Endovascular aneurysm repair (EVAR) is indicated in high-risk patients for conventional surgery, with anatomic conditions for endoprosthesis implantation. Low morbidity, mortality and physiological aggressiveness have been expanding the indications for its use. Still, EVAR is questionable in younger patients, with a low surgical risk and a prolonged life expectancy. Abdominal aortic aneurysms (AAA) are rare in human immunodeficiency virus (HIV) infected patients in western countries and have singular characteristics: an unknown etiology, multiple arterial involvement, poor open surgery results and risk of infection transmission to surgeons. For these reasons EVAR opened new therapeutic perspectives in this group of patients. We present our experience with two HIV patients in whom an AAA was diagnosed, one with a 10cm diameter treated by EVAR, excluded with an aorto-uni-iliac endoprosthesis, other followed regularly, describing their features and therapeutic results. The reported cases allow us to speculate on the importance of anti-retroviral and endovascular treatments reducing the inflammatory process on the arterial wall, with a consequent delay in aneurysm growth and even its regression, which reinforces the possible relevance of EVAR as a first line treatment for this particular pathology.
O tratamento endovascular do aneurisma da aorta (EVAR) está indicado em doentes de alto risco cirúrgico e com condições anatómicas favoráveis para implantação da endoprótese. As reduzidas morbimortalidade e agressividade fisiológica desta técnica expandiram as suas indicações, sendo ainda questionável para doentes jovens, de baixo risco e sobrevida prolongada. A associação entre aneurismas da aorta abdominal (AAA) e infeção vírus da imunodeficiência humana (HIV) é rara no mundo ocidental, tendo ainda várias particularidades: uma etiologia desconhecida, envolvimento arterial múltiplo, maus resultados no tratamento por cirurgia convencional e risco de transmissão do HIV. Apresentamos a nossa experiência em 2 pacientes com infeção HIV e AAA, um com 10cm de diâmetro, excluído por EVAR com endoprótese aorto-uni-ilíaca; outro seguido regularmente, descrevendo as suas características e resultados. Estes casos permitem-nos refletir sobre a importância do tratamento antiretrovírico e endovascular na redução do processo inflamatório da parede arterial, condicionando um atraso na progressão do aneurisma ou mesmo a sua regressão, abrindo perspetivas para uma nova indicação para EVAR.
Epidemic infection by the human immunodeficiency virus (HIV) continues to progress despite the global efforts to reduce it. The largest number of patients with HIV/AIDS is located in sub-Saharan Africa (60% of the world's total)1,4 an area where health care resources are scarce and access to anti-retroviral treatment is limited. In this disease, non-atherosclerotic vasculopathies, in which aneurysms are included, occur at a later stage and are a hallmark of advanced disease, and that may be the reason why they are more commonly reported in Africa.1,6,8,9
The treatment of abdominal aortic aneurysms (AAA) can be performed, as is known, by conventional surgery or endovascular repair (EVAR); however, it is still a matter of dispute in this pathology, which one could be better. Aneurysms associated with HIV have particular characteristics and pose specific problems: the etiology is unknown, patient life expectancy is reduced compared to the general population, there is an increased infection risk due to immunosuppression leading to poorer short and medium term conventional surgery results and there is a risk of HIV infection transmission to surgeons.
Treatment with highly active antiretroviral therapy (HAART) has a proven efficacy in improving overall survival by inhibiting viral replication,10,14 but its effect in the natural history of aneurysmal disease is not well defined.1,6–9,15
Case reportsWe present two case reports of HIV infected patients in whom an AAA was diagnosed.
Case 1A 51-year-old male patient, of mixed European and African ancestry, smoker, with hypertension and HIV infection detected in 1999 following the diagnostic work-up for asthenia. The infection was transmitted by sexual intercourse. The initial results of laboratory tests showed 500,000 RNA copies/ml, 24 CD4+ T lymphocytes and treatment with HAART was initiated. In the same year, the patient developed atypical mycobacteria pneumonia treated with rifampicin, ethambutol and pyrazinamide. In 2002, he endured weighty personal problems that led him to abandon HAART. In 2005 he had an acute myocardial infarction: coronary angiography revealed dilated arteries with slow flow and stenotic disease of the first marginal artery, not amenable to revascularization.
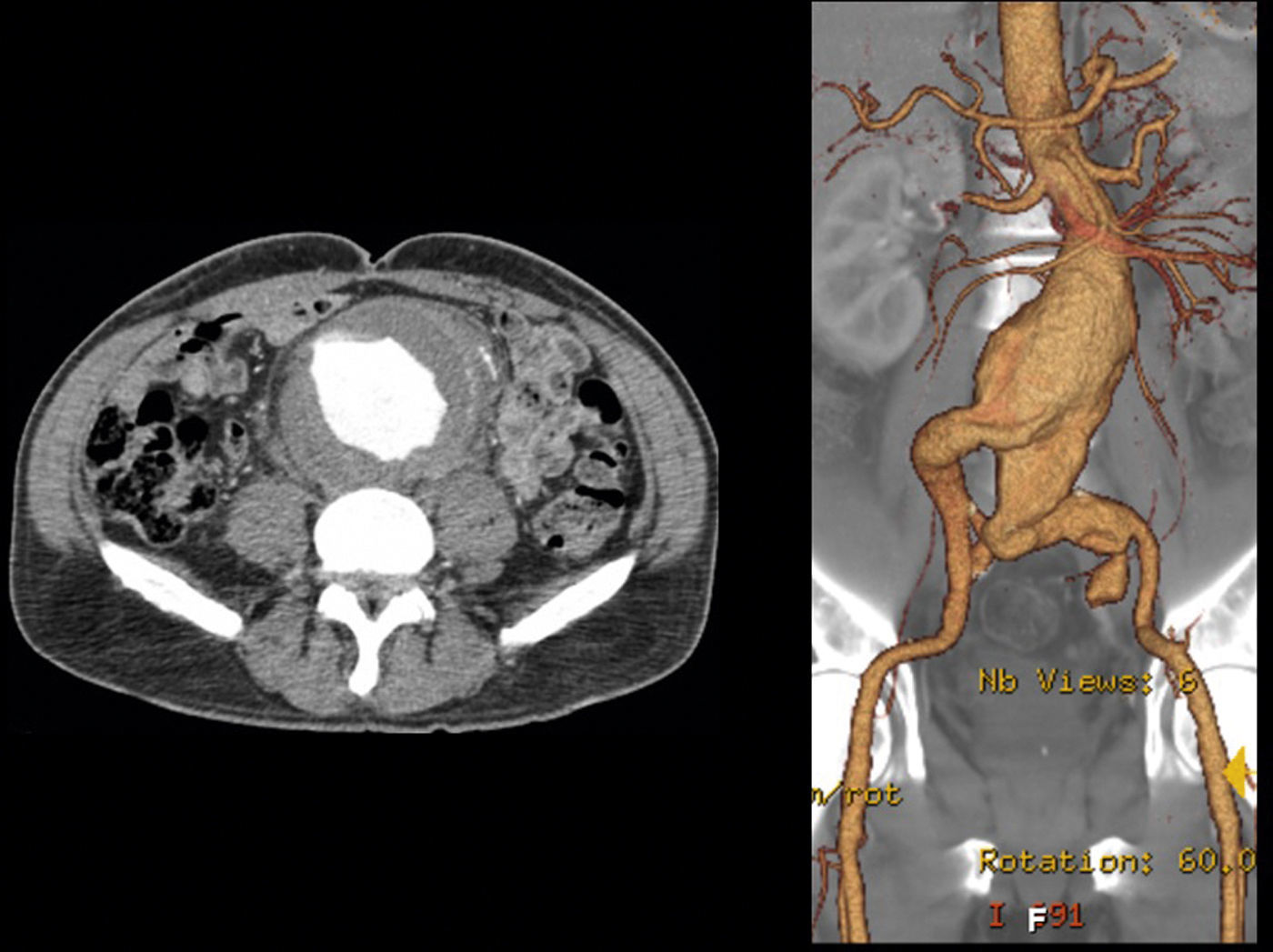
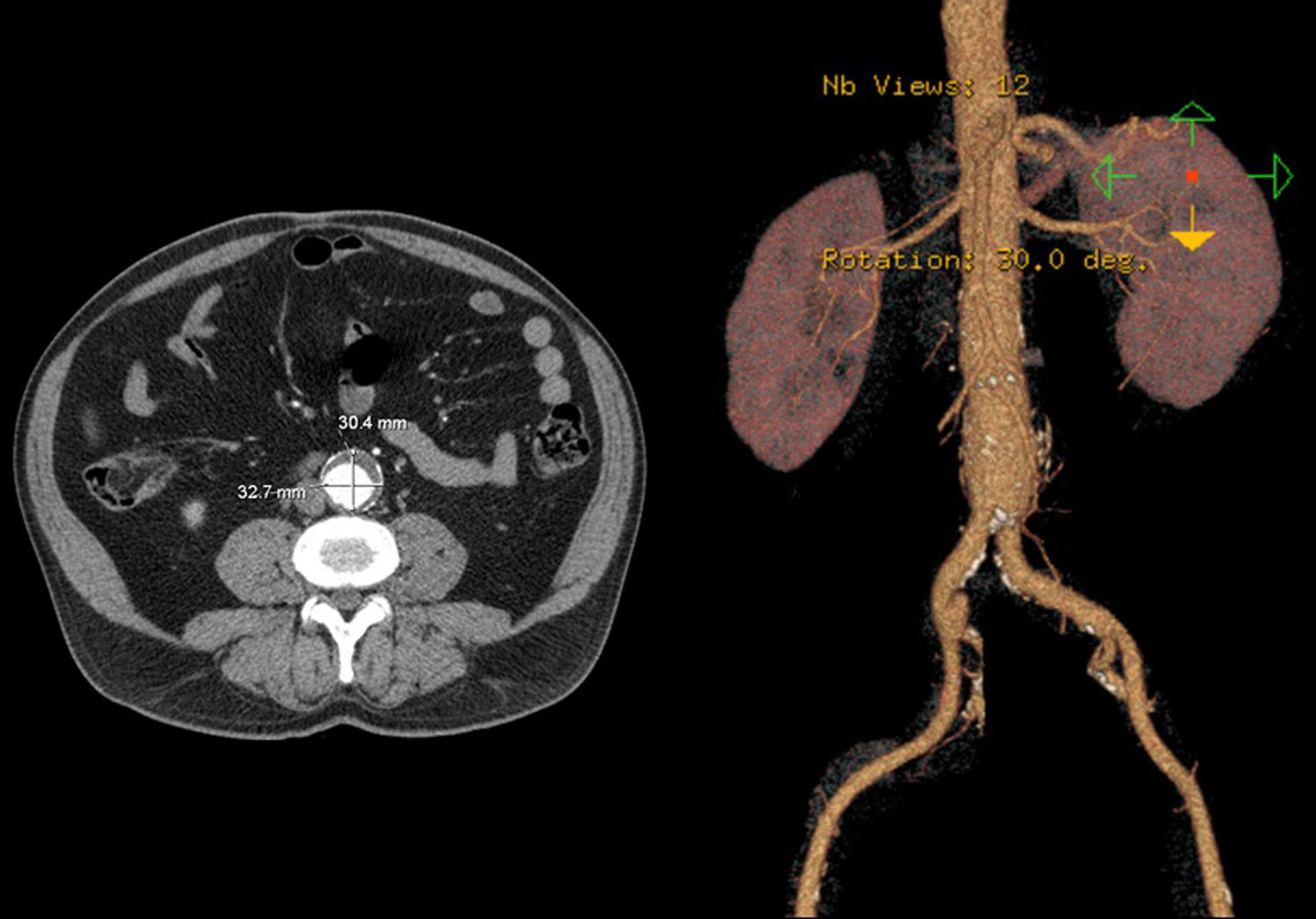
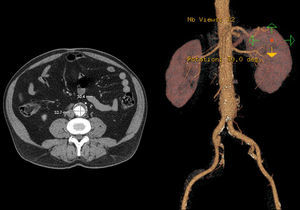
In 2008 he was admitted to the emergency department (ER) with diffuse abdominal pain and a large pulsatile expandable abdominal mass, hypertension and anemia (Hb 6.8g/dl). A CT scan of the abdomen showed an infra-renal aorto-iliac aneurysm of 10cm diameter and no rupture evidence (Image 1). At that moment the patient had 14,731 RNA copies/ml and 81 CD4+ T lymphocytes. He was transferred to the Department of Angiology and Vascular Surgery on intravenous perfusion of labetalol for blood pressure control and resumed HAART. During hospitalization he had numerous episodes of abdominal pain associated with hypertensive crisis, performing several CT's that excluded AAA rupture. Blood, urine and sputum cultures were negative for bacteria, as well as fungi and serological tests showed presence of IgG antibodies for Herpes Simplex 1, 2 and Varicella-Zoster virus.
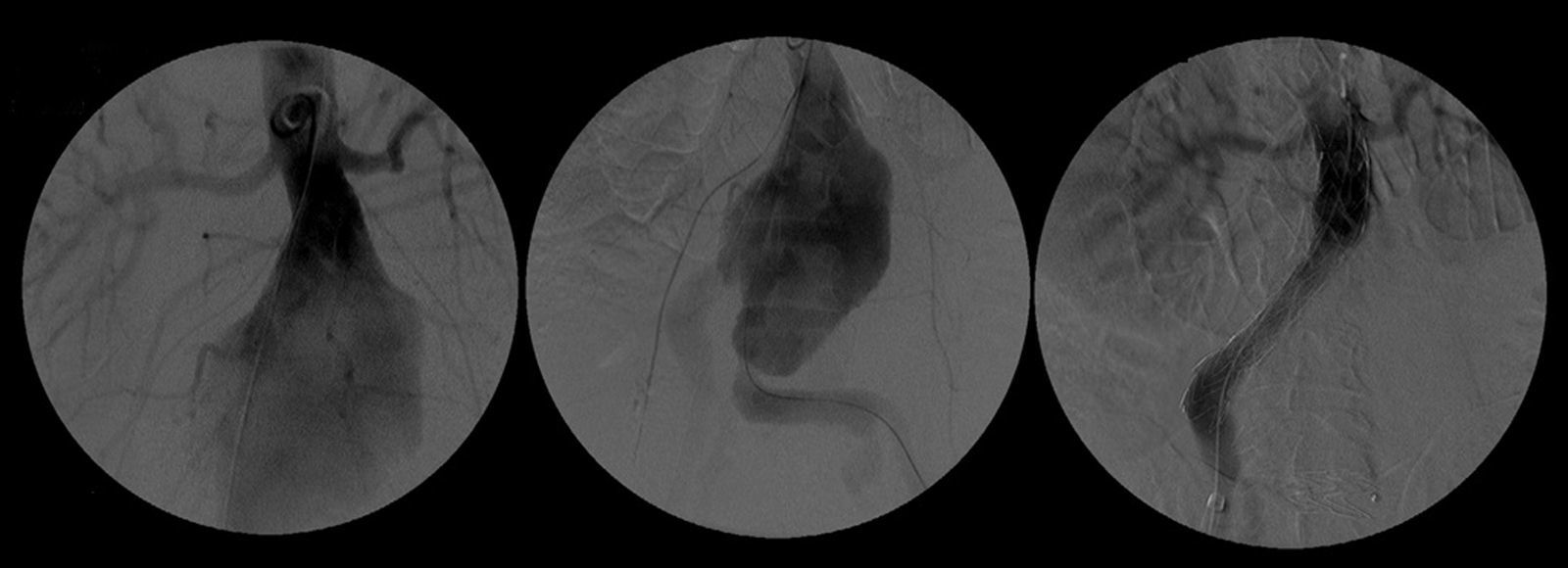
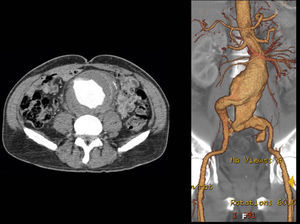
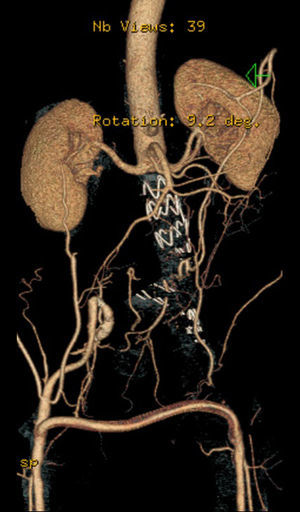
After a HAART cycle, which allowed a decrease of the viral load to 212 RNA copies/ml, EVAR was performed under general anesthesia, by surgical exposure of both common femoral arteries. An aorto-uni-iliac Endurant Medtronic® endoprosthesis (with a proximal diameter of 28mm and distal diameter of 24mm) was implanted through the right iliac axis, followed by occlusion of the common and external left iliac arteries (with 24 and 14mm occlusion stents) and a femoro-femoral 8mm PTFE crossover bypass (Image 2). The option for the mono-iliac endoprosthesis was due to the aneurysmal extension to the left common iliac artery and severe tortuosity of the left iliac axis. The postoperative period was complicated by an ileus.
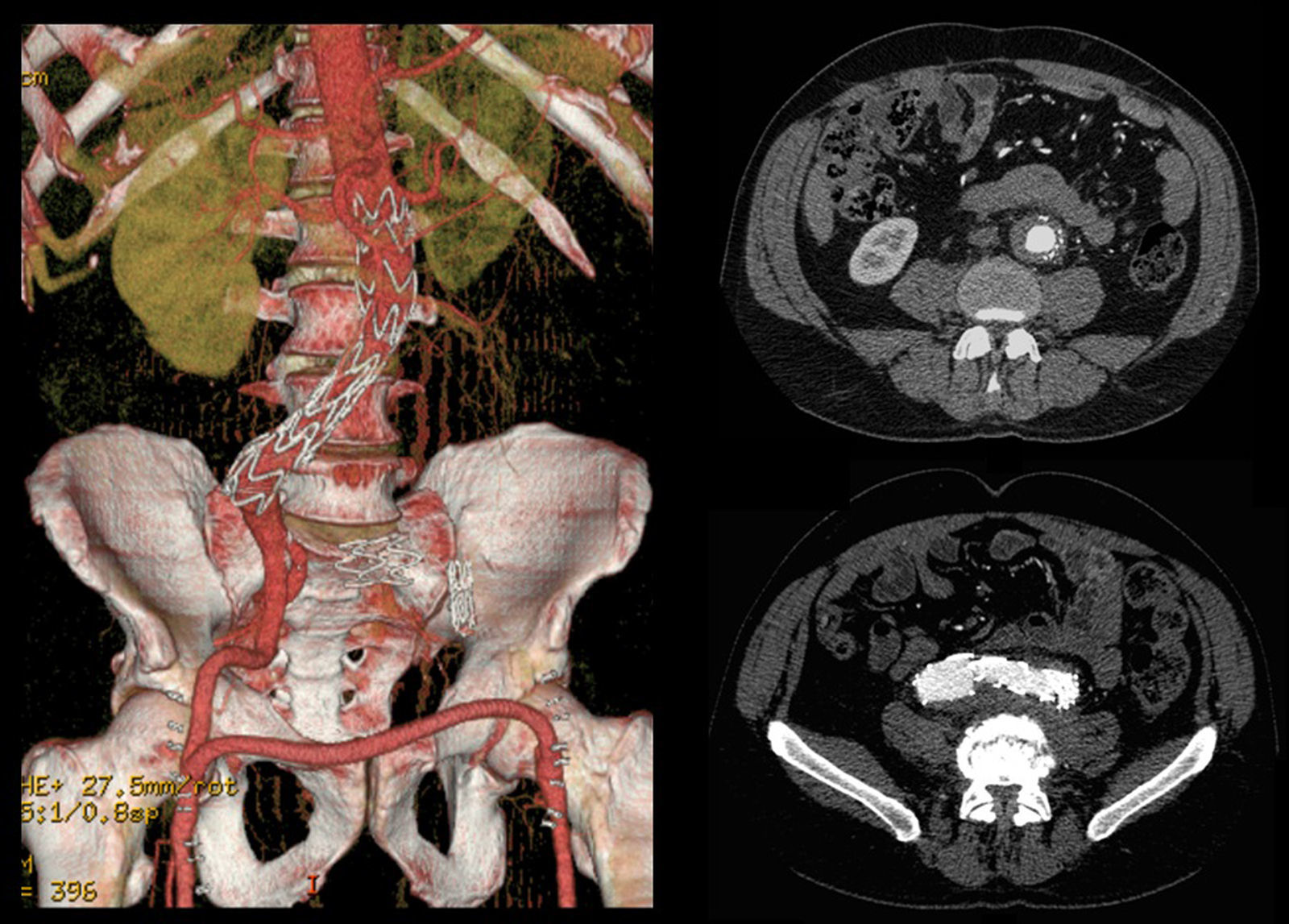
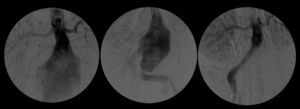
A control CT performed three months later showed no endoleaks and at 9th month it revealed a proper sealing and a complete regression of the aneurysm with a good match between the endoprosthesis and native arteries (Image 3). However, at 18 months the patient started complaining of bilateral intermittent, non-disabling, buttock claudication for distances of 500 meters, without palpable pulses in the lower limbs. A CT revealed occlusion of the endoprosthesis and patency of the femoro-femoral crossover bypass (Image 4). After a detailed analysis of the previous CT images it was concluded that the aneurysmal regression changed the endoprosthesis conformation within the common iliac artery, leading to apposition of its distal end to the arterial wall, a fact that may have caused its occlusion.
In 2009 the patient was admitted to the ER with severe headache and diagnosed with a left middle cerebral artery aneurysm, which was surgically treated using a Yasargyl 11mm clip. The postoperative period was uneventful. At our last evaluation he still has stable non-disabling claudication, without any evidence of aorto-iliac aneurysm in control CT's.
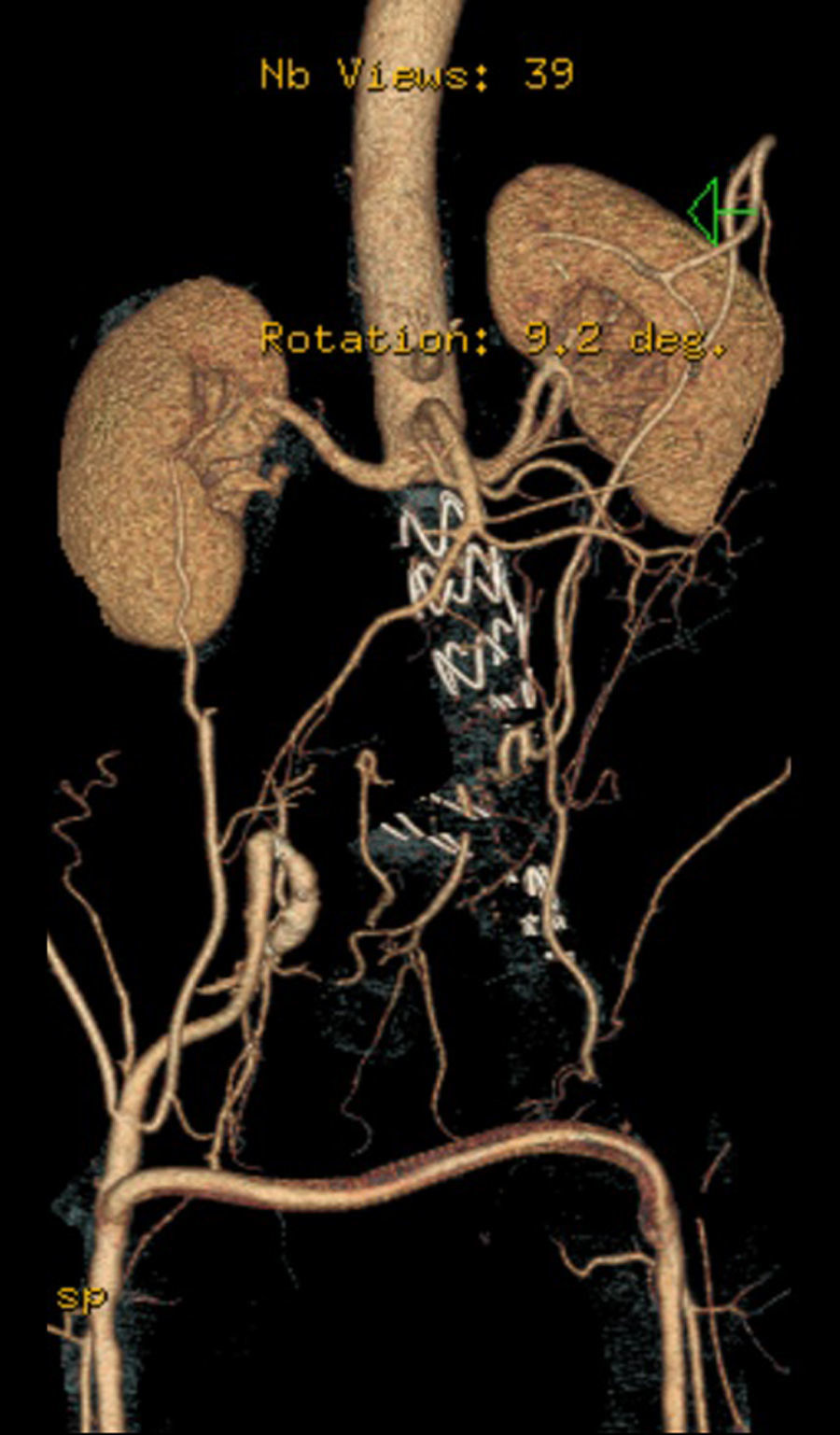
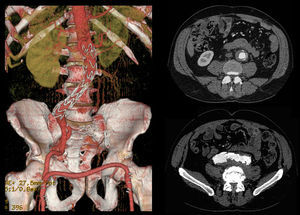
Case 256 year-old male, Caucasian, smoker, with hypertension and dyslipidemia. He had syphilis infection twenty-three years earlier and two myocardial infarctions in 1999 and 2004, the last one treated with percutaneous revascularization of the right coronary artery. In 2000 he was diagnosed with HIV infection, transmitted through sexual contact, having at the time 400,289 RNA copies/ml and 197 CD4+ HAART was initiated resulting in a decrease in RNA counts to less than 50/ml and CD4+ T lymphocytes increase to more than 500. In 2007, in a routine abdominal ultrasound, he was diagnosed with 3.6cm diameter AAA, confirmed by CT. He has been followed regularly as an outpatient, asymptomatic, with a stabilized aneurysm diameter (Image 5).
DiscussionThe occurrence of aneurysms in patients with HIV infection, particularly in the aorta, poses several particular questions concerning its etiology, the best form of treatment and also ethical considerations.
Regarding the etiology, they can be secondary to a bacterial or fungal infection of the aortic wall or of a pre-existing aneurysm5,13 as a result of the immunodeficiency. They can also be secondary to a leukocytoclastic vasculitis of the peri-adventitial blood vessels’ and vasa-vasorum caused by an HIV direct action on the endothelium or by deposition of immune-complexes.2,7,12 Regardless of the etiology, aneurysms associated with HIV often involve multiple arteries, most frequently the carotid and femoral vessels and more commonly younger patients, the male gender and African ancestry.6,8
The first report of an aneurysm associated with HIV comes from by Sinzobahamvya in Zimbabwe15 and the two largest published series in the literature by Robbs1 and Botes9 are from South Africa including a total of 111 and 24 patients, respectively.
From an ethical point of view, these patients should be offered the best treatment available and simultaneously the one that has a less likelihood of HIV transmission to surgeons.5
The best therapy is not yet defined. The results of conventional surgery are dismal11 due to a high rate of infectious complications, but they are highly dependent on multiple factors such as the CD4+ lymphocyte count, the level of RNA copies, the serum albumin levels and type of transmission (drug-addiction, homosexual, heterosexual or other).3 Endovascular treatment, as a less aggressive therapy, with more favorable short-term results, may have a particular interest in these patients. As an additional advantage it allows a reduction of HIV transmission risk to the surgical team due to a reduced use of blades and needles, reduced contact with open surfaces, less blood loss, shorter operative time and hospitalization. It is important though, to still take into account general measures for contamination prevention.
As the majority of the AAAs associated with HIV are reported in Africa, where EVAR is infrequently performed due to less resources, there cannot be withdrawn any conclusions from the literature concerning the utility of this technique, in this group of patients. In our experience with these two patients, we could observe in the first case a complete and surprising regression of a 10cm aorto-iliac aneurysm nine months after its exclusion, which leads us to speculate that associated with the reduction of the circulating virus load by HAART, the physical barrier of the endoprosthesis between arterial wall and the virus and/or their immune-complexes might have conditioned a reduction of the inflammatory process, with a subsequent aneurysm regression. In the second case we can also speculate that the maintenance of a low level of circulating copies of HIV RNA by HAART could be responsible for the stabilization of the aneurysm diameter.
ConclusionsThe HIV infection continues to progress despite the efforts made for its control. The clinical consequences are likely to expand, particularly in the vascular territory, in the form of aneurysms, which are late manifestations of the disease. The reported cases allow us to speculate on the importance of HAART in reducing the inflammatory process on the arterial wall, with a consequent delay in aneurysm growth or even its regression. Our option for an endovascular treatment in the first described case was decided after a reflection of our group regarding this situation and its related pathophysiology. We have obtained a complete regression of a large 10cm aorto-iliac aneurysm, which reinforces the possible relevance of EVAR as a first line treatment for this particular pathology. It is however necessary to acquire more experience with this technique in this subset of patients and call for publication from centers with larger number of patients with this condition so that more clear data can be obtained.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.