INTRODUCTION
Helmintes are able to induce different diseases in humans, by direct action of the parasite, by the immunologic response induced in the host or by both mechanisms. Anisakis simplex is a worm, common parasite of fish and shellfish, acquired by humans by eating raw or undercooked fish which contain the third stage larvae of A. simplex.
Parasitic activity of the living larva in humans is called anisakiasis and symptoms are produced by penetration of the gastrointestinal mucosa by the larvae. In this sense, cases have been described of eosinophilic gastroenteritis, granulomas and abscesses with eosinophilia, fever and abdominal pain, acute appendicitis, mechanical ileus, gastritis and epigastric pain. Other organs like lungs, spleen, liver, pancreas, peritoneum, lymph nodes, amygdales and oesophagus are rarely affected. The survival of the larva usually does not exceed few weeks at the most.
Gastro allergic anisakiasis1,2 is an acute and auto-limited reaction induced by the living larva, in which abdominal symptoms (nauseas, vomits, abdominal pain, diarrhoea) coexist with hypersensitivity symptoms, such us urticaria, angioedema and anaphylaxis. Diagnosis is made by: 1) suggestive anamnesis after raw or undercooked fish consumption; 2) gastroscopy; 3) total IgE increase; 4) significance levels of specific IgE to A simplex and 5) peripheral eosinophilia (non constant).
Anisakiasis prophylaxis can be made by freezing all fish destined to consumption during 48 hours, or by industrial smoking at 60 °C at least for 10 minutes. However, termostabile proteins from A. simplex are also able to induce urticaria, angioedema or anaphylaxis by ingestion3, behaving like a real food allergy, in which prophylaxis would be to avoid the consumption of any kind of fish and shellfish.
In addition occupational asthma and conjunctivitis, contact dermatitis and arthralgia/arthritis by A. simplex exposition have been described also.
Nephrotic syndrome (NS) is characterized by albuminuria, hypoalbuminemia, hyperlipidemia and edema and it is the common final point of diverse pathological processes which alter permeability of glomerular capillary wall. Nephrotic range proteinuria (> 3 g/day) is characteristic of glomerular diseases, and it is observed rarely in tubulo-intersticial and vascular diseases of the kidney. It could be suffered as a primary or secondary consequence of multiple illnesses, like helmintic parasite infection by filarias4, Echinococcus5, Strongyloides6 and specially Schistosoma7-9.
CASE
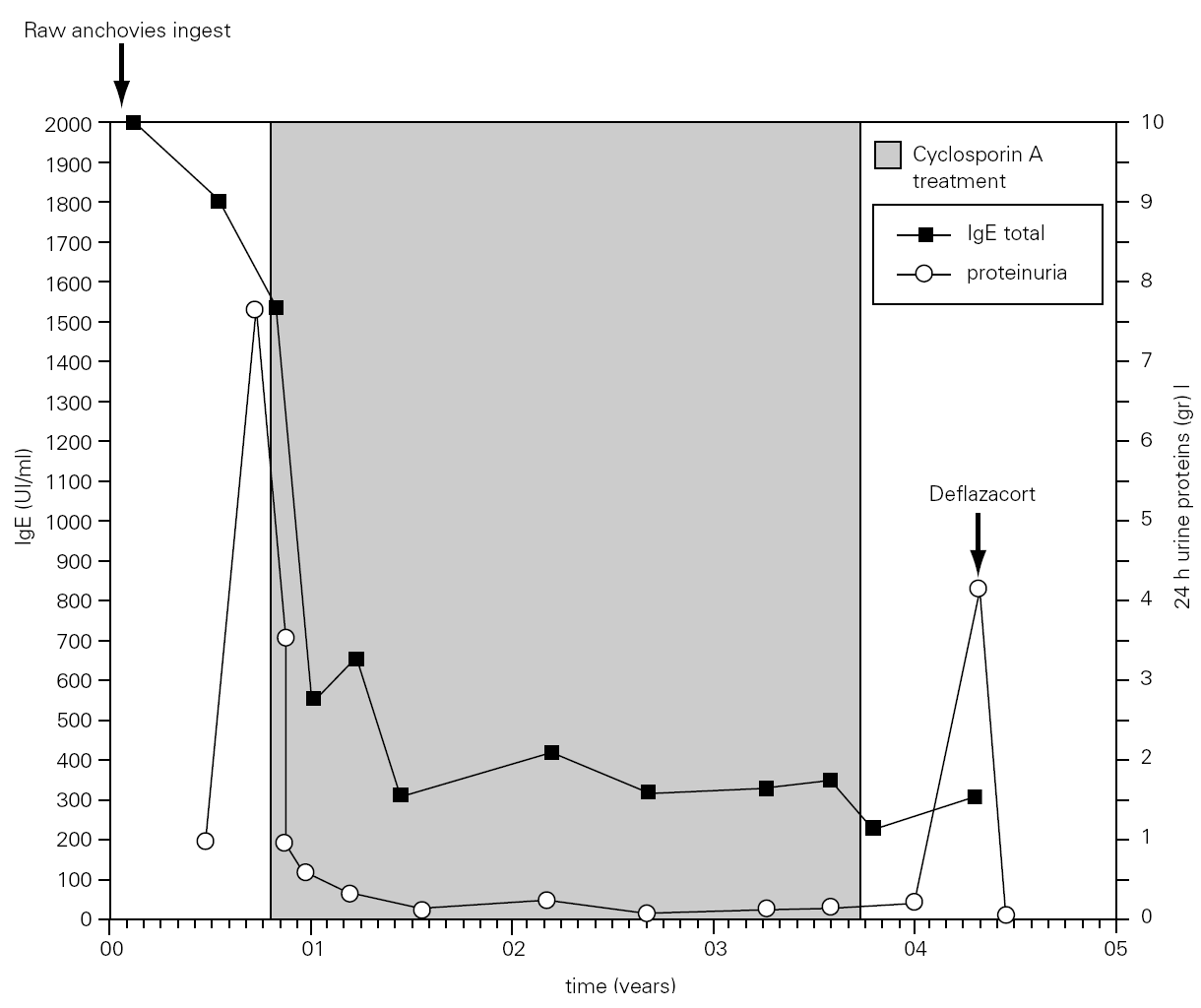
We present a case of a 60-year-old woman, housewife, non atopic, who suffered from arterial hypertension and was dealt with fosinopril a year ago. She referred periodic episodes of generalized urticaria and facial angioedema during the last years, which were controlled after treatment with antihistamines and systemic steroids. She suffered from another generalized urticaria episode, accompanied of facial angioedema and nauseas which needed emergence assistance nine hours after eating raw anchovies. Three weeks later hypoproteinemia, (5.4 g/dl), hypoalbuminemia (2.5 g/dl), and increase of α -2 and β -globulins was observed. Later she exhibited edemas in legs and eyelids, dyspepsia and general discomfort. In analysis, proteinuria (892.5 mg urine protein/day) and hypercholesterolemia were detected. Blood pressure and renal function were normal. A diet in absence of fish and seafood and urticaria-angioedema and nephrotic syndrome study were instituted. Six months later proteinuria rose up to 7.7 g urine protein/day (microalbuminuria of 1564 mg/dl and urine protein of 2672 mg/dl) and oral cyclosporin A (Sandimmun® Neoral) 3 mg/kg/day treatment was established with a spectacular and quickly decreases of the urine protein level (fig. 1). This treatment was maintained during three years (fig. 1) with closely monitored serum levels. Renal function was not altered in any time of pursuit. Seven months after CsA suppression proteinuria appeared again (3.824 mg urine protein/day) and steroid treatment was instituted with a quick restoration of urine protein value (fig. 1). The patient was stable during one year of treatment with low doses of steroids remaining in remission during one year after suppression of them. Also the patient has not presented new episodes of urticaria-angioedema.
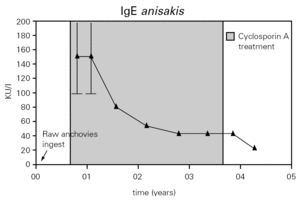
Figure 1.--Values of serum total IgE and urine protein. The total IgE was raised three week after the ingestion of raw anchovies and it decreased progressively to the six months. A great and rapid fall of urine proteins levels and total IgE was observed after initiation of cyclosporin A (CsA) treatment 3mg/kg/day. Seven months after CsA suppression, nephrotic syndrome relapsed and treatment with deflazacort was established.
MATERIALS AND METHODS
Anisakis simplex and Ascaris sp. specimens were ground in a pool of liquid nitrogen into a course "powder" of frozen fragments in a mortar and extracted by magnetic stirring in agitation in 50 mM phosphate-buffered saline (PBS) at pH 7.5 during 4 h at room temperature. After centrifugation, supernatant was dialyzed against water. The dialyzed extract was filtered through a 0.22 μm-pore diameter membrane and freeze-dried.
Prick-test with extracts from A. simplex (IPI, International Pharmaceutical Immunology), common inhalants (mites, fungi, pollens, and animal dander), latex, some fish species (raw and fried anchovies and sardine) and other standard foods (fish, seafood, fruits, nuts, milk, egg, vegetable and flours) (Bial Aristegui Laboratories, Bilbao, Spain) were performed according to standard procedure. Histamine phosphate (10 mg/ml) and sterile 0.9 % saline were used as positive and negative controls, respectively.
Total IgE was determined by the CAP System IgE FEIA (Pharmacia Diagnostics, Uppsala, Sweden), following manufacturer's instructions. Specific IgE to A. simplex, Ascaris sp. and Echinococcus were assessed by the CAP System RAST FEIA (Pharmacia Diagnostics, Uppsala, Sweden).
Specific IgG, IgM and IgA to extract of A. simplex were valued means ELISA. It was the greater dilution than it gives rise at the end of the colorimetric reaction, to an absorbance, at least twice that a serum control (pool of serum of nonallergic people) to the same dilution.
DS-PAGE and immunoblotting to A. simplex and Ascaris sp. was made as well as a blotting-inhibition between Anisakis and Ascaris extracts.
HLA class I (A, B and C) serologic typing was performed following a standard complement-dependent microcytotoxicity assay (Van Rood, 1979)10.
Serological tests, haematology, biochemistry and urine parameters were analyzed by standard methods.
RESULTS
Prick-test with A. simplex extract was positive (wheal diameter 7 mm). Positive control histamine hydrochloride 10 mg/ml was 6 mm. Negative results were obtained with prick test to raw and fried anchovy and sardine, standard fish, seafood and other common food allergens, latex, house dust mites, moulds, pollens and animal dander.
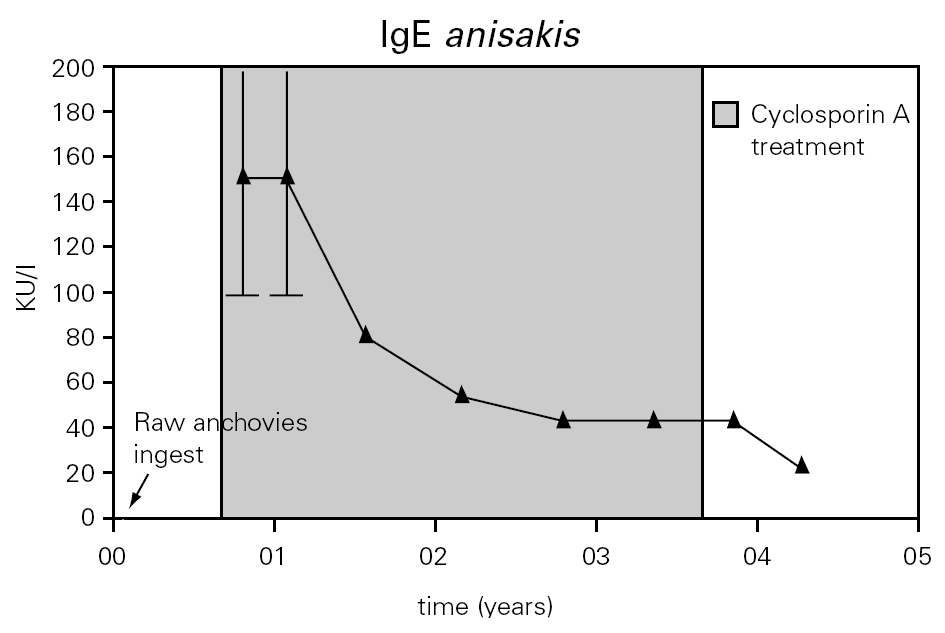
The total IgE was raised to 2000 UI/ml three weeks after the ingestion of raw anchovies and it diminished progressively in six months (fig. 1). Specific IgE antibodies to A. simplex (fig. 2) and Ascaris sp. extracts were > 100 UI/ml and 4, 51 UI/ml, respectively. Specific IgE against Echinococcus was negative. The evolution of total and specific IgE to A. simplex serial values during several years decreased until 262 kU/l and 23.5 kU/l respectively (figs. 1 and 2).
Figure 2.--Anti-A. simplex IgE serum levels were over 100 kU/l during the first months of a diet in absence of fish and seafood. Later it descended gradually during the treatment with cyclosporine A.
The titles obtained for other anti-A. simplex specific immunoglobulins were: IgG: 1/1280; IgM: 1/40; IgA: 1/160.
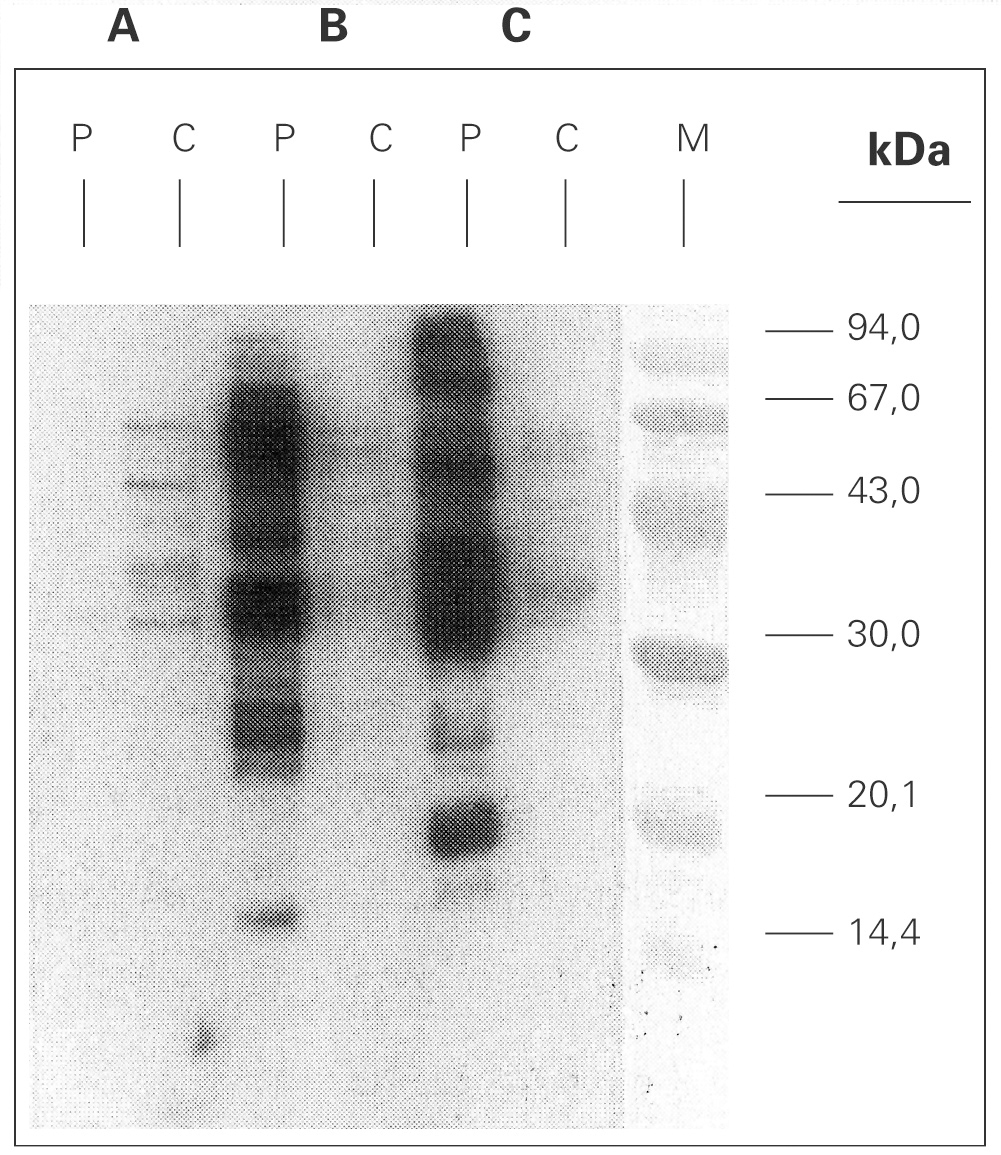
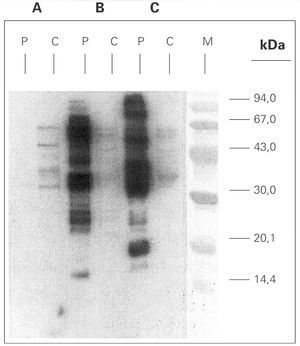
A. simplex and Ascaris sp. extracts were analysed by means of SDS-PAGE technique, and Coomassie Brilliant Blue stained method showed protein bands ranging from 14 to > 90 kDa (data not shown). Immunoblotting with A simplex extract showed IgE-binding bands of 16, 23, 25, 26, 28, 30, 33, 37, 42, 48, 58 and 67 kDa (fig. 3). IgE-binding bands weren't detected when Ascaris sp was blotted. Immunoblotting-inhibition results revealed a slightly cross-reactivity between A. simplex and Ascaris sp protein extracts (data not shown).
Figure 3.--SDS-PAGE Immunoblotting. A: Ascaris sp. extract B: Anisakis simplex extract (+b -mercaptoethanol) C: Anisakis simplex extract (b -mercaptoethanol). P: patient serum C: control sera (pool of sera from non atopic subjects) M: molecular mass marker.
Class I HLA expressed by the patient was type HLA-B12.
523.6 eosinophils/μl (8.5 %) in peripherical blood was observed only one time. Other blood counts, erythrocyte sedimentation rate, coagulation study and blood values of glucose, creatinine, urea, uric acid, bilirubin, alanine aminotransferasa, aspartate aminotransferasa, gamma-glutamyl-transpeptidase, alkaline phosphatase, creatine phosphokinase, lactate dehydrogenase, C-reactive protein, ions, thyroidal hormones, C3, C4, ASLO, rheumatoid factor, ANA, anti-DNA, anti-cardiolipin, immunocomplexes and serum total IgG, IgA and IgM were normal or negative. Cholesterol and triglyceride serum levels were discretely elevated until suppression of CsA. Serological study for hepatitis A, C, B, cytomegalovirus, Epstein-Barr virus, VIH, Toxocara canis, Toxoplasma, Leishmania, Treponema, Brucella and Mycoplasma was normal or negative. Urine biochemistry and sediment were also normal in different times, excluding urine protein, with no abnormal proteins (Bence-Jones, Immunofixing). Parasites in feces were determined, obtaining negative results. Chest and abdomen X-ray were normal. A thoracic and abdominal computerized tomography showed only residual apical lesions in superior lobule of left lung. Seven months after the treatment with CsA began a gastroduodenoscopy with multiple biopsies was carried out, which revealed the presence of an acute and chronic gastritis in antro, body and duodenal bulb and a non-specific chronic inflammation of distal duodenum. Helicobacter pylori, worms or eggs from A. simplex were not seen.
DISCUSSION
Anchovies are frequently contaminated by A. simplex in Spain, a country where anchovies marinated in vinegar are consumed more and one of the main causes of allergic symptoms by A. simplex. Exists a high degree of sensibilitation (prick-test +) to A. simplex in Spain11 so it supposes until 52 % of the cases of food allergy and up to 10 % of the cases of anaphylaxis and urticaria acute3.
Gastroallergic anisakiasis after eating raw fish is a frequent pathology due to the presence of living A. simplex larvae in some fish species. Gastroscopy examination to look for these larvaes within the following hours after the appearance of the symptoms is the most common procedure to diagnose the parasitized condition1. Unfortunately that is not often possible and the diagnosis depends essentially on clinical findings and indirect complementary diagnostic tools like the presence of a skin prick test (SPT) such as to the parasite or raised serum levels of total and specific IgE against A. simplex in the first thirty days after infection returning to the previous values to the six months2. In our patient it could not be confirmed by the gastroscopy exploration. Gastroallergic anisakiasis in this patient was diagnosed by a suggestive anamnesis, very raised serum levels of total IgE three week after the ingestion of raw anchovies and reduction to the six months, a positive skin prick tests with A. simplex extract, a high level of serum specific IgE to A. simplex (> 100 UI/ml) and after ruled out the possibility of fish allergy.
Furthermore living larvaes in a habitual consumer of raw anchovies could be the cause of the previous urticaria-angioedema episodes. An allergy response to termoestabile allergens from A. simplex could contribute to aggravate the symptoms also. The patient has not presented new episodes of urticaria-angioedema and the levels of total and specific IgE decreased with diet in absence of fish and seafood but it was treated with cyclosporine and this fact may have influence too12 (figs. 1 and 2). Specific IgE to Ascaris was detected but the low value obtained and immunoblotting-inhibition results let us to point at cross-reactivity13 as the cause.
A.simplex IgE-immunoblott with patient serum showed multiple IgE-binding bands between 20 kDa and 99 kDa. This broad range of molecular mass for IgE-binding proteins is a common fact in blotts performed with sera from A. simplex sensitized patients14.
On the other hand and refered to the nephrotic syndrome appeared in the patient the minimal changes glomerulonephritis (MCGN) is one of the most frequent responsible entities of this syndrome in adults and it is suspected to be the cause in this case. A high frequency of HLA B12 allele has been found in patients with MCGN15,16 suggesting a genetic predisposition. Clinically these patients present nephrotic syndrome and benign urinary sediment. Children present a selective proteinuria and it is typically no selective in adults. Spontaneous remission is relatively frequent in childhood but is less common in adults who can show relapses. MCGN is highly steroids responsive and carries an excellent prognosis. However there are many other options of treatment. Cyclosporine in low doses is one of them and can induce remission in 60-80 % of patients.
The ethiology of minimal change disease is unknown and the vast majority of cases are idiopathic. Occasionally it develops after infection by helmintos parasites and allergic diseases17. Thus it has been described associated to filarias4, Echinococcus5, Strongyloides6, Schistosoma7, inhalation of chironomid larvae18 and seasonal allergic rhinitis19.
So, we were in presence of an allergic patient to A. simplex who suffered from a nephrotic syndrome and it expressed haplotype HLA-B12. Renal biopsy was not made due to our clinical and analytical suspicion of MCGN and the good response to treatment with CsA and steroids.
In conclusion, nephrotic syndrome outbreaks coincident with episode of gastroallergic anisakiasis and absence of other more evident causes would show the existence of a possible etiologic link between a minimal change glomerulonephritis and gastroallergic anisakiasis. In our knowledge it is the first described case for Anisakis simplex allergy and nephrotic syndrome.
Correspondence:
José Meseguer Arce
Sargento Ángel Tornel, 11, 3.º-C
30009 Murcia. Spain
E-mail: jos_arc@ono.com