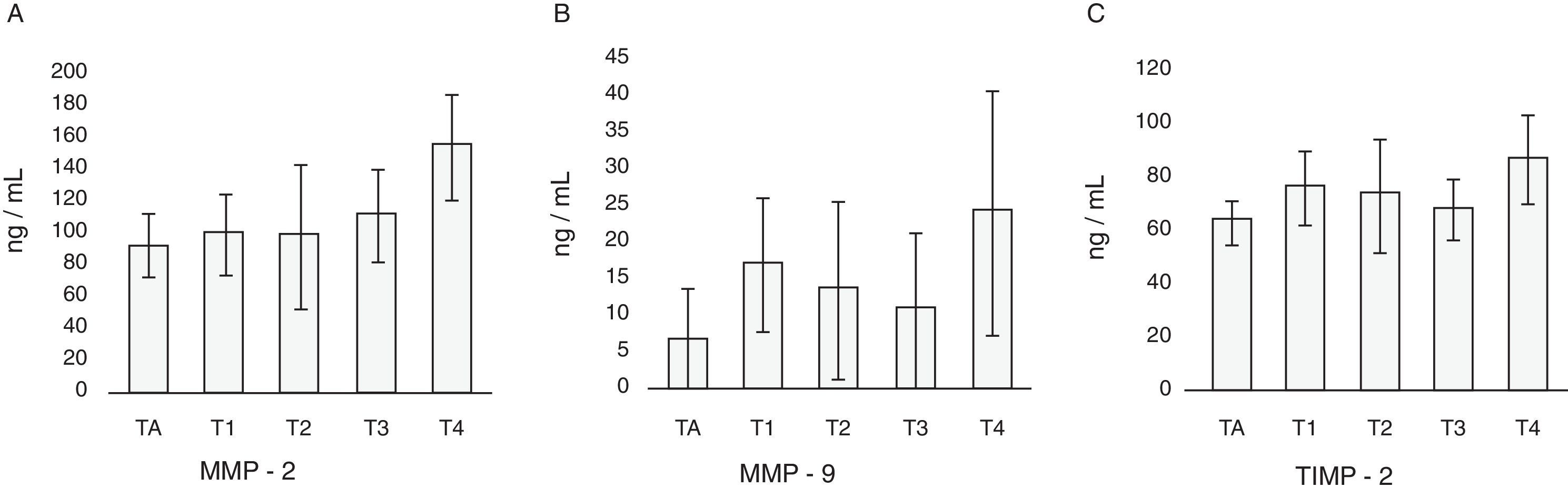
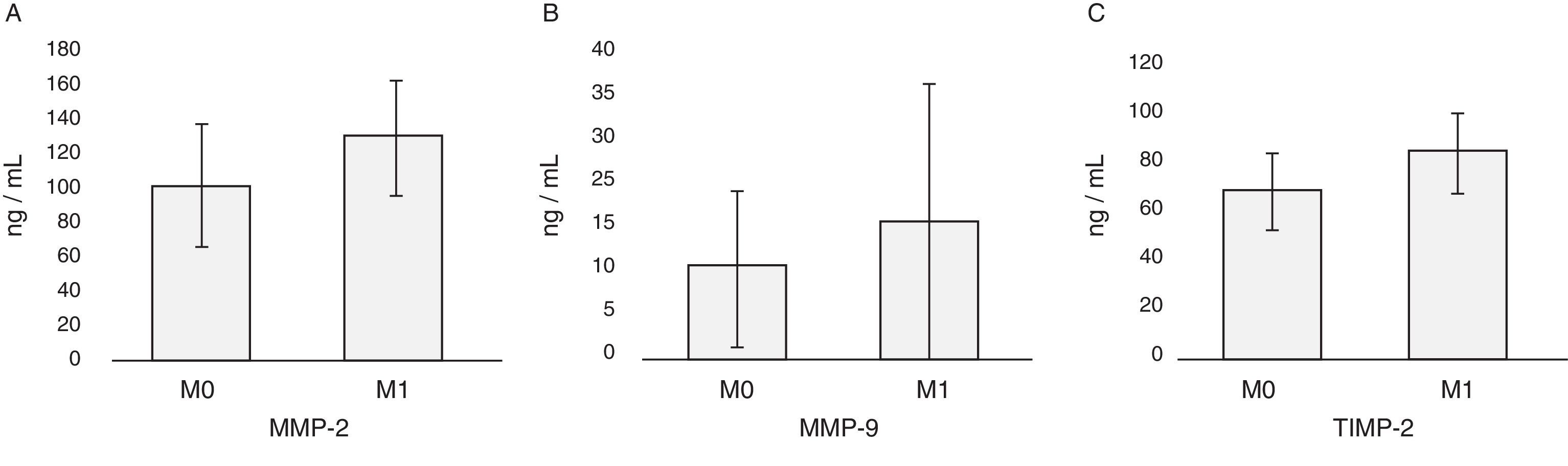
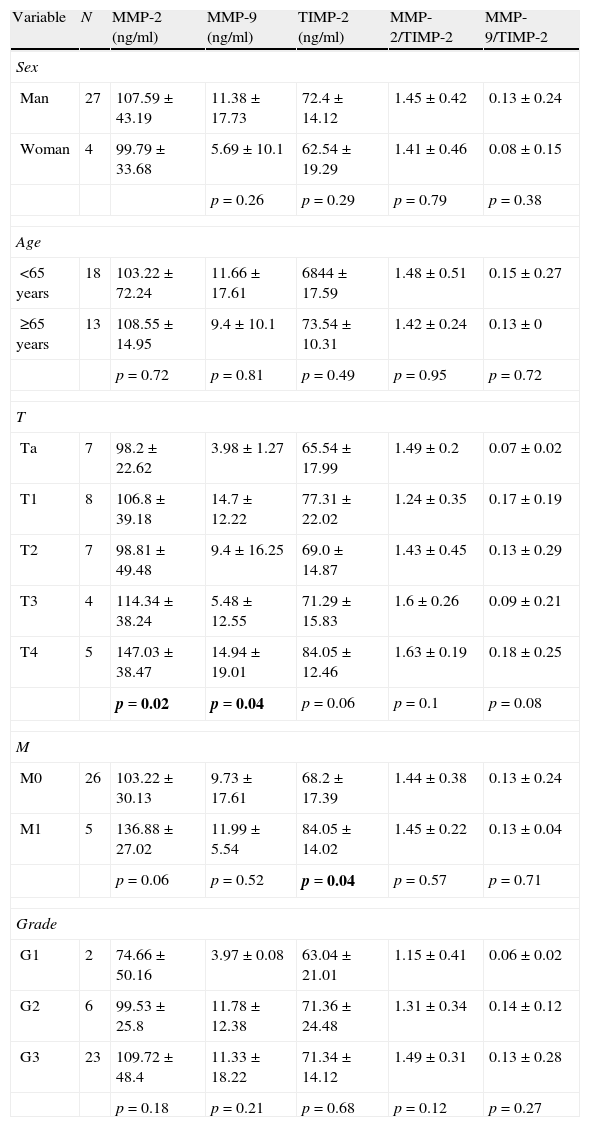
In patients with cancer no differences in the levels of MMP-2, MMP-9 and TIMP-2 with respect to sex or age of the patients are found (Table 2). The highest values of MMP-2 correspond to T3 and T4 tumors, and the values of MMP-9 and TIMP-2 to T4 tumors (Table 2; Fig. 3). Nor are observed differences related to MMP-2/TIMP-2 ratios (p=0.1) or MMP-9/TIMP-2 (p=0.08). With respect to the category M, levels of MMP-2, MMP-9 and TIMP-2 were higher in metastatic patients at diagnosis; only the difference between the values of TIMP-2 reached statistical significance (Mann–Whitney U; p=0.04) but it was not the case with MMP-2 (p=0.06) or MMP-9 (p=0.52) (Table 2; Fig. 4). There is no discrimination between patients with or without metastasis MMP-2/TIMP-2 ratios (p=0.573) or MMP-9/TIMP-2 (p=0.707). Neither MMP-2/TIMP-2 ratios (p=0.573) nor MMP-9/TIMP-2 (p=0.707) discriminates between patients with or without metastasis. None of the compounds tested show differences in the cancer patients groups according to the histologic grade (Table 2).
2 Serum values of MMP-2, MMP-9, TIMP-2 and their respective ratios in patients with bladder cancer according to clinicopathologic variables.
| Variable | N | MMP-2 (ng/ml) | MMP-9 (ng/ml) | TIMP-2 (ng/ml) | MMP-2/TIMP-2 | MMP-9/TIMP-2 |
| Sex | ||||||
| Man | 27 | 107.59±43.19 | 11.38±17.73 | 72.4±14.12 | 1.45±0.42 | 0.13±0.24 |
| Woman | 4 | 99.79±33.68 | 5.69±10.1 | 62.54±19.29 | 1.41±0.46 | 0.08±0.15 |
| p=0.26 | p=0.29 | p=0.79 | p=0.38 | |||
| Age | ||||||
| <65 years | 18 | 103.22±72.24 | 11.66±17.61 | 6844±17.59 | 1.48±0.51 | 0.15±0.27 |
| ≥65 years | 13 | 108.55±14.95 | 9.4±10.1 | 73.54±10.31 | 1.42±0.24 | 0.13±0 |
| p=0.72 | p=0.81 | p=0.49 | p=0.95 | p=0.72 | ||
| T | ||||||
| Ta | 7 | 98.2±22.62 | 3.98±1.27 | 65.54±17.99 | 1.49±0.2 | 0.07±0.02 |
| T1 | 8 | 106.8±39.18 | 14.7±12.22 | 77.31±22.02 | 1.24±0.35 | 0.17±0.19 |
| T2 | 7 | 98.81±49.48 | 9.4±16.25 | 69.0±14.87 | 1.43±0.45 | 0.13±0.29 |
| T3 | 4 | 114.34±38.24 | 5.48±12.55 | 71.29±15.83 | 1.6±0.26 | 0.09±0.21 |
| T4 | 5 | 147.03±38.47 | 14.94±19.01 | 84.05±12.46 | 1.63±0.19 | 0.18±0.25 |
| p=0.02 | p=0.04 | p=0.06 | p=0.1 | p=0.08 | ||
| M | ||||||
| M0 | 26 | 103.22±30.13 | 9.73±17.61 | 68.2±17.39 | 1.44±0.38 | 0.13±0.24 |
| M1 | 5 | 136.88±27.02 | 11.99±5.54 | 84.05±14.02 | 1.45±0.22 | 0.13±0.04 |
| p=0.06 | p=0.52 | p=0.04 | p=0.57 | p=0.71 | ||
| Grade | ||||||
| G1 | 2 | 74.66±50.16 | 3.97±0.08 | 63.04±21.01 | 1.15±0.41 | 0.06±0.02 |
| G2 | 6 | 99.53±25.8 | 11.78±12.38 | 71.36±24.48 | 1.31±0.34 | 0.14±0.12 |
| G3 | 23 | 109.72±48.4 | 11.33±18.22 | 71.34±14.12 | 1.49±0.31 | 0.13±0.28 |
| p=0.18 | p=0.21 | p=0.68 | p=0.12 | p=0.27 | ||
Values are expressed as medians±interquartile range. p-Value corresponds to Mann–Whitney U test when comparing two variables and Kuskal–Wallis test when comparing >2. Bold values reach statistical significance.
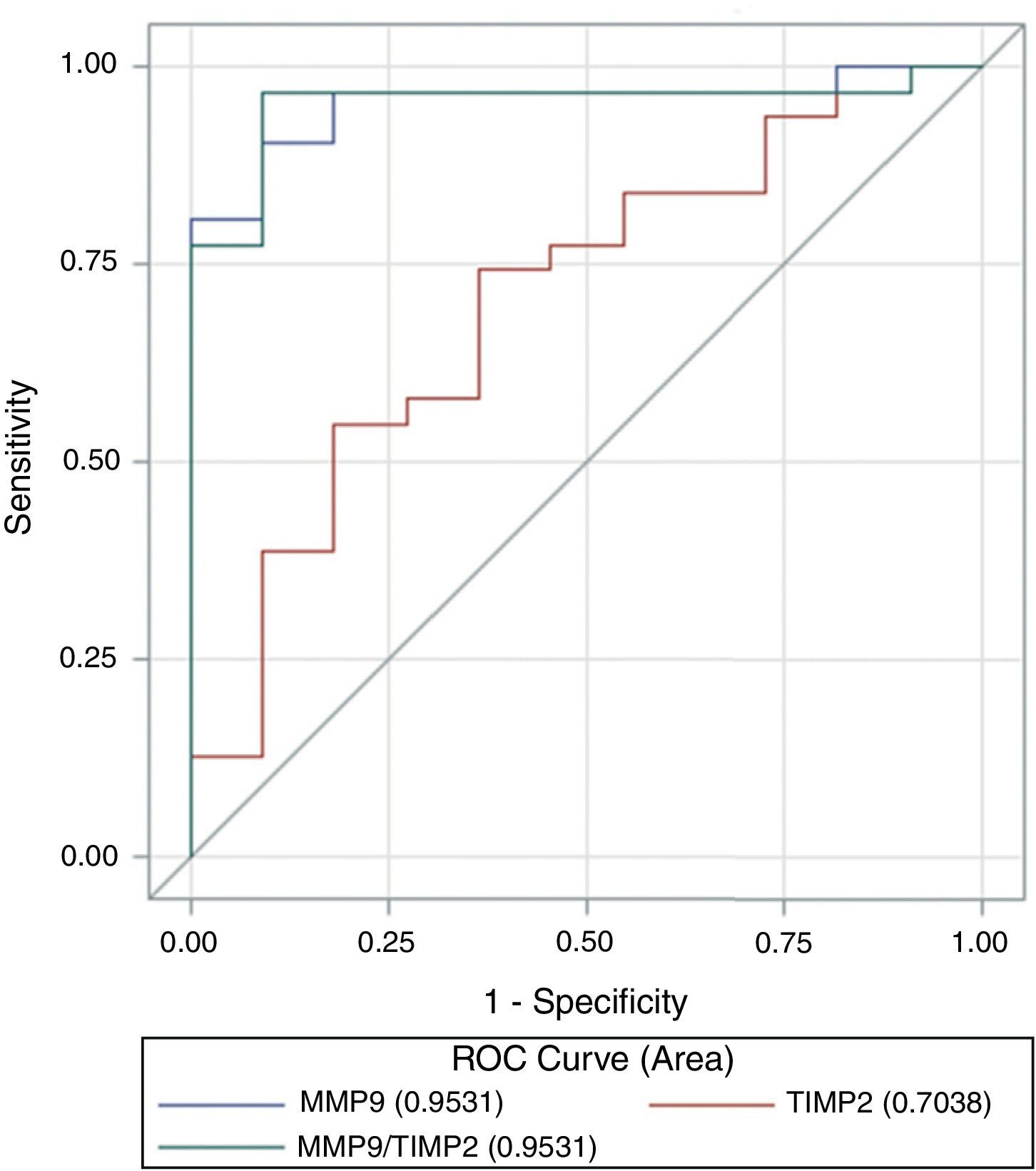
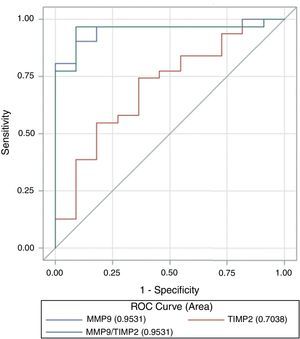
ROC curve analysis revealed that both MMP-9 (AUC=0.95, 95% CI 0.89–1.0) and the ratio MMP-9/TIMP-2 (AUC=0.95, 95% CI 0.88–1.0) discriminate between cancer patients and controls with equivalent diagnostic accuracy. However, the capacity of TIMP-2 to set the discrimination was lower (ABC=0.7, 95% CI 0.52–0.89) (Fig. 5). The difference between areas under the curve was statically significant between MMP-9 and TIMP-2 (Z test, p=0.009) and between MMP-9/TIMP-2 and TIMP-2 (p=0.015). MMP-9 cut-off value of 3.93ng/ml provided discrimination between cancer patients and controls, with a sensitivity and specificity to predict the occurrence of cancer in this series of 90 and 81%, respectively. Similarly, the optimal MMP-9/TIMP-2 cut-off was 0.053ng/ml, with a sensitivity of 96% and specificity of 84% (Table 3).
Cut-off points for MMP-9 and MMP-9/TIMP-2 to discriminate cancer and control.
| Cutoff | Sensitivity (%) | Specificity (%) | VP | VN | FP | FN |
| MMP-9 (ng/ml) | ||||||
| 7.37 | 61.29 | 61.29 | 19 | 11 | 0 | 12 |
| 4.1 | 80.65 | 71.55 | 25 | 10 | 1 | 6 |
| 3.93 | 90.32 | 81.23 | 28 | 10 | 1 | 3 |
| 3.39 | 96.77 | 42.23 | 30 | 5 | 6 | 1 |
| 0.73 | 96.77 | 14.96 | 30 | 2 | 9 | 1 |
| MMP-9/TIMP-2 | ||||||
| 0.124 | 61.29 | 61.29 | 19 | 11 | 0 | 12 |
| 0.059 | 77.42 | 68.33 | 24 | 10 | 1 | 7 |
| 0.053 | 96.77 | 87.68 | 30 | 10 | 1 | 1 |
| 0.033 | 96.77 | 60.41 | 30 | 7 | 4 | 1 |
| 0.010 | 96.77 | 14.96 | 30 | 2 | 9 | 1 |
Values in bold correspond to the optimal cut-off points showing maximum values of sensitivity and specificity.
Many authors have investigated the role of MMPs and more specifically of gelatinases (MMP-2 and/or MMP-9) on bladder neoplasia, applying different approaches for studying the activity of these enzymes ECM degradation.5 However, we are increasingly aware that these substances are less related to the matrix, as originally interpreted, and that is a class of enzymes ubiquitous in nature related to own communication between cells.7 No wonder therefore their role in cardiovascular, neurodegenerative diseases and tumor progression.15
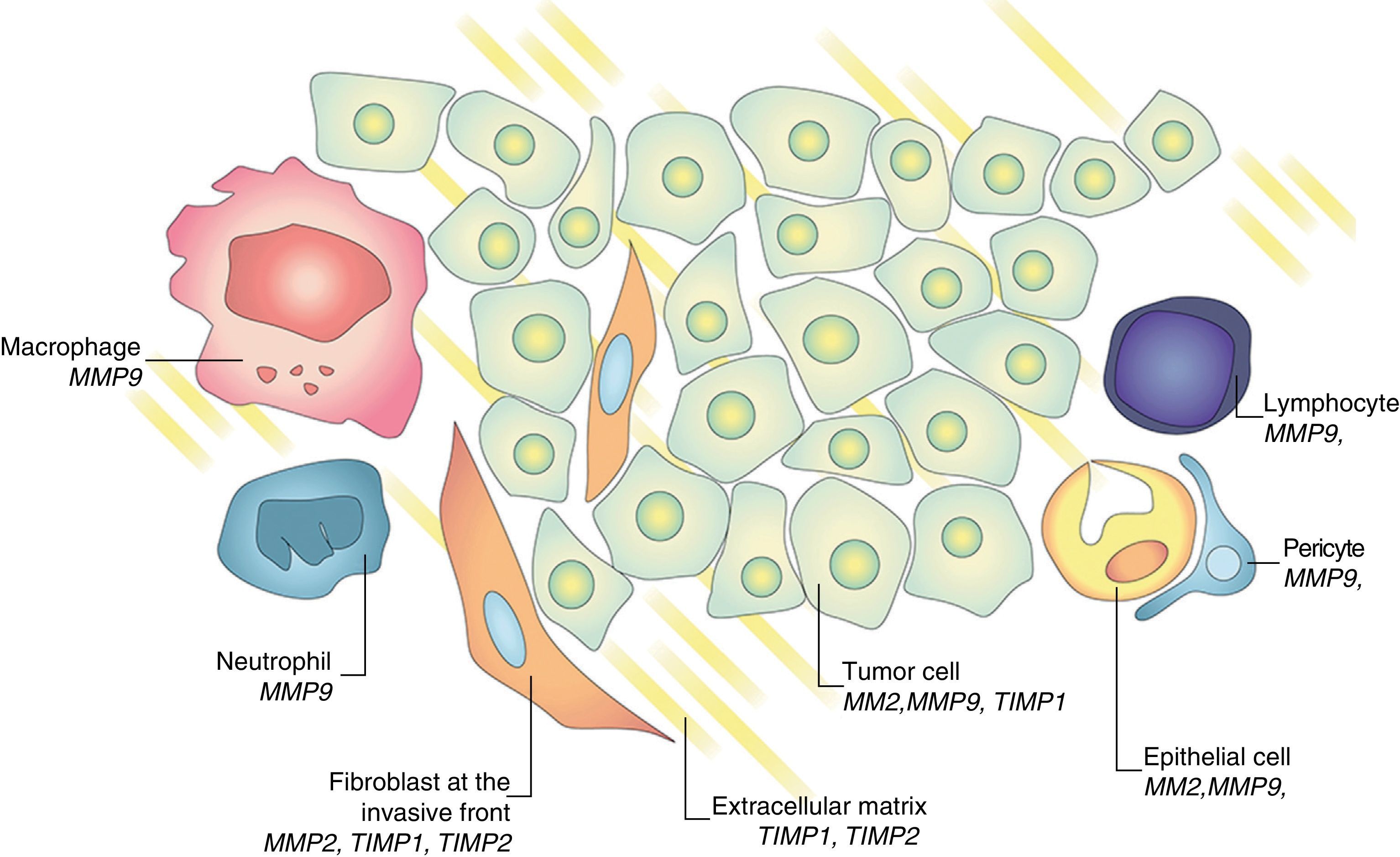
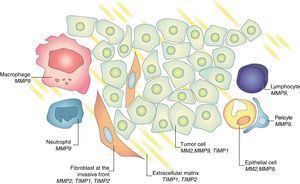
Regulatory process exists between metalloproteinases and tissue inhibitors in tumor progression and metastasis development.15,16 Gene expression and activation of MMPs are associated with the invasive potential of tumor cells. It was initially considered that the MMPs were produced exclusively by tumor cells. This concept was replaced by the more widespread belief that some MMPs are mainly produced by the stroma; for example, stromelysin (MMP-3) is produced by tumor-fibroblasts. Similarly, TIMP-1 and TIMP-2 are produced in both neoplastic cells and stromal counteracting collagenase and gelatinase activity (Fig. 6). This inhibitors family promotes tumor proliferation or induces apoptosis in some systems and inhibits tumor growth and promotes cell death in others.16 This apparently contradictory response of TIMP-2, which on the one hand acts as a growth factor and facilitates the development of metastasis while on the other hand inhibits angiogenesis is known as homeostatic response to high expression of MMP-2.6 The expression of these substances can be studied in different ways, evaluating their presence in tumor tissue and in healthy stroma interacting with tumor environment itself, in the urine of patients, serum, and even in the peripheral blood mononuclear cells.5,14
Several studies have confirmed that the immunohistochemical expression (IHC) of MMP plays a prognostic role in bladder cancer. Grignon et al. were pioneer when they established that an elevated expression of TIMP-2 in both neoplastic cells and in the surrounding stroma, relates to worse survival when associated with loss of collagen type immunostaining iv.8 Other studies confirmed that the IHC expression of MMP-9,17,18 MMP-2,19,20 MMP-721 in paraffin tissue has implications for poor prognosis. An analysis of 1176 genes associated with bladder cancer showed expression of MMP-2 and TIMP-2 by RT-PCR in tissue in all patients who died of the disease.22 Therefore, there is a significant body of knowledge that supports the fact that the expression of MMPs in tissue is an essential step in tumor progression of bladder cancer.5,15
Nevertheless, from the clinical standpoint, there are two areas of greater interest with important implications in the diagnosis, prognosis, assessment of disease and monitoring: 1) detection of such markers in biological fluids and 2) accurate evaluation of their significance when present in urine or serum. Using quantitative gelatin zymography the detection of MMP-2 and MMP-9 in urine in invasive bladder cancer11 and its correlation with tumor stage and grade23–25 were confirmed; suggesting, even that urinary MMP-9 could be used as a possible marker for developmental control of patients.13 Other authors measured gelatinase expression in bladder cells exfoliated and found a significant correlation between pro-MMP-9 expression and the detection of neoplasia.24 The study by ELISA in the supernatant of urine may be the most simple to carry out and one of the most researched.17,26 Eissa et al. showed that detection of MMP-9 may be useful to increase the sensitivity of urine cytology.27 Other authors also proposed that this marker in urine correlates with stage and tumor size.18 More recently, Offersen et al. proposed the determination of MMP-9 in the urine of patients with bladder cancer as an independent prognostic factor.28
To determine the levels of MMP and other related substances, serological studies using ELISA technique have been carried out. Naruo et al. found that TIMP-1 level in patients with advanced or metastatic tumors was almost twice as high compared with controls.29 Initially Gohji et al. found no differences in circulating levels of MMP-2 between the surface and controls neoplasia, although they did detect higher levels of MMP-2 in advanced disease.9 Later, the same group documented that a high risk for recurrence and progression was proportional to the ratio of MMP-2/TIMP-210.10 In this regard, the determination of serum MMP-9 is recognized as a molecular factor which promotes genesis and predicts progression of bladder cancer30 and breast cancer.31,32 Some authors have also proposed that the MMP-2/TIMP-2 ratio, measured in serum by ELISA, is associated to bladder cancer with poor prognosis33; the MMP-9/TIMP-1 ratio has been considered too a predictor of recurrence in non-muscle-invasive bladder cancer.18
These data seem highly concordant with those detected by our series. We confirm the role of MMP-9 and TIMP-2 for predicting the development and progression of bladder cancer. Actually, in our experience the detection of MMP-9 and MMP-9/TIMP-2 ratio determination by ELISA behave as useful clinical markers for disease detection. MMP-9 and MMP-2 show higher levels in patients with advanced lesions and TIMP-2 levels increase in patients with metastatic disease at diagnosis. Therefore, these results support those observed by detecting mRNA for MMP-2, MMP-9 and TIMP-2 using RT-PCR in real time in peripheral blood mononuclear cells from the same patients.14 We corroborate the finding that gelatinase activity, especially modified by TIMP-2, represents a new way to understand molecular staging bladder cancer using non-specific markers of vesical cancer.14,34 ELISA technique to determine protein levels of MMP-9 and TIMP-2 provides a quantitative and simpler measurement, while the measurement of the mRNA levels of these same elements by RT-PCR in real time which provides only semiquantitative determination; i.e., comparison between groups in relative terms. Additionally, certain discrepancies are expected between mRNA levels at steady state and the corresponding enzyme activity due to different regulation at post-transcriptional level. We propose cut-points of MMP-9 and MMP9/TIMP-2 that allow efficient discrimination between cancer patients and healthy controls. Applying effectiviness criteria, isolated determination of MMP-9 might be a better procedure for the discrimination, due to the fact that it implies a significant reduction in cost without loss of diagnostic accuracy.
We must insist that MMP-9 is a marker of tumor environment and not a marker of neoplasia; therefore, it is not a specific cancer marker. In fact, MMP function in non-neoplastic conditions with abundant angiogenesis as gestation, the healing of injuries or various inflammatory diseases has been well documented.31,35 MMP-9 is expressed predominantly in neutrophils, macrophages and mast cells, rather than in neoplastic cells themselves.6,31 The local inflammatory reaction often associated with advanced neoplasia may also stimulate the overexpression of MMP-2, MMP-9 and TIMP-2. The inflammatory reaction, thus understood, would act as an accomplice of carcinogenesis and/or tumor progression.6 The total MMP-9 activity of the host may contribute to enhance both neovascularization and tumor invasion.36
Certainly the presence of metastasis or circulating tumor cells may contribute to the increased expression of gelatinase in patients with advanced tumors; however, this does not justify the findings in healthy controls or patients with genuine superficial neoplasia (Ta), who also show detectable levels. This fact severely limits the specificity of this new marker. Despite the growing body of evidence, these preliminary results should obviously be taken with caution. Further large-scale prospective multicenter studies to evaluate the role of this marker for molecular diagnosis and staging of bladder cancer could corroborate those findings.
FinancingThis work has been partially supported by financial assistance promoted by IPSEN Pharma.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors acknowledge to Juan Dorado (Project Manager, Biostatistics, Pértica) for his statistical support and José Domínguez (Medical Documentation Service, Hospital Universitario de Getafe) for his iconographic support.
Please cite this article as: Ramón de Fata F, Ferruelo A, Andrés G, Gimbernat H, Sánchez-Chapado M, Angulo JC. El papel de la metaloproteinasa de la matriz MMP-9 y del inhibidor tisular de metaloproteinasa TIMP-2 como marcadores séricos de cáncer vesical. Actas Urol Esp. 2013;37:480–488.