Endovascular treatment of complex thoracoabdominal aneurysms with branched and fenestrated grafts (B/F-EVAR) has become the first option for patients with suitable anatomy and very high risk for open surgery, who would likely be refused for open repair. We present a case of a patient with a type III thoracoabdominal aneurysm submitted to endovascular repair with a custom-made endograft with branches to the celiac trunk and SMA, and a fenestration to the left renal artery. During the procedure there was the need to do a laparotomy to allow for retrograde catheterization of the SMA, with technical success. In this case, the retrograde access was of utmost importance for the completion of the procedure. Different techniques for target vessel catheterization, such as the one we describe, should be part of a vascular surgeon's resources in B/F-EVAR procedures.
O tratamento endovascular de aneurismas toraco-abdominais complexos com endopróteses ramificadas e/ou fenestradas (B/F-EVAR), coloca-se como primeira opção em doentes com risco cirúrgico muito elevado e anatomia favorável, e que potencialmente seriam recusados para tratamento cirúrgico convencional. Os autores apresentam um caso clínico de um doente com um aneurisma da aorta toraco-abdominal tipo III submetido a B/F-EVAR com endoprótese custom-made com ramificações para o tronco celíaco e artéria mesentérica superior, e fenestração para a artéria renal esquerda. Durante o procedimento houve necessidade de realizar laparotomia mediana para cateterização retrógrada da AMS, concretizada com sucesso. Neste caso, a cateterização retrógrada da AMS foi fulcral para o sucesso técnico da intervenção. Técnicas de cateterização alternativas como a descrita devem ser parte do armamentário de recurso na execução de casos de B/F-EVAR.
Since endovascular aneurysm repair (EVAR) was introduced,1 technology has evolved and custom-made fenestrated grafts have been made available since 1999,2 which allowed to take the endovascular repair to even more complex aortic aneurysms, namely juxtarenal, suprarenal and thoracoabdominal (TAAA) ones. Besides fenestrations, custom-made branched grafts have also been developed and used to repair TAAA.3 This has become a feasible technique with promising short and mid-term results reported in high volume centers.4,5 These techniques bring a possibility of endovascular repair to patients with aneurysms of great anatomical complexity whose open surgical treatment would probably require a supra-celiac clamping of the aorta.
However, despite careful planning of the procedures, difficulties in target vessel catheterization may occur during branched and fenestrated EVAR (B/F-EVAR), and bailout techniques can be used.6,7 We report a case of a successful retrograde catheterization of the superior mesenteric artery (SMA) using supra-umbilical laparotomy during B/F-EVAR.
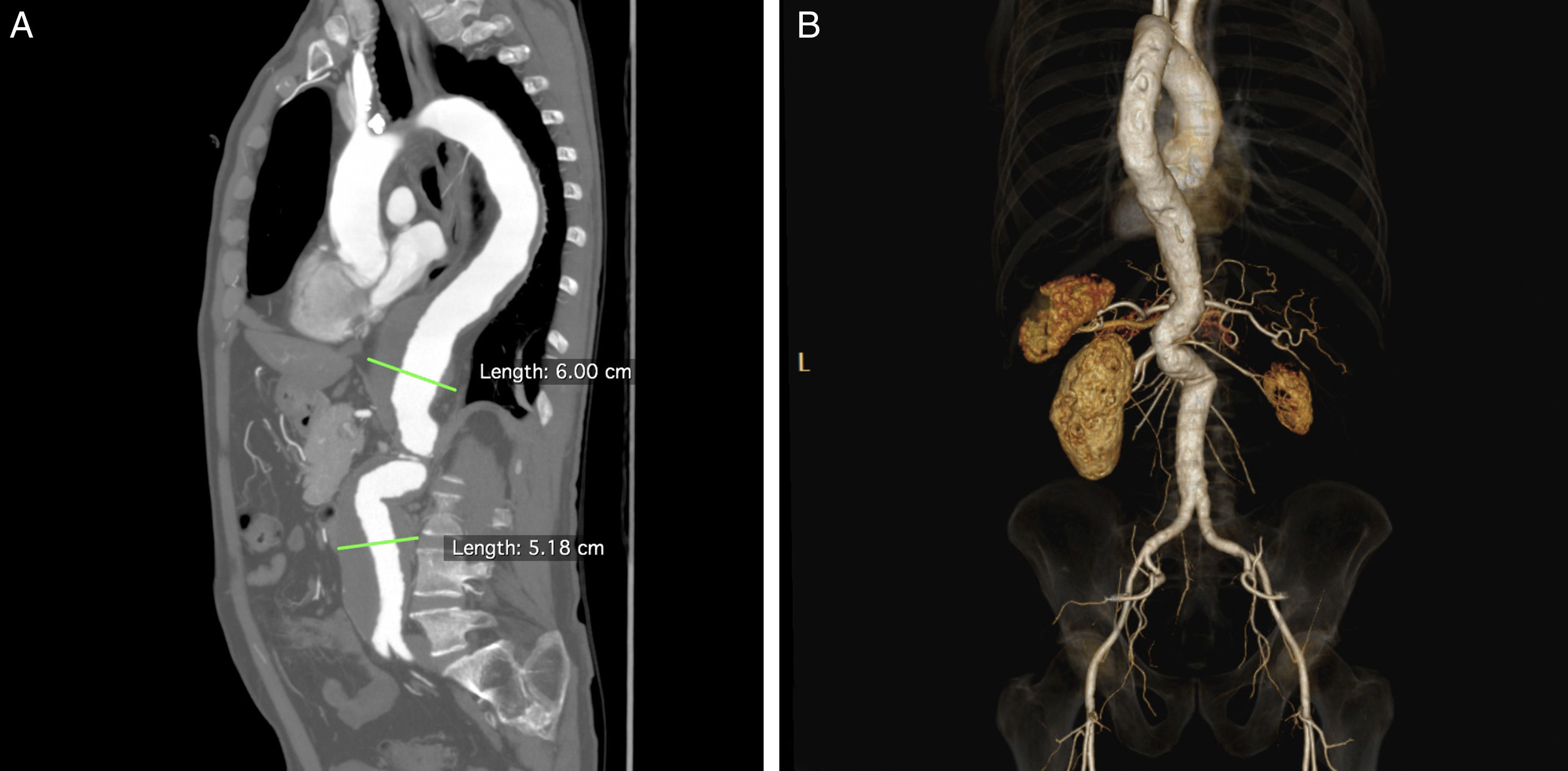
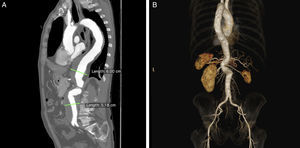
Clinical caseA 70-year-old caucasian male, with known history of chronic obstructive pulmonary disease (COPD) with severe bronchic and bronchiolar obstruction on respiratory tests, stage 38 chronic kidney disease with an eGFR of 38.5mL/min/1.73m2, arterial hypertension and smoking in the past, was sent from another Hospital's outpatient clinic to our Vascular Surgery Department with the diagnosis of type III TAAA with 60mm diameter (Fig. 1A). The patient was considered of high risk for open surgery, and proposed for endovascular repair with a custom-made graft.
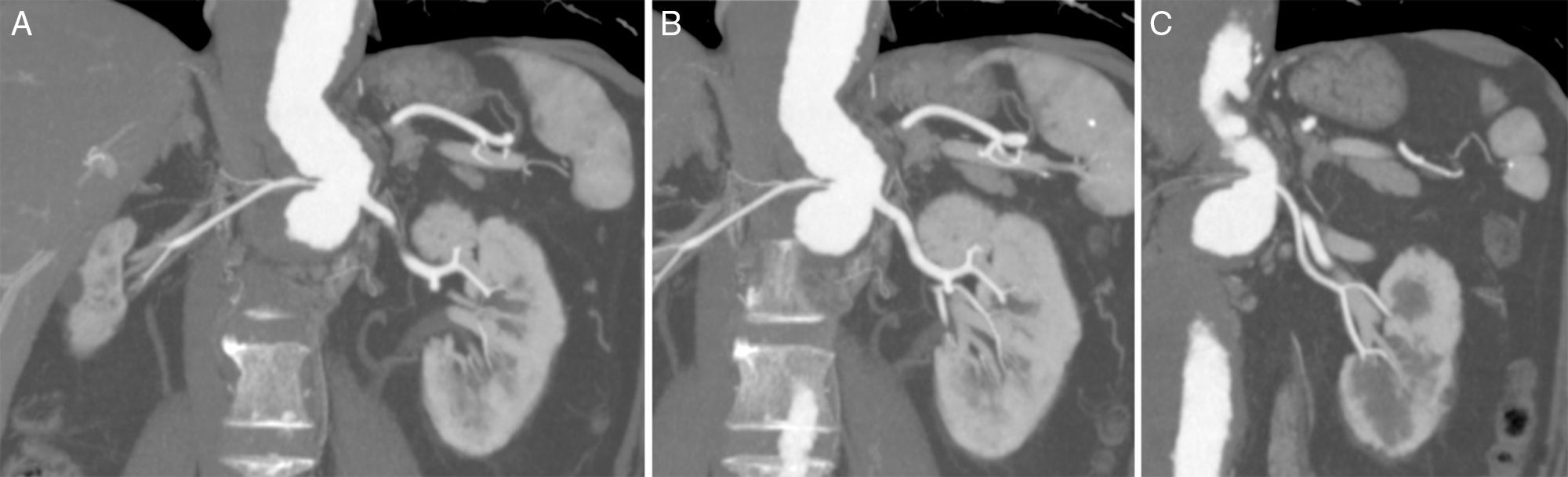
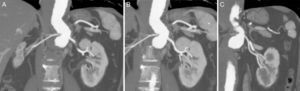
Regarding planning for B/F-EVAR, several technical details had to be considered due to the patient's anatomy, namely: the tight double angulation of the aorta below the origin of the celiac trunk (CT) and SMA (Fig. 1B); a thin right renal artery (RRA) with an atrophic kidney (Fig. 2A); two left renal arteries (LRA), with the thinner one going to lower kidney pole (Fig. 2B and C); and the fairly low diameter of the aorta at the level of the renal arteries.
After thorough planning, a customized Cook Zenith® graft (William A. Cook Australia Ltd., Brisbane, Australia) was designed, and included a proximal tapered thoracic component, a main body with branches to the CT (8×18mm, diameter×length) and SMA (8×18mm) and a fenestration (6×8mm, width×height) for the main LRA, and a distal bifurcated graft plus contralateral limb. As part of the plan for this main procedure, the patient underwent coil embolization of the RRA and thinner LRA 2 weeks before B/F-EVAR, without any complications. It was not feasible to build a graft with 2 fenestrations for the left renal arteries because they arose from the aorta at almost the same level and there was only 1h 45min between them, and a minimum of 2h 15min was needed for this to be possible.
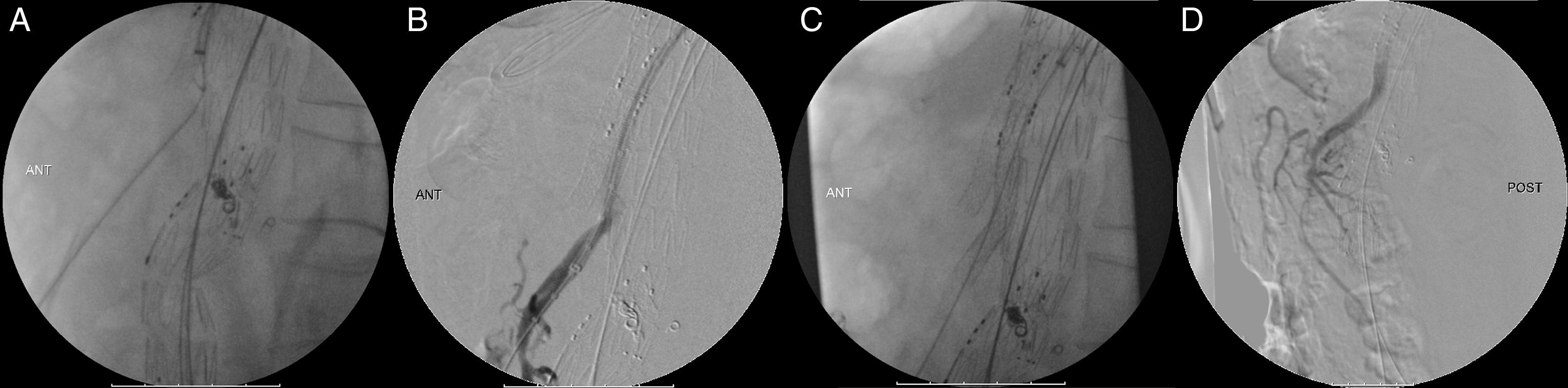
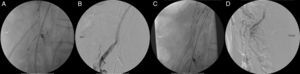
The patient underwent the procedure under general anesthesia, a spinal catheter for cerebrospinal fluid (CSF) drainage was placed the day before, contrast-induced kidney injury prophylaxis was done with pre-procedural intravenous hydration with saline and N-acetylcysteine, and both femoral and left axillary arteries were used as access using surgical exposure. The main LRA and CT were catheterized without issues. We were not able to catheterize the SMA using either the axillary or the femoral access, mainly due to the angulation of the aorta which led practically to the occlusion of the SMA's ostium by the main graft's body. After all the failed attempts to catheterize the SMA endovascularly, a median laparotomy was performed, and the SMA was retrogradely catheterized. The guidewire easily entered the endograft's branch retrogradly, and the SMA was stented with success (Fig. 3A–D) which allowed for completion of the B/F-EVAR, with an estimated partial bowel ischemia time of 1h.
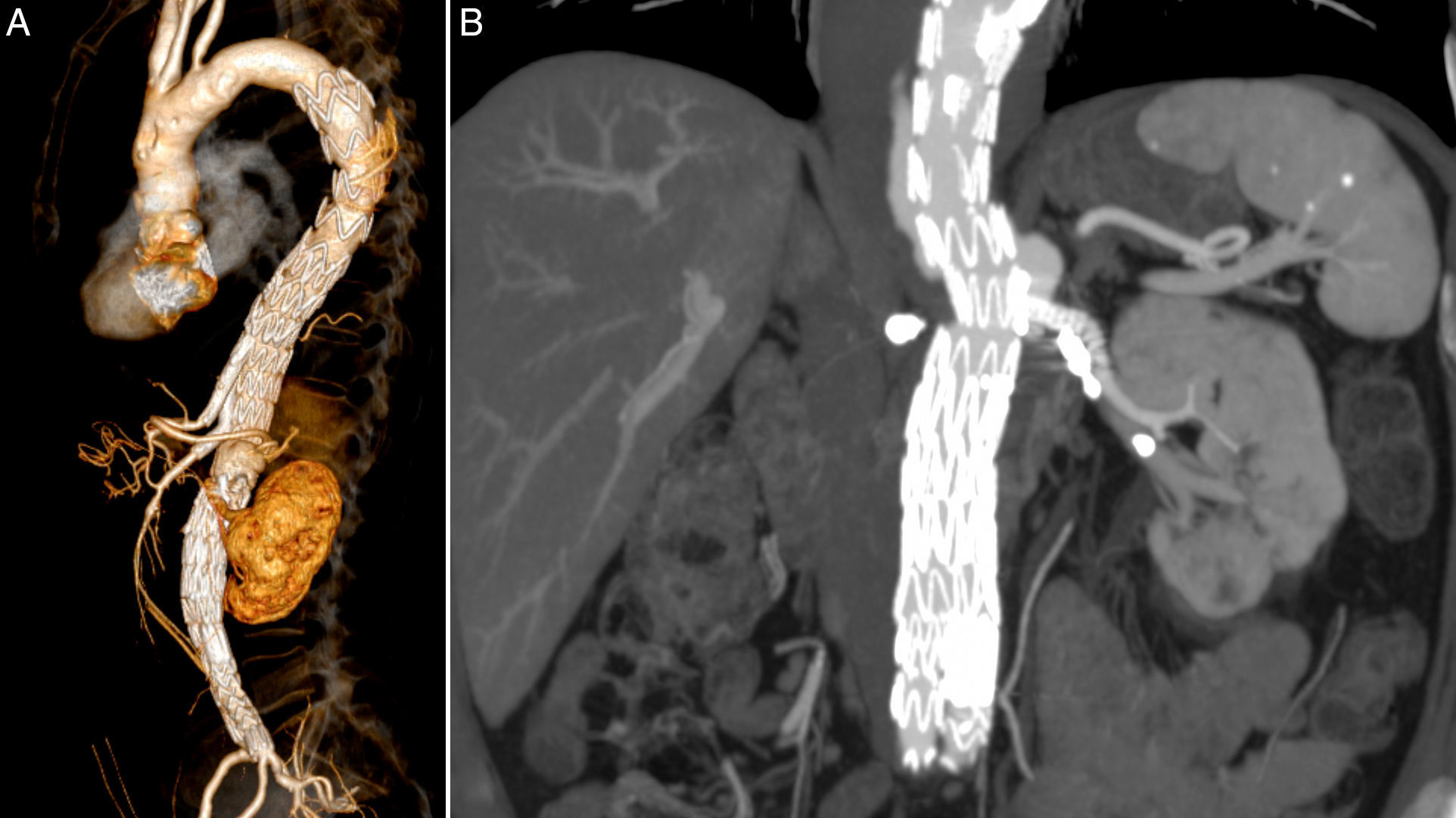
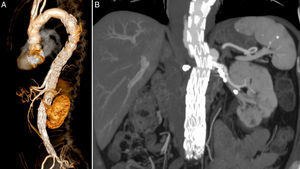
The patient was transferred to the intensive care unit (ICU), where he stayed for 6 days. There was a transient deterioration of kidney function, AKIN II, that recovered to pre-operative values with medical therapy alone and no need for dialysis. In the first 8h after the surgery, the patient had transient intra-abdominal hypertension (IAH) with a pressure of 24mmHg and high lactates of 40mg/dL, that resolved with medical treatment, namely sedation and analgesia. 24h after the surgery the patient developed paraparesis which required vasoactive support with norepinephrine in order to achieve mean arterial pressures (MAP) of 90–100mmHg, and lowering the spinal cord drain pressure to 5mmHg, to completely revert the neurological deficits successfully within another 24h; a neurological evaluation was done hourly in the first 12h. The patient was discharged 14 days after surgery. The first control CT angiogram was only at 6 months considering the patient's chronic kidney disease, and showed both branches and LRA stent patent (Fig. 4A and B).
DiscussionFenestrated and branched stent grafts are becoming more frequently used in our department, which allows us to offer treatment to an even bigger group of patients. However, with that comes more challenging patients and anatomies that were probably refused for open surgery before, and make these cases very complex. Very accurate planning of these procedures and grafts is crucial, and sometimes it might be difficult to predict how a graft will behave intra-operatively. The risk of failure to catheterize a target vessel, although very low,7 can occur and the surgeon has to decide whether an open surgical approach should be used to salvage the procedure.
In the case we report, we consider that the severe double-angulation of the visceral aorta probably contributed to the impossibility of SMA antegrade catheterization, with the SMA branch being put against the aortic wall curvature, which made catheterizing the target vessel undoable. The decision was to do a laparotomy and catheterize the SMA retrogradely, with success. The patient did have apparent bowel ischemia, with temporary raise in intra-abdominal pressure (IAP), but that was managed in a conservative way.
A recent report from Oikonomou et al.7 relates difficulty in target vessel catheterization with its anatomy in the case of fenestrations; in branched grafts they consider the main cause of failure is probably related to graft planning or deployment, rather than anatomy.
ConclusionTarget vessel catheterization may prove to be impossible intra-operatively during a F/B-EVAR, even with exhaustive planning. Retrograde target vessel using open surgery is one alternative for the surgeon in such cases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.