Disseminated BCG infections among other complications of Bacillus Calmette–Guérin (BCG) vaccine are rare and have occurred in children with immunodeficiency disorders such as mendelian susceptibility to mycobacterial disease (MSMD) which could be due to defects in some elements of IL-12/IFN-γ axis. MSMD-causing mutations have been identified in 10 genes during the last two decades. Among them, mutations in the IL12Rβ1 and IFNγR1 genes constitute about 80% of recorded cases of MSMD syndrome. The aim of this study was to investigate IL-12Rβ1 and IFN-γR1 deficiencies in patients with disseminated BCG infection.
MethodsThis study was performed on 31 children with disseminated BCG infections who referred to children's medical center. Whole blood cell culture was performed in presence of BCG, IL-12 and IFN-γ stimulators. The supernatants were assayed for IFN-γ and IL-12p70 by ELISA method. In order to evaluate IL12Rβ1 and IFN-γR1 receptors expression, flow cytometry staining was performed on the patients’ T-cells stimulated with PHA.
ResultsFlow cytometry staining of 31 Iranian patients with disseminated BCG infections with the average age of 43 months showed lack of the expression of IL-12Rβ1 and IFN-γR1 genes in PHA-T-cells of the nine and one patients, respectively in whom the incomplete production of IFN-γ and IL-12 was reported by ELISA. Among these 10 patients, eight cases had related parents (80%).
ConclusionIt is recommended that to avoid BCG complications, screening be performed for MSMD before BCG inoculation in individuals with positive family history of primary immunodeficiency diseases and inhabitants of areas with high frequency of consanguinity.
Bacillus Calmette Guérin (BCG) is an attenuated strain of Mycobacterium bovis that is currently used as a live vaccine to prevent early-life infections of Mycobacterium tuberculosis.1,2 The BCG vaccine was developed by Albert Calmette and Camille Guerin and first administered to infants between 1908 and 1921.2,3 According to World Health Organization (WHO) recommendation, vaccination with BCG of all infants takes place in several countries, especially in highly endemic countries or neighboring such regions, to prevent from miliary and meningeal forms of tuberculosis (TB).2 Although BCG vaccination is safe in most children,4 it may cause some complications such as cellulitis and abscesses at the site of inoculation, regional lymphadenitis and disseminated BCG infection or BCGosis. The most frequent complications are purulent regional lymphadenitis, and bone BCG infection as the second most frequent.5
Disseminated infections are even scarcer and the estimated incidence has been 0.1–4.3 per one million vaccinated children but in recent years has increased up to 0–1/100,000 vaccinated children.5–7
Disseminated BCG infections have mostly occurred in children with immunodeficiency disorders such as mendelian susceptibility to mycobacterial disease (MSMD) with underlying genetic defects, and it is hardly seen in apparently normal individuals.5
MSMD is a rare congenital syndrome8 that predisposes otherwise apparently healthy individuals, with no obvious abnormalities in their routine hematological and immunological tests9 to infections caused by weakly virulent mycobacteria, such as BCG and environmental mycobacteria (EM).10,11
The interleukin (IL)-12/interferon (IFN)-γ dependent signaling pathway plays a central role in controlling mycobacterial infections.12 Therefore, molecular defects in this pathway could lead to an increased susceptibility to mycobacterial diseases.13 According to previous reports, ten genes, namely IL-12B, IL-12RB1, IFN-γR1, IFN-γR2, STAT1, IKBKG, CYBB, TYK2, IRF8 and ISG15, have been identified as causing MSMD when they exhibit germline mutations.14 Either autosomal recessive (AR) mutations in IL-12RB1 or autosomal dominant (AD) mutations in IFN-γR1 have been reported in most MSMD patients.13 Autosomal recessive IL-12Rβ1 deficiency is the most common genetic etiology of MSMD15 and mutations in the IL-12RB1 and IFN-γR1 genes account for about 80% of all recorded cases of MSMD syndrome.16
However, most of the studies on BCGosis are based on case reports; there are a few large sample studies on the immunogenetics of BCGosis up to now.4
The aim of this study was to evaluate probable IL-12Rβ1 and IFN-γR1 deficiencies as the most common etiologies of MSMD in patients suffering from disseminated BCG infection.
Material and methodsPatientsThis case series study was performed at the pediatric infectious diseases research center of Tehran University of Medical Sciences, during a three-year period from 2013 to 2015. Thirty-one unrelated patients who had disseminated BCG infection were enrolled in this study.
Inclusion criteria were:
Positive history of the inoculation of BCG vaccine; two or more signs and symptoms of a systemic syndrome compatible with mycobacterial disease containing fever, weight loss, lymphadenopathy (LAP) or cutaneous abscesses, pneumonia, osteomyelitis and hepatosplenomegaly (HSM); evidence of BCG infection including a histopathological demonstration of acid-fast bacilli at two or more anatomic sites far from the region of vaccination such as lymph nodes or cutaneous abscesses outside the region of inoculation, liver biopsy, gastric aspiration and bone marrow aspiration.17
MSMD was suspected in these patients after exclusion of other common immunodeficiency disorders such as severe combined immunodeficiency (SCID) and chronic granulomatous disease (CGD).
The study was conducted in accordance with the Helsinki Declaration. Informed consent was obtained from patient's parents.
Whole-blood activationHeparinized blood samples were activated with BCG, BCG+ IL-12p70 and BCG+ IFN-γ as described previously.13,18 Briefly, heparinized blood was diluted 1:2 in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD, USA)18 supplemented with 10% FBS (Gibco BRL, Gaithersburg, MD, USA), 100U/mL penicillin and 100mg/mL streptomycin (Gibco BRL, Gaithersburg, MD, USA).13
Aliquots of diluted blood were distributed into the wells of a 48-well plate and incubated in four sets of conditions (1mL/well): with medium alone, with live BCG (M. bovis BCG, Pasteur strain, MOI 20:1), with BCG plus IFN-γ (5000IU/mL; Macs; Miltenyi Biotec), or with BCG plus IL-12p70 (20ng/mL; Macs; Miltenyi Biotec). Supernatants were collected after 48h, incubating at 37°C in an atmosphere containing 5% CO2/95% air and centrifuged at 1000g for 5min.
Cytokine (IFN-γ and IL-12p70) concentrations in supernatants were analyzed by ELISA, using the human IFN-γ and IL-12p70 ELISA development kits (Mabtech AB, Sweden), according to the manufacturers’ guidelines. Results for cytokines level are expressed in pg/mL/106 peripheral blood mononuclear cells (PBMCs).18
Cell culture and flow cytometryFor the production of phytohemagglutinin (PHA)-activated T cells, peripheral blood mononuclear cell (PBMCs) was isolated by centrifugation on a Ficoll-Hypaque gradient (Sigma, St Louis, MO, USA). Cells were resuspended in RPMI 1640 medium supplemented with 10% FBS, 100U/mL penicillin and 100mg/mL streptomycin (Gibco BRL, Gaithersburg, MD, USA) and activated by incubation with PHA (Sigma, St Louis, MO, USA) for 72h, followed by activation with IL-2 (50IU/mL; Sigma, St Louis, MO, USA) for 48h.
Evaluation of IL-12RB1 and IFN-γR1 receptors expression on PBMCs was performed by flow cytometry which consists of a series of steps of preparation and staining. The procedure was followed by labeling with appropriate antibodies for analysis with a FACScan flow cytometer and the FlowJo 7.5 software, as described previously.10
Data analysis was performed using SPSS software. Descriptive statistics (frequency, percentage, mean and standard deviation) was used for statistical analysis.
ResultsIn this survey, 31 children with the clinical symptoms of disseminated BCG infection were enrolled. All the children had been inoculated with BCG at birth under the national BCG vaccination program. Fourteen patients were male (45%) and 17 patients were female (55%), within a range of six to 127 months (mean: 43±27.36 months). Around more than half of the patients (18 of 31, 58%) had parental consanguinity. Onset age was between 0.1 and 108 months and until the end of the study all patients were alive. Salmonellosis occurred in eight (26%) patients, while two patients were reported with oral candidiasis (6%).
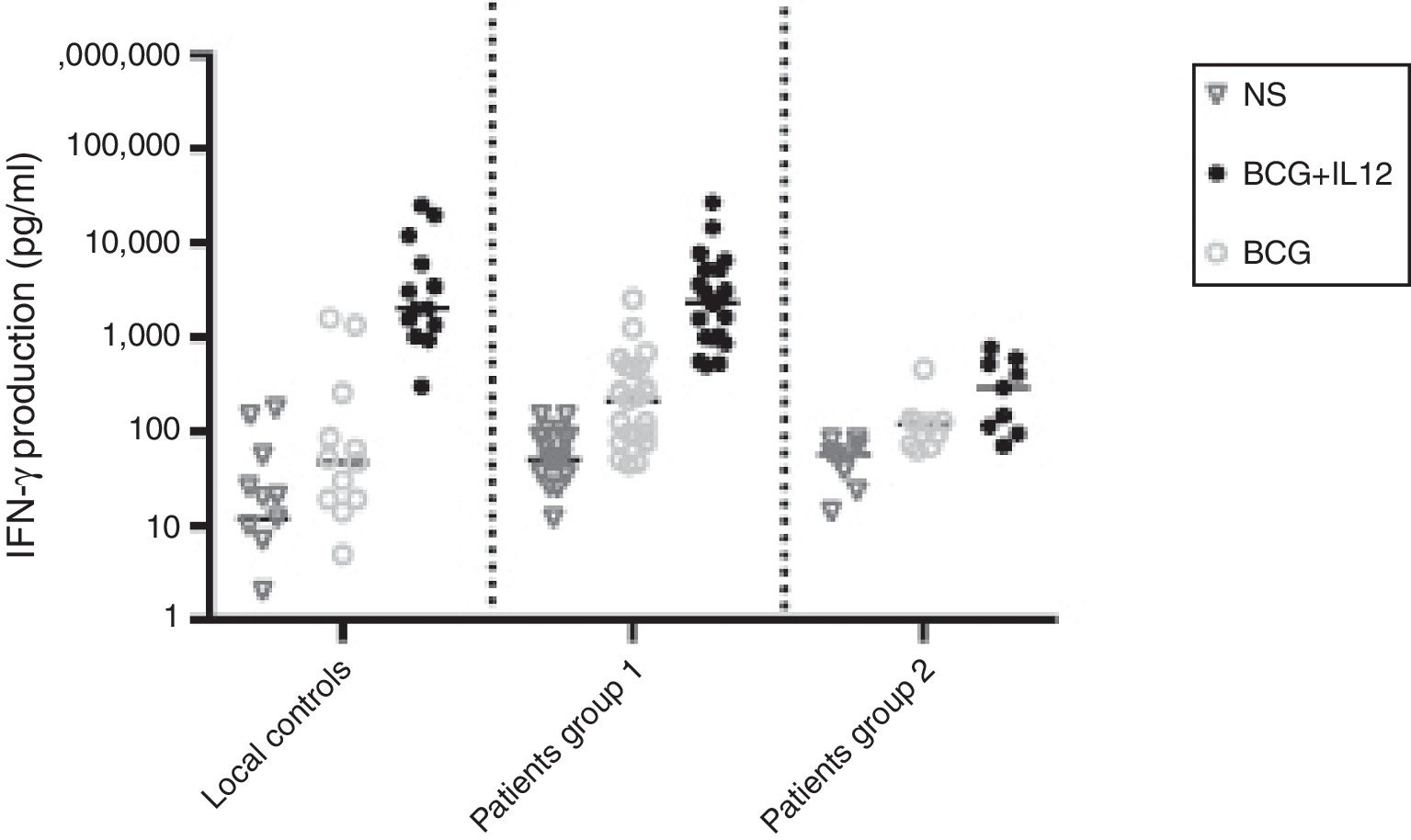
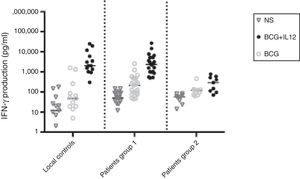
The production of IFN-γ was evaluated in diluted whole blood in response to stimulation with BCG and with BCG plus IL-12, as an effective inducer of IFN-γ, in all patients and 13 control individuals (Fig. 1).
IFN-γ production in the supernatants of whole blood cells from patients with disseminated BCG infection, unstimulated or stimulated by BCG alone or BCG plus cytokine, as detected by ELISA. First column belonged to 13 normal individuals, second column (which was named as “patients group 1”) to 22 patients without impaired production of IFN-γ and third column (which was named as “patients group 2”) to nine patients with impaired production of IFN-γ.
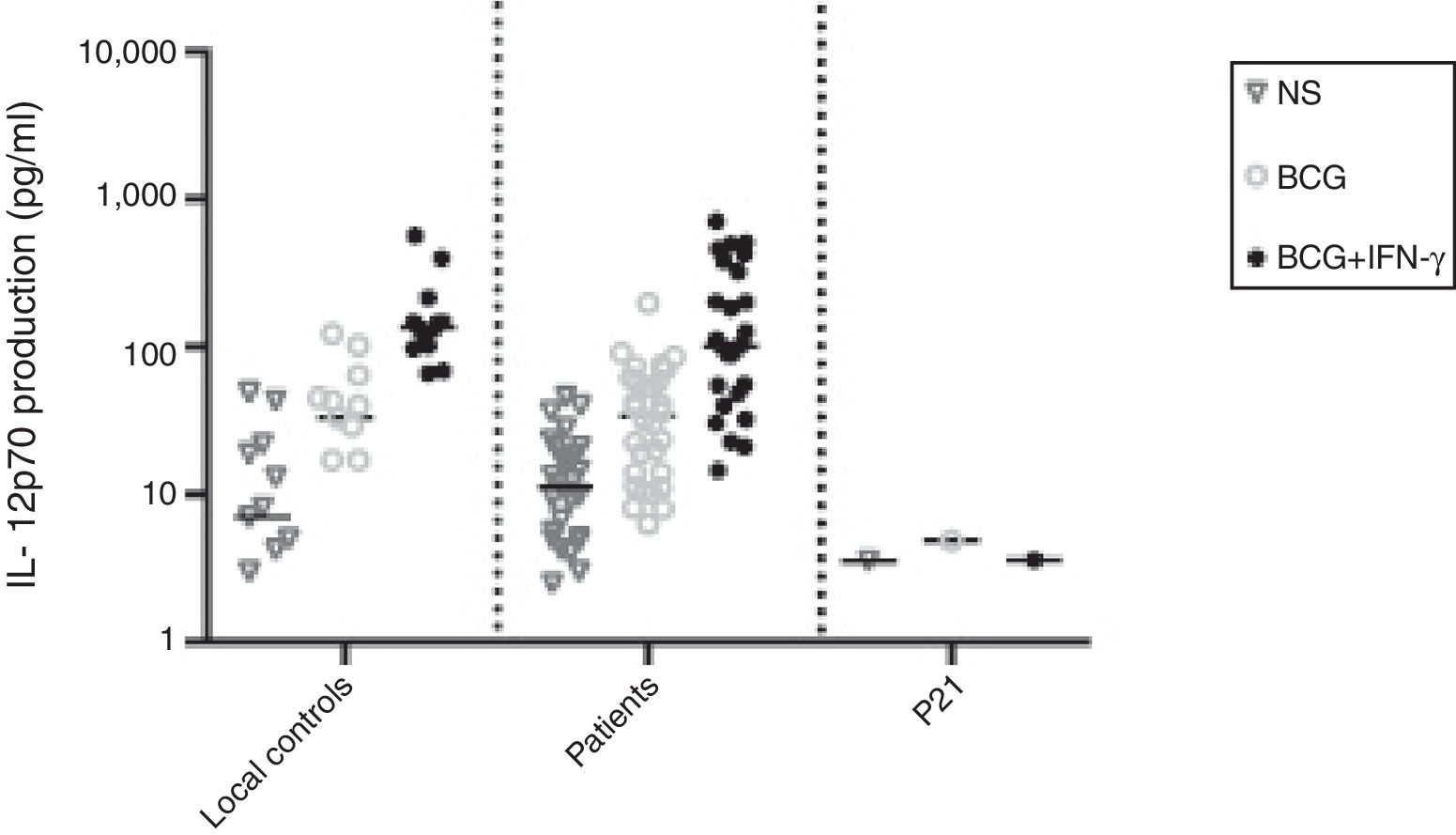
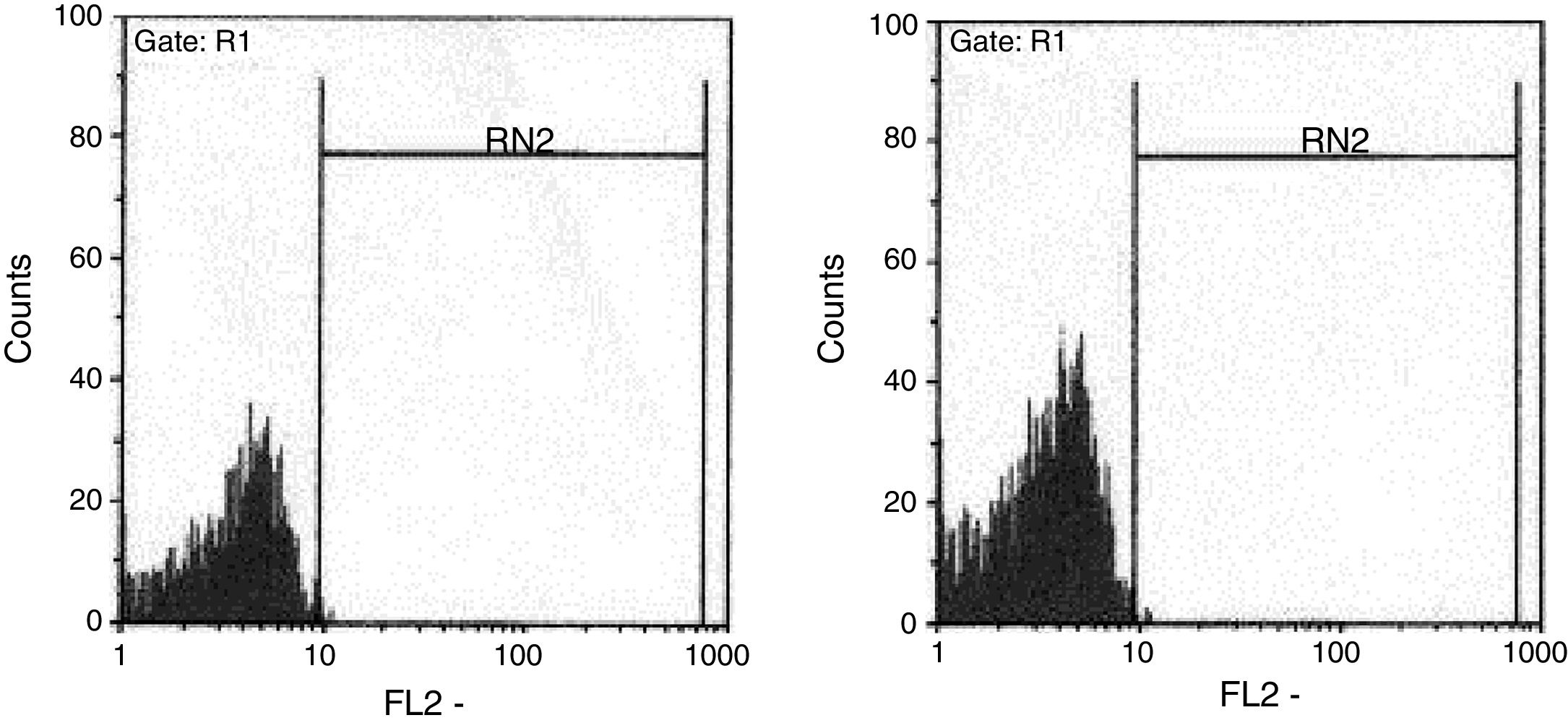
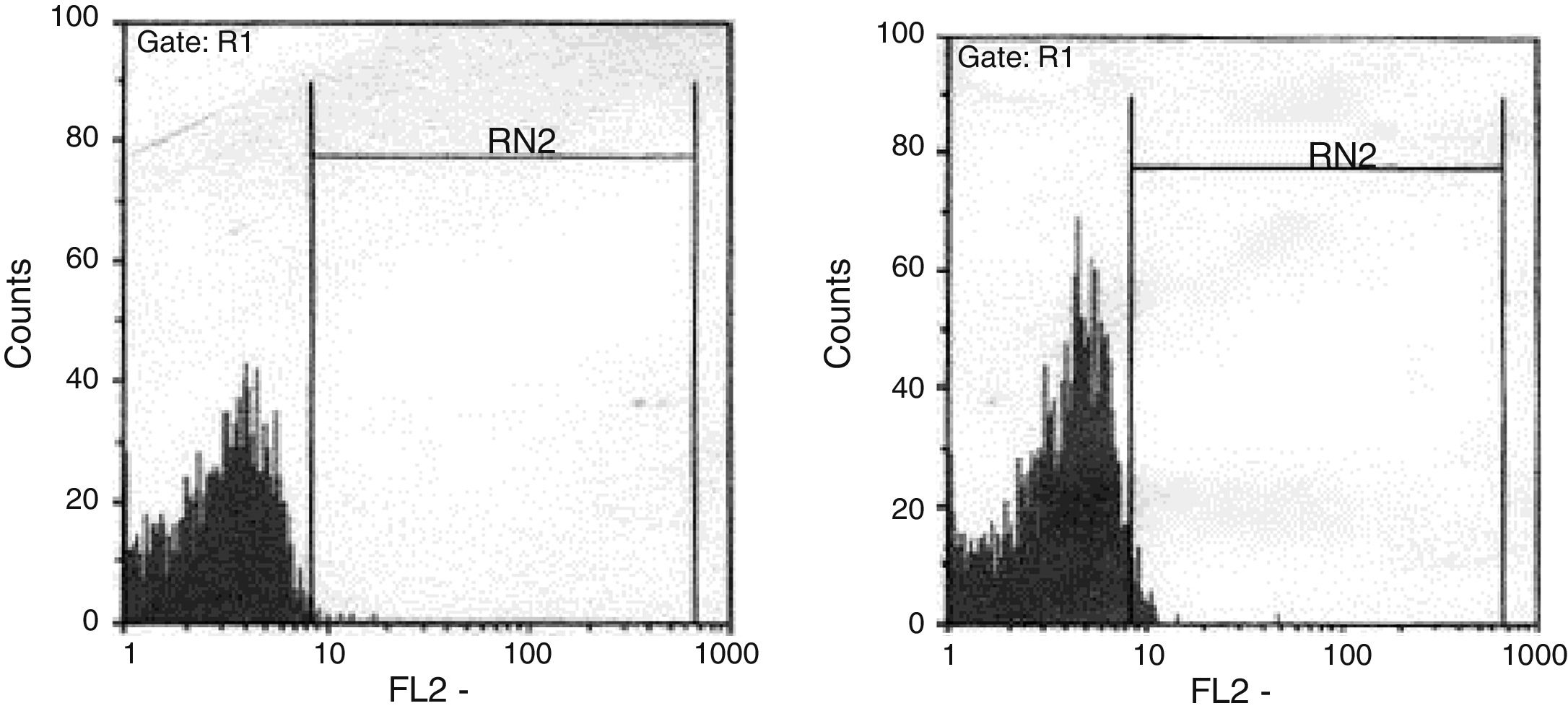
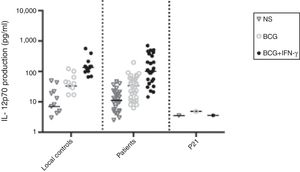
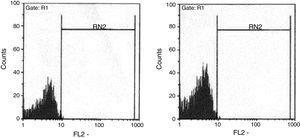
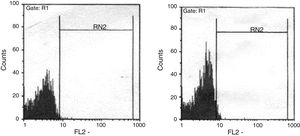
The ratio of stimulated level of IFN-γ with BCG+ IL-12p70 to its stimulated level with BCG should only be above 10 in the normal population.13 After analysis, the results illustrated impaired response to IL-12 and also impaired production of IFN-γ in nine of our patients (mean: 335.57±254.23pg/mL) in whom the mentioned ratio was below 10. Generally, the blood cells from the patients tested displayed an impaired response to IL-12, intensely suggesting that these patients had complete IL-12Rβ1 deficiency.10 Also, the ratio of stimulated level of IL-12p70 with BCG+ IFN-γ to its stimulated level with BCG should only be above two in the normal population13 and the ratio was lower than the normal range in only one patient (P21) (Fig. 2). Then IL-12Rβ1 and IFN-γR1 expression on the surface of T-cell blasts was assessed by flow cytometry with specific antibody reactivating with the extracellular domain of both receptors. No IL-12Rβ1 molecules were detected on the surface of cells from those nine patients who had impaired response to IL-12 (one of which has been shown in Fig. 3). In the remaining 22 patients as controls, IL-12Rβ1 receptor expression was normal. Additionally, there were no IFN-γR1 molecules on the surface of cells from one patient who had impaired response to IFN-γ (Fig. 4). At each stage of flow cytometry, receptor expression of a patient was compared with a normal individual.
IL-12p70 production in the supernatants of whole blood cells from patients with disseminated BCG infection, unstimulated or stimulated by BCG alone or BCG plus cytokine, as detected by ELISA. First column belonged to 13 normal individuals, second column to 30 patients without impaired production of IL-12 and third column to one patient with impaired production of IL-12.
Among the nine patients with disseminated BCG infection who had IL12Rβ1 deficiency, six patients were female, with the average age of 36.9±32.9 months. Consanguineous was found in seven of these patients (78%) and family history of disseminated BCG infection or immunodeficiency was seen in six patients (67%). It should be mentioned that two of them had siblings who displayed the same symptoms. The only patient with IFN-γR1 deficiency was five years old who had related parents with positive family history.
DiscussionBCG vaccine is currently the only one licensed vaccine against TB which is a live attenuated strain of M. bovis.19 It is one of the most widely used of all current vaccines, reaching >80% of neonates and infants in countries where it is part of the national childhood immunization program.4 However, the existence of numerous complications ranging from local inflammatory reactions to disseminated diseases and even deaths associated with the vaccine is probable.7
According to previous studies, the immunological condition of children is a vital factor in BCG infection.4 Casanova et al.20 and Norouzi et al.2 reported that 50% to 76% of BCG-infected patients had immunodeficiency.4 These results show that immunogenetic factors are critical, as these can cause BCGosis/BCGitis.
As the IL-12/IFN-γ intercellular pathway seemed to have a central role in immunity against mycobacteria and some other intracellular microorganisms, numerous studies have focused on this pathway during the last two decades. Since discovering the IFN-γ receptor gene defect as a condition associated with mycobacterial infection in 1996, several genes have been recognized in which their mutations can lead to susceptibility of mycobacterial infections. Among them, IL-12Rβ1 gene seems to be the most common genetic etiology of MSMD.13
Although there is not a defined genetic etiology in about half of all the patients with MSMD,21 flow cytometry has proved to be useful in patients with genetic defects associated with MSMD by focusing on the assessment of specific surface protein expression and cell function analysis.1
In the current study, 31 children with clinical features of disseminated BCG infection were recruited and evaluating on MSMD was conducted, and among them nine children with IL-12RB1 deficiency and one child with IFN-γR1 deficiency were recognized. In the Ying et al. study in China from 74 patients, 14 cases suffered from disseminated BCG infection and of those, two cases had been reported with IL-12Rβ1 deficiency and two patients had a mutation in IFN-γR1 gene.4 Also in the Sadeghi-Shanbestari et al. study, 11 out of 48 infants had disseminated BCG infection, of which two cases had IL-12Rβ1 deficiency.5 The frequency of both studies on IL-12Rβ1 deficiency is lower than the results reported in this study.
More than 70 unique IL-12RB1 mutations with a very uneven distribution have been reported including 1791+2T>G, 580+1G>A and T355del which occur in introns 15, intron 6 and exon 10, respectively.
In the current study, all the patients were alive at the end of the investigation. Lotte et al. identified 60 cases of BCG dissemination for which the mortality rate was 50% and cellular immunodeficiency was identified as the principal risk factors for fatal outcome.5 One of the fundamental causes of the lower mortality rate in this study was to evaluate patients in which other common immunodeficiency diseases were excluded and only the MSMD immunodeficiency was suspected, while in the vast majority of the studies,4,5 patients with immune deficiencies such as CGD and SCID have also been studied.
AR forms of MSMD are most prevalent in regions exposed to tuberculosis with high rate of consanguineous marriages.22 In this study parental consanguinity was observed in 18 of 31 patients (58%) with disseminated BCG infection which is compatible with the Sadeghi-Shanbestari et al. report in which more than half of the patients had related parents.5 Besides, in the study of Casanova et al., parental consanguinity was found in 30% of the families.5,23 In this study, seven out of nine patients (78%) with IL-12Rβ1 deficiency and one patient with IFN-γR1 deficiency had related parents. Generally, it is not surprising that recessive traits are repeatedly involved in countries in which the rate of consanguinity is so high, such as Iran (38%).24 Low consanguinity and more restricted BCG vaccination policy may clarify why there were no North American and Australian patients diagnosed.10
In this study, nine children (29%) had family history of disseminated BCG infection or primary immunodeficiency disease, while in Parvaneh et al. study only one child (9%) and in Sadeghi-Shanbestari et al. study about one third of patients had family history of disease which was similar to our results. Interestingly, seven of nine patients with family history of disease had deficiency, which in six of them was IL-12Rβ1 deficiency.
By and large, it seems MSMD has a considerable role in the etiology of disseminated BCG infection, as 10 patients in this study have been reported with IL-12Rβ1 or IFN-γR1 deficiency. Besides, evaluation on other involving genes in MSMD in the patients who have not been identified with defects in the current study could be our next program.
Moreover, it could be recommended that in our country with a high incidence of tuberculosis and high mortality in patients with primary immunodeficiency; inoculation of BCG vaccine should be postponed for a few months in the families with history of inherited immunodeficiency or disseminated BCG infection to investigate for MSMD along with other screening tests for other underlying immunodeficiency diseases.
Conflict of interestThe authors have no conflict of interest to declare.
This study was Reihaneh Hosseinpour Sadeghi's postgraduate thesis and was supported by a grant (grant number: 90-04-88-16468) from Tehran University of Medical Sciences to Dr. Setraeh Mamishi.