Nonalcoholic fatty liver disease (NAFLD) is a chronic pathological liver disease caused by excessive buildup of fat in liver cells [1]. NAFLD can range from simple fat accumulation in the liver to nonalcoholic steatohepatitis (NASH) alone or with fibrosis, which may subsequently lead to cirrhosis and liver cancer [2]. NAFLD is found in 25.2% of the population worldwide and poses serious health risks [3]. It is the hepatic manifestation of metabolic syndromes, such as obesity, dyslipidemia, insulin resistance, glucose intolerance and type 2 diabetes mellitus [4,5]. There are currently no pharmacological agents that are approved for NAFLD or NASH treatment [6]. Therefore, the development of treatments for NAFLD, especially the incurable NASH, is an unmet and urgent medical need.
A growing body of work indicates that adhering to the Mediterranean diet, supplementing with probiotics, antioxidants, polyphenols or specific nutrients with hepatoprotective effects may improve liver enzyme levels and hepatic steatosis in NAFLD, or slow its progression [7–10]. Silymarin is a potent liver-tropic antioxidant extracted from milk thistle (Silybum marianum). This extract is comprised of several antioxidant substances, the most abundant of which are silibine A and B and the flavonoid taxifolin [11]. Silymarin has multiple hepatoprotective effects, including antioxidant activity, cell regeneration, increased protein synthesis, and anti-inflammatory and antifibrotic properties [12]. As a result, silymarin has been proposed as a therapeutic approach for NAFLD [13].
NAFLD has a complex pathogenesis and can be caused by impaired metabolism and inflammation. Studies have shown that NAFLD is the hepatic manifestation of metabolic syndromes such as obesity, dyslipidemia, hypertension and type 2 diabetes mellitus (T2DM) [14]. In addition, NAFLD can also result from increased oxidative stress, which increases lipid peroxidation and reactive oxygen species production within hepatocytes and thereby leads to mitochondrial dysfunction and lipotoxicity [15,16]. Some studies showed that silymarin can improve insulin resistance by inhibiting the production of pro-inflammatory cytokines through inhibition of NF-κB signaling [17]. The anti-inflammatory and anti-fibrotic effects of silymarin are mediated by upregulated adiponectin expression, downregulated resistin expression, inhibition of NF-κB and related pathways, reduced activation of hepatic stellate cells, and decreased expression of interleukins (IL-2 and IL-4), TNF-α and IFN-γ[18].
Previous studies have suggested that silymarin should be initiated as early as possible in patients with fatty liver disease when the regenerative potential of the liver is still high [19,20]. Silymarin is a promising botanical treatment for NAFLD patients, and its efficacy has been investigated in several randomized-controlled trials (RCTs). However, a systematic evaluation of the efficacy of silymarin in NAFLD is currently lacking. This meta-analysis aims to systematically evaluate the efficacy of silymarin in NAFLD patients by characterizing its effects on energy metabolism, liver injury, liver histology and anthropometric parameters in order to provide support for its clinical application in NAFLD.
2Materials and MethodsThis study was conducted according to the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement [21] and was registered with PROSPERO (CRD42023398590).
2.1DatabasesRelevant articles were retrieved from PubMed, Embase, the Cochrane Library, Web of Science, clinicaltrails.gov, and China National Knowledge Infrastructure.
2.2Search strategySearch terms related to “silymarin” and “nonalcoholic fatty liver disease” were used to retrieve records from the databases. The search strategy and results are detailed in Supplementary Table S1.
2.3Study selectionStudies were searched and selected independently by three researchers. The titles and abstracts of the identified studies were first screened, then the full texts of potential studies were further assessed based on the eligibility criteria. The selected full texts were compared among the three researchers, and any disagreement or discrepancy was resolved by discussion and voting.
2.4Inclusion and exclusion criteriaClinical studies were included if they meet the following criteria: (1) Study design: RCTs; (2) Participants: NAFLD patients, without age, gender, or race restrictions; (3) Intervention: Silymarin or silymarin complex; (4) Comparators: Same treatment as the intervention group except for silymarin; (5) Outcomes: 1) Energy metabolism indicators: Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), fasting insulin (FI), and homeostatic model assessment of IR (HOMA-IR); 2) Liver injury indicators: Alanine aminotransferase (ALT) and aspartate aminotransferase (AST); 3) Liver histological indicators: Fatty liver index, fatty liver score, and hepatic steatosis grade; 4) Anthropometric indicators: Body mass index (BMI), waist circumference (WC), and hip circumference (HC).
Exclusion criteria: (1) Alcoholic steatohepatitis, alcoholic fatty liver, cirrhosis or liver cancer; (2) Received additional medication(s) or has genetic predisposition (single nucleotide polymorphisms); (3) Underwent liver transplantation; (4) Conference papers, abstracts, non-original research, case reports, and non-peer-reviewed articles (e.g., conference materials and thesis).
2.5Quality assessmentThe titles and abstracts of retrieved records were independently assessed by two authors to exclude irrelevant studies. Full texts of selected articles were then assessed individually based on the eligibility criteria. The risk of bias in eligible RCTs was evaluated using the Cochrane Collaboration's Risk of Bias (RoB) tool.
2.6Data synthesis and analysisStatistical analyses were performed by RevMan v5.3 (The Cochrane Collaboration, Copenhagen, Denmark). Given the different methods used to measure outcomes in each original study, continuous and categorical variables were pooled using standard mean difference (SMD) and odds ratio (OR), respectively. If data were available and sufficient, subgroup analyses were also performed according to the type of disease (NAFLD/NASH), type of intervention (silymarin, silymarin complex), and duration (<12; ≥12 to ≤24; >24). Heterogeneity was assessed using the Cochran's Q test (I2 statistic). A fixed-effects model was used when the I2 is <50%; otherwise, a random-effects model was used. Source of heterogeneity was identified by sensitivity and subgroup analyses. All statistical tests are two-tailed with a significance level of 0.05. Sensitivity analysis was performed to identify the stability of the results. Publication bias was assessed using a funnel plot if a given outcome measure is reported in over 10 studies.
3Results3.1Study selectionA total of 1,039 articles (up until February 26, 2023) were initially retrieved according to the search strategy, and 26 RCTs were included in the meta-analysis (Figure 1).
3.2Basic characteristics and quality assessmentThe 26 included studies [22–41] involved a total of 2,375 patients. The characteristics of the included studies are summarized in Table 1. The risk of bias assessment showed that the included studies had medium to low overall risk (Fig. 2). Since allocation concealment and blinding methods were unclear in most included studies, a clear judgement could not be made in these areas, resulting in reduced quality of evidence.
Characteristics of included studies
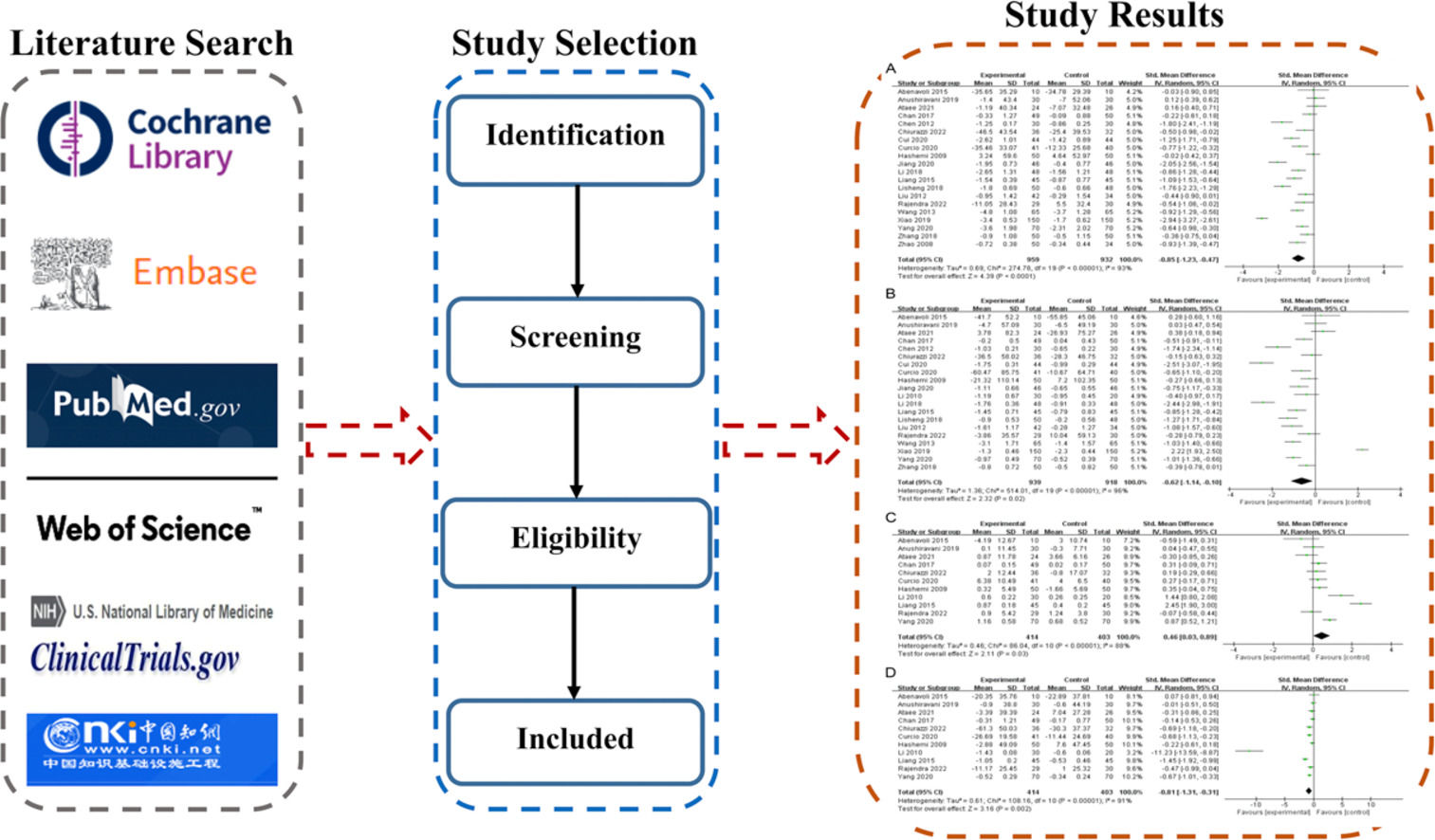
TC was reported in 20 studies involving 1,891 patients. As shown in Fig. 3A, silymarin significantly decreased TC level in NAFLD patients (I2 = 93%, random-effects model; SMD = -0.85 (95% CI [-1.23, -0.47], P<0.0001). Subgroup analysis of the included studies revealed significant differences in TC levels between types of intervention [silymarin (95%CI = -1.03[-1.49, -0.58]) vs. silymarin complex (95%CI = -0.32[-0.64, 0.01]), P = 0.01], types of disease [NAFLD (SMD[95%CI] = -0.93[-1.33, -0.53]) vs. NASH (95%CI = -0.12[-0.40, 0.16]), P = 0.001], and treatment duration [<12 weeks (95%CI = -1.04[-1.43, -0.66]) vs. ≥12 to ≤24 weeks (95%CI = -0.86[-1.30, -0.42]) vs. >24weeks (95%CI = -0.22[-0.61, 0.18]), P = 0.010] (Figures S1-S3).
3.3.2TGMeta-analysis of TG (20 studies, n = 1,857) showed that TG was significantly different between the two groups [SMD = -0.62, (95% CI [-1.14, -0.10], P = 0.02, I2 = 96%, random-effects model]. Subgroup analysis revealed a significant difference in TG level between treatment duration [<12 weeks (95%CI = -2.47[-2.86, -2.09]) vs. ≥12 to ≤24 weeks (95%CI = -0.41[-0.95, 0.13]) vs. >24 weeks (95%CI = -0.51[-0.91, -0.11]), P<0.00001] (Figures S4-S6). These results suggested that silymarin can effectively reduce TG in NAFLD patients (Fig. 3B).
3.3.3HDL-CEleven studies involving 818 patients assessed HDL-C. Meta-analysis showed that HDL-C was significantly different between the two groups [SMD = 0.46 (95%CI [0.03, 0.89], P = 0.03, I2 = 88%, random-effects model]. Subgroup analysis demonstrated a significant difference in HDL-C level between types of intervention [silymarin (95%CI = -1.03[-1.49, -0.58]) vs. silymarin complex (95%CI = -0.32[-0.64, 0.01]), P = 0.03] (Figures S7-S9). These findings suggest that silymarin can increase HDL-C in NAFLD patients (Fig. 3C).
3.3.4LDL-CMeta-analysis of 11 studies involving 817 patients showed that LDL-C level was significantly lower in the experimental group than in the control group [SMD = -0.81, (95% CI [-1.31, -0.31], P = 0.002, I2 = 91%, random-effects model)], suggesting that silymarin reduces LDL-C in NAFLD patients (Fig. 3D).
3.3.5FBGFBG was reported in 7 studies (n = 537) and was significantly lower in the experimental group than in the control group [SMD = -0.09, (95% CI [-0.25, 0.08], P = 0.33, I2 = 0%, random-effects model)] (Fig. 4A).
3.3.6FIMeta-analysis showed that the pooled FI (2 studies, 160 patients) was significantly lower in the experimental group than in the control group [SMD = -0.59, (95% CI [-0.91, -0.28], P = 0.0002, I2 = 0%, random-effects model)], suggesting that silymarin can reduce FI level in NAFLD patients (Fig. 4B).
3.3.7HOMA-IRHOMA-IR was pooled from 4 studies (n = 318) and was found to be significantly lower in the experimental group than in the control group [SMD = -0.37, (95% CI [-0.77, 0.04], P = 0.08, I2 = 64%, random-effects model)] (Fig. 4C).
3.4Effect of silymarin on liver injury3.4.1ALTALT was pooled from 23 studies (n = 2,138) and was found to be significantly lower in the experimental group than in the control group [SMD = -12.39 (95% CI [-19.69, -5.08], P<0.00001, I2 = 98%, random-effect model)], which implies that silymarin can reduce ALT level in NAFLD patients (Fig. 5A).
3.4.2ASTTwenty-two studies with 2,091 patients reported AST. Meta-analysis showed that the experimental group had significantly lower AST level than the control group [SMD = -10.97 (95% CI [-15.51, -6.43], P<0.00001, I2 = 96%, random-effects model)], which demonstrates that silymarin lowers AST level in NAFLD patients (Fig. 5B).
3.5Effect on liver histological changes3.5.1Fatty liver indexTwo studies containing 79 patients evaluated fatty liver index. Meta-analysis revealed a significantly lower fatty liver index in the experimental group than in the control group [SMD = -6.64 (95% CI [-10.59, -2.69], P = 0.0010, I2 = 16%, fixed-effect model)], which affirms that silymarin can reduce FLI in NAFLD patients (Fig. 6A).
3.5.2Fatty liver scoreTwo studies involving 206 patients reported the fatty liver score, and meta-analysis revealed a significantly lower fatty liver score in the experimental group than in the control group [SMD = -0.51 (95% CI [-0.69, -0.33], P<0.00001, I2 = 0%, fixed-effects model)]. This suggests that silymarin can lower fatty liver score in NAFLD patients (Fig. 6C).
3.5.3Hepatic steatosis gradeHepatic steatosis grade was pooled from 7 studies (n = 492) and was found to be significantly higher in the control group than in the experimental group [OR = 3.25, (95% CI [1.80, 5.87], P<0.0001, I2 = 0%, random-effects model)]. This result shows that silymarin can improve hepatic steatosis in NAFLD patients (Fig. 6D).
3.6Effect on anthropometric parameters3.6.1BMISeven studies with 416 patients assessed BMI. Meta-analysis showed that BMI was significantly lower in the experimental group than in the control group [SMD = -0.19 (95%CI [-0.39, 0.00], P = 0.05, I2 = 33%, fixed-effects model)], suggesting that silymarin can lower the BMI of NAFLD patients (Fig. 7A).
3.6.2WCMeta-analysis of 3 studies (n = 148) showed that WC was similar between the two groups [SMD = 0.02 (95% CI [-0.31, 0.34], P = 0.92, I2 = 0%, fixed-effects model)] (Fig. 7B).
3.6.3HCSimilarly, HC (2 studies, 88 patients) was similar between the two groups [SMD = -0.16, (95% CI [-0.58, 0.26], P = 0.46, I2 = 0%, fixed-effects model)] (Fig. 7D).
3.7Sensitivity analysis and publication biasSensitivity analysis of all parameters showed that the results were robust. The funnel plots of TC, TG, HDL-C, HDL-C, ALT and AST were all symmetrical, indicating the absence of publication bias (Figures S10-S15).
4DiscussionThe present study evaluated the effects of silymarin on energy metabolism, liver injury, liver histology and anthropometric parameters in NAFLD patients. Our results showed that silymarin plays a role in regulating energy metabolism, attenuating liver damage, and improving liver histology, and may, therefore, be a potential treatment for NAFLD.
NAFLD is a hepatic manifestation of dyslipidemia [4]. Elevated TC and TG levels are important risk factors for NAFLD [42], which is characterized by low HDL-C and high LDL-C levels [43,44]. Consistent with previous studies, we found that silymarin can improve blood HDL-C level and reduce blood TC, TG, LDL-C levels in NAFLD patients [45–48]. Insulin resistance and glucose metabolism dysfunction are typical clinical symptoms of NAFLD and metabolic syndrome [49], and elevated blood glucose can be found in 70-80% of NAFLD patients [1]. It was shown that silymarin can lower blood glucose, insulin and HOMA-IR [50,51], but the exact mechanism by which silymarin affects glucose levels is unclear. However, since silymarin is a powerful antioxidant, its effect on glucose levels may be mediated by inhibiting lipid peroxidation [52]. In addition, silymarin acts as an inhibitor of aldose reductase and can reduce insulin levels by inhibiting insulin secretion in response to glucose stimulation [53]. Silymarin was found to be effective in ameliorating insulin resistance in NAFLD mainly by reducing visceral fat, enhancing lipolysis, and suppressing gluconeogenesis [54]. We also found that silymarin can lower FI level and potentially FBG and HOMA-IR. The absence of significant differences in FBG and HOMA-IR may be related to the small number of included studies, and greater emphasis should be placed on the changes in FBG and HOMA-IR in subsequent studies to ascertain these findings. Collectively, our data indicate that silymarin may be involved in the regulation of energy metabolism of NAFLD patients. Although changes in energy metabolism have been suggested to affect the anthropometric parameters of NAFLD patients, we did not observe any significant differences in BMI, WC and HC between the experimental and control groups.
Silymarin also has a protective effect on the liver, as indicated by reduced AST and ALT levels in the experimental group. Elevated ALT and AST levels are biomarkers for liver injury and were shown to be associated with the occurrence and development of NAFLD. Furthermore, a higher ALT level is correlated with a higher incidence of NAFLD progression to cirrhosis or hepatocellular carcinoma [55,56]. It was reported that silymarin is a beneficial treatment for cirrhotic diabetic patients due to its ability to lower AST and ALT levels [57]. There are two mechanisms by which silymarin protects liver cells. First, silymarin protects intact liver cells or cells not yet irreversibly damaged by reducing oxidative stress and consequent cytotoxicity [58]. It can stabilize membrane permeability by suppressing lipid peroxidation, thereby maintaining the level of the antioxidant glutathione in the liver [59]. Second, silymarin exerts anti-inflammatory effects by inhibiting NF-κB to reduce inflammatory cytokine production in the liver parenchyma and interacting with protein kinase to downregulate cyclooxygenase-2 [58,60].
Furthermore, we found that silymarin can decrease fatty liver index and fatty liver score and improve hepatic steatosis grade in NAFLD patients. NAFLD is a clinicopathologic syndrome characterized by hepatic steatosis. Fatty liver index, fatty liver score and hepatic steatosis grade are often used to assess the severity of disease [61,62]. Oxidative stress, increased lipid peroxidation and decreased antioxidant status can all contribute to NAFLD progression. Silymarin was reported to protect the liver from oxidative stress, inflammation, steatosis, and fibrosis [63,64]. Because of the limited histological data, hepatic fibrosis was neither considered nor evaluated. In addition, silymarin downregulates the mRNA expression of enzymes responsible for de novo lipogenesis such as sterol-regulatory element binding protein (SREBP1c), fatty acid synthetase (FAS), and acetyl-CoA carboxylase 1 (ACC1), which consequently phosphorylates AMP-activated protein kinases in diabetic obese mice with NAFLD [65,66]. However, because of the limited histological data, hepatic fibrosis was neither considered nor evaluated.
Our subgroup analysis showed that TC and HDL-C levels are significantly different between different interventions. Silymarin alone was superior to silymarin complex in reducing TC and increasing HDL-C levels in NAFLD patients. Drug interactions may impair the efficacy of silymarin, and hence more consideration should be given to silymarin in the treatment of NAFLD. When using silymarin complex, care should be taken to ensure that the dosage of silymarin is sufficient and that other ingredients do not affect its efficacy. In addition, subgroup analysis based on type of disease revealed that silymarin has a greater effect on TC reduction in patients with NAFLD than in patients with NASH. NASH is a more severe form of NAFLD characterized not only by hepatic steatosis, but also by hepatic lobule inflammation, balloon-like changes in the liver cells, and fibrosis. Combined with the results of this study, silymarin should be initiated as early as possible to maximize its effect on delaying NAFLD progression [67]. Furthermore, TC and TG levels were also significantly different between different durations of treatment. Notably, the duration of treatment was 12-24 weeks in most included studies, and there were only few studies that examined a duration of <12 weeks or >24 weeks, which may result in bias in the results.
There are several limitations in this study. First, the types, the doses of silymarin and lifestyle management of the patients were different among the included RCTs. Second, the design of a few RCTs was not well standardized, which may affect the effectiveness of the evaluation. Furthermore, because of the limited histological data, hepatic fibrosis was neither considered nor evaluated. Last, different measurement methods used in each study may introduce bias in the results, such as when pooling the data for hepatic steatosis grade.
5ConclusionsSilymarin can regulate energy metabolism, attenuate liver damage and improve liver histology in NAFLD, and is thus a promising treatment for NAFLD. However, the results of this study should be interpreted with caution due to its limitations. Further studies with more adequate and proper methodological design are warranted to confirm these findings.
Availability of data and materialsThe data that support the findings of this study are available from the corresponding author upon reasonable request.
FundingThis study was supported by National famous traditional Chinese medicine Maudxi inherit- ance studio construction project (China Medical Office teaching Letter [2018] 119), Henan Province Chinese medicine scientific research special topic (2022ZY1167), National Clinical Research Base of Traditional Chinese Medicine, Henan Provincial Health Commission (2021 JDZX2014). The fund was not involved in any study design, data collection, analysis and interpretation, report writing, and article submission for publication.
CRediT authorship contribution statementShudi Li: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. Fei Duan: Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. Suling Li: Conceptualization, Data curation, Software, Visualization, Writing – original draft, Writing – review & editing. Baoping Lu: Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.