Liver diseases are a growing global health concern, and early detection through biochemical markers is essential to prevent the progression of manageable conditions and reduce the eventual need for urgent interventions. Serum biochemical tests are crucial to identify hepatic tissue injury and assessing the aetiology, staging and prognosis of chronic liver disease. Different biomarker patterns provide insights into injury, function, or development of advanced fibrosis. Tests indicative for liver injury primarily include serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH), while alkaline phosphatase (ALP) is more commonly associated with cholestasis; γ-glutamyl transferase (GGT) may be an additional index of cholestasis, as well as an indicator of metabolic liver diseases or alcohol use disorder. Liver function is assessed by total and direct bilirubin, serum albumin, and prothrombin time measurement. Severe liver fibrosis is usually suspected based on reduced platelet count, often integrated in simple algorithms such as the Fibrosis-4 (FIB-4) index [1]. Other scores include the Aspartate Aminotransferase to Platelet Ratio Index (APRI) [2], and transient elastography and ultrasound associated ARFI techniques [3]. More complex parameters rely on direct markers of fibrogenesis, hyaluronate, type IV collagen, procollagen III, and laminin, which are usually not routinely tested, while some of them have been integrated in the Enhanced Liver Fibrosis (ELF™) panel [4]. Alpha-fetoprotein (AFP) measurement reflects extensive cell proliferation and is often associated with hepatocellular carcinoma.
Among all indicators of liver injury, ALT is the most sensitive as it is predominantly expressed in the liver, unlike AST which is also present in heart, muscles and kidney [5]. Upon cellular damage, ALT is released into the bloodstream and is detectable at the onset and throughout the process of liver injury [6]. In most hepatic diseases, elevated ALT levels contribute to predict clinical outcomes, helping to assess disease severity, and to determine prognosis [7]. Moreover, ALT elevation in relation to levels of other relevant biomarkers, may provide clues on disease etiology.
Despite the availability of an easy and inexpensive biochemical test, that potentially allows to recognize liver abnormalities at an initial stage, the global incidence of liver diseases is on a swift rise, resulting in approximately two million annual fatalities, predominantly due to cirrhosis and liver cancer [8]. A relevant example is represented by metabolic dysfunction-associated steatotic liver disease (MASLD, previously NAFLD), a stable and potentially reversible condition which currently affects 15 %−40 % of the adult population in western and eastern countries [8–10]. Moreover, it is estimated that 20–30 % of cases worsen to metabolic dysfunction associated steatohepatitis (MASH, previously NASH), a clinical condition affecting 1 %−7 % of the adults in eastern and western countries which is now the leading cause of liver fibrosis, cirrhosis, and eventually hepatocarcinoma (HCC) [9]. Yet, these figures may be underestimated as hepatic dysfunction often occurs in asymptomatic patients [11]. Therefore, early identification in primary care is crucial to allow a timely referral to specialized centres.
This expert opinion aims to highlight the clinical significance of elevated ALT as an early marker of different chronic liver diseases. Early detection can help in predicting adverse clinical outcomes at the primary care level, helping to prevent the progression of manageable liver conditions and ultimately reducing the need for urgent referrals.
2Factors influencing ALT levels and clinical interpretationFactors such as gender, age, body mass index (BMI), environment, and exercise influence ALT levels [12,13]. As a result, the upper limit of normality (ULN) fluctuates among clinical laboratories and populations. The proposed ULN for ALT in both genders, without evident risk factors for liver diseases, ranges from 29 to 33 IU/L for males and 19 to 25 IU/L for females [5,14]. Nevertheless, these thresholds cannot univocally identify or exclude severe liver disease. A large-scale population study, involving different cohorts, has suggested redefining healthy ALT ranges based on the latest clinical evidence, to optimise the sensitivity in detecting liver diseases [15].
Physiological ALT levels are generally lower in women compared to men [5,14,16]. In addition, metabolic risk factors or physical inactivity, which are associated with obesity [17–19] and type 2 diabetes [20], are often associated with elevated ALT values. Concerning ethnic differences, Hispanics display a higher prevalence of elevated ALT levels compared to Caucasian, Black and Asian populations [21,22]. Elevated ALT values should prompt a thorough assessment of medical history, physical examination, and the measurement of additional liver biomarkers in order to provide a comprehensive evaluation of liver status [5,23]. In particular, the assessment of patient's medical history in terms of risk factors for comorbidities, alcohol consumption, use of potentially hepatotoxic drugs or dietary and herbal supplements, and a family history of liver disease followed by the evaluation of ALT and major liver biomarkers, can help determine the aetiology of liver injury. In this respect, determination of AST may be particularly helpful, and together with the calculation of the AST/ALT ratio, is a useful biomarker to assess the prognosis of liver diseases. Altered AST/ALT levels are reported to be predictive of cancer initiation and overall negative outcome, with an increased risk of all-cause mortality [24,25]. Over the years, the performance of the AST/ALT ratio has further improved, providing reliable predictions on liver disease severity and liver related mortality that are population-specific [26,27].
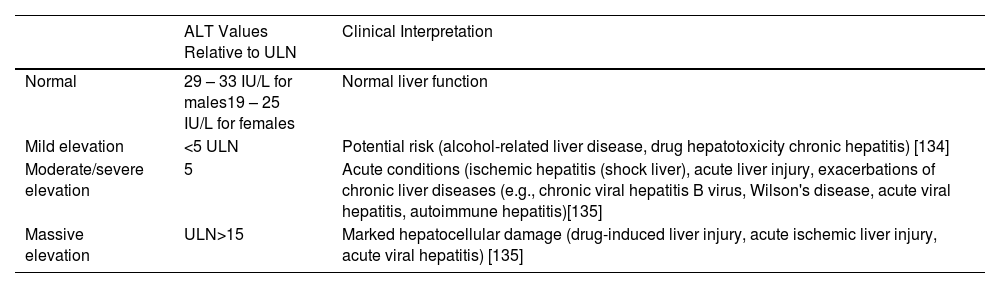
According to ULN values, aminotransferase abnormality can be classified into mild (<5 times the ULN), moderate/severe (5 to 15 times the ULN) and massive (concentration >10,000 IU/L) (Table 1) [5,28].
ALT levels and clinical interpretations based on ULN.
| ALT Values Relative to ULN | Clinical Interpretation | |
|---|---|---|
| Normal | 29 – 33 IU/L for males19 – 25 IU/L for females | Normal liver function |
| Mild elevation | <5 ULN | Potential risk (alcohol-related liver disease, drug hepatotoxicity chronic hepatitis) [134] |
| Moderate/severe elevation | 5 | Acute conditions (ischemic hepatitis (shock liver), acute liver injury, exacerbations of chronic liver diseases (e.g., chronic viral hepatitis B virus, Wilson's disease, acute viral hepatitis, autoimmune hepatitis)[135] |
| Massive elevation | ULN>15 | Marked hepatocellular damage (drug-induced liver injury, acute ischemic liver injury, acute viral hepatitis) [135] |
This classification may guide the initial diagnosis. Furthermore, concomitant to ALT and AST alterations, out-of-proportion values of ALP, GGT, and bilirubin generally suggest a mixed hepatocellular-cholestatic pattern.
The subsequent section provides the clinical interpretation of ALT elevation associated with different liver diseases.
3The role of ALT elevation in different liver diseases3.1Viral hepatitisA mild increase in ALT or AST is a common clinical manifestation of chronic hepatitis B and C. These viral chronic infections are accompanied by the risk of advancing to fibrosis and cirrhosis in one-fourth of cases [29]. Notably, nearly 80 % of infections are asymptomatic and undiagnosed [29,30]. Thus, screening for these viral infections should be prioritized as a first-line investigation whenever mild ALT elevations are observed. Acute hepatitis is associated with a moderate to severe elevation in transaminase levels, particularly with an increase in ALT over AST [28]. However, acute HCV infection is typically anicteric and asymptomatic, and therefore rarely diagnosed [31]. Patients with chronic hepatitis D also exhibit elevated ALT levels [32].
Following viral transmission, transaminase levels may be useful in identifying the phase of infection. In the early phase of HBV infection, liver inflammation is scarce, but as the infection progresses to the 'seroclearance' phase where immune-mediated mechanisms clear infected hepatocytes, transaminase levels rise due to increased liver activity [30,33]. This elevation requires close monitoring to assess the progression of the infection and liver function.
Additional evidence highlights the prognostic value of transaminase levels during treatment, underscoring their importance in monitoring therapeutic response and disease progression. However, persistent ALT flares in the case of both chronic HCV or HBV infection could be a signal of protracted liver injury with advanced fibrosis and cirrhosis [34–36]. Due to the effectiveness of direct antiviral agents in patients with HCV infection, the serum level of ALT is no more crucial in the choice to refer for treatment; indeed, all the patients with detectable HCV-RNA should be addressed to virus elimination, including patients with normal ALT. In the setting of chronic HBV hepatitis, the serum level of ALT is still relevant for the therapeutic choices as an ALT level persistently higher than 2 x ULN is a clear indication for treatment of patients with HBeAg positive infection and HBV-DNA level higher than 20,000 IU/ml or with HBeAg negative infection and HBV-DNA level higher than 2,000 IU/ml [33].
AST/ALT ratio values exceeding 1 in infected patients confer a significant risk of developing cirrhosis [35,37]. This supports the role of transaminase levels as a surveillance marker in hepatitis patients, useful for monitoring in hepatocellular carcinoma programs [38].
3.2Metabolic dysfunction-associated steatotic liver disease (MASLD)MASLD is an accumulation of fat in hepatocytes linked to systemic metabolic dysfunction [10,39]. This term replaces the previous nomenclature of MAFLD/NAFLD. The prerequisites for diagnosing MASLD are related to metabolic risks such as type 2 diabetes mellitus and obesity. The MASLD nomenclature allows the inclusion of different stages of liver disease including isolated liver steatosis (metabolic dysfunction associated steatotic liver, MASL), metabolic dysfunction associated steatohepatitis (MASH), as well as fibrosis and cirrhosis [10,40].
Several studies have demonstrated a close association between ALT elevations and liver fat accumulation in MASLD patients [41–44], pointing out that persistent elevation of this biomarker even within the normal range is associated with an increased incidence of MAFLD [45] and with increased liver-related mortality [10]. Indeed, patients with MASLD and normal ALT levels may have undetected inflammation resulting in steatohepatitis. Normal ALT levels can result in failure to prescribe the necessary additional tests to obtain a diagnosis. In this regard, a recent report found that 33 % of MASLD/MAFLD patients with ALT ranging from 31 to 54 IU/L in primary care had advanced fibrosis or cirrhosis [46]. Additional evidence found that 37.5 % of MASLD/MAFLD cases with normal ALT had advanced fibrosis [47]. Nonetheless, only a portion of these patients had ALT that would fall within the ‘revised’ normal values, indicating that labelling these patients as having ‘normal ALT’ can be misleading. Overall, these findings underscore the relevance of ALT as a predictive marker for steatosis-related complications and emphasise the need to update ALT reference ranges [7,48,49] to prevent underdiagnosis and facilitate effective risk stratification in primary care.
3.3Alcohol-related liver disease/acute alcoholic hepatitisAlcohol-related liver disease (ALD) significantly contribute to the global burden of liver disease. The definition of excessive alcohol consumption has been suggested to be > 140 g and 210 g per week in woman and man, respectively [5]. Excessive alcohol intake may lead to a wide spectrum of severe liver-related complications, ranging from steatosis to acute alcoholic hepatitis with or without cirrhosis. Although transaminase levels may remain unchanged or show elevations that are not proportional to the extent of liver damage, they are an important biomarker in cases of suspected ALD [50]. Typically, abnormal transaminase values are up to 5 times the ULN, with AST two- to three-fold higher than ALT and AST/ALT ratio of at least 2:1 driven by piridoxin deficiency often found in ALD patients [51]. However, individuals with elevated ALT and moderate alcohol intake have an increased risk of all-cause mortality [52]. ALD individuals have usually increased serum levels of GGT and the contemporary detection of increased transaminases with AST/ALT ratio higher than 2 may reinforce the clinical suspicion of ALD. Due to the harmful effect of alcohol on the progression of hepatic damage, guidelines recommend complete abstinence for subjects with ALD to improve survival [53].
3.4Drug- and herbal-induced liver injuryA number of pharmaceutical compounds (especially antibiotics, anticonvulsants, and statins) are responsible for drug-induced liver injury (DILI), together with herbal and dietary supplements (HILI), causing variable aminotransferase elevations. DILI can be classified as intrinsic or idiosyncratic. Intrinsic DILI is dose-related and predictable and usually occurs within days from the first intake. On the contrary idiosyncratic DILI is not dose-related, is unpredictable and occurs after days to weeks after the first intake of the drug. Exclusion of other causes of liver injury and discontinuation of the medication may help the identification of DILI, which results in a transaminase decrease by 50 % and recovery of overall hepatic function [54].
A multicenter study found that DILI ranked third among diseases causing markedly elevated levels of aminotransferases (>400 U/L), potentially leading to fatal consequences [55]. Corroborating evidence indicates that in clinical cases of DILI, a sharp increase in AST and an AST/ALT ratio greater than 1.5 are negative prognostic indexes of death and liver transplantation [56,57]. In particular, AST is a key indicator of poor prognosis as the release of AST from mitochondrial hepatocytes, over ALT, reflects more severe hepatocyte injury [56].
The Guidelines on DILI management report that an increase of 5 times above ULN for ALT, or 3 times above ULN together with elevated bilirubin are key thresholds in identifying severe DILI [58]. Regular monitoring of ALT, especially in case of suspected liver injury due to drug exposure or when using drugs with known hepatotoxic effects, is recommended. Overall, the severity of impending liver damage is closely linked to the specific drug involved and the type of liver injury, whether with a hepatocellular, cholestatic, or mixed pattern [56]. Notably, even within therapeutic doses, the use of acetaminophen can lead to elevated ALT levels in one-third of users, making it the most frequent cause of DILI [59]. However, acetaminophen hepatotoxicity is more frequently intrinsic and related to excessive intake ranging between 4 and 10 grams per day for several days and remains the most common underlying cause of drug-related acute liver failure that requires urgent treatment, including liver transplantation [58,60,61].
Concerning HILI, the primary cause of liver injury is often linked to both herbal remedies, especially plants containing pyrrolizidine alkaloids, and nutritional supplements, whose ingredients are frequently unknown, complicating diagnosis. Due to worldwide increase of HDS-related liver injury and the unclear injury patterns associated with these products, efforts have focused on chemical analyses and the development of informative databases on their harmful effects, to raise awareness among consumers and regulatory bodies [62].
3.5Hepatocellular carcinomaALT and AST have historically been viewed as liver injury markers in hepatology [24]. However, their role in HCC management is relatively limited and must be interpreted cautiously within the broader clinical context.
In viral hepatitis-related HCC, transaminase levels can serve as surrogate markers for viral replication activity. Elevated ALT/AST levels in HBV or HCV-associated HCC may indicate ongoing viral replication, which can potentially accelerate hepatic decompensation and complicate treatment decisions [63]. However, normal transaminase levels do not exclude significant underlying liver disease or tumor progression.
Particularly in MASLD-associated HCC, transaminase levels can be misleadingly normal despite advanced liver disease and HCC development [64]. This emphasizes the unreliability of using transaminases alone as prognostic markers in HCC surveillance or management.
The primary utility of transaminases in HCC lies in their role as complementary markers when assessing liver function reserve, particularly in conjunction with other parameters like albumin, bilirubin, and coagulation factors. They should not be used as isolated marker for staging, prognostication, or treatment selection in HCC patients.
A marked elevation of transaminases, particularly when accompanied by increased GGT or alkaline phosphatase, may suggest an infiltrative pattern of hepatocellular carcinoma. However, this association is primarily based on clinical experience and case reports, rather than robust scientific evidence.
For optimal HCC management, clinicians should rely on validated staging systems (such as Barcelona Clinic Liver Cancer, BCLC) [65], imaging studies, and comprehensive liver function assessment rather than transaminase levels alone. The latter serve merely as adjunct markers in the complex decision-making process of HCC management.
3.6Autoimmune and cholestatic liver diseasesAutoimmune hepatitis (AIH) is an immune-mediated liver disease, potentially leading to cirrhosis. Elevated levels of ALT and immunoglobulin (Ig), in the presence of autoantibodies such as ANA, are optimal markers to suspect the condition. Typically, diagnosis relies on ALT levels exceeding 5 times ULN and IgG at least 2 times ULN, together with positive assay for non-organ specific autoantibodies and moderate or severe inflammation in liver biopsy. Notably, the International Autoimmune Hepatitis Group has introduced a diagnostic scoring system, considering not only laboratory and histological parameters but also responses to therapy, to enhance the assessment of AIH [66]. ALT remains an unsuitable surrogate marker of histologic liver examination to assess disease progression, as normal ALT levels do not rule out the presence of liver fibrosis and severe cirrhosis, making the liver biopsy necessary [66,67]. On the other hand, ALT normalization is a mandatory feature of the success of therapy and for the definition of remission.
Nonetheless, during immunosuppressive therapy, reduced ALT levels are associated with the improvement of liver inflammation, overall reflecting lifelong survival [68,69]. Thus, clinicians should consider the stabilization of ALT a crucial goal to achieve at follow-up [69].
Other immune-mediated liver diseases can display a cholestatic component where inflammation and destruction of the intrahepatic bile ducts are the main features of primary biliary cholangitis and primary sclerosing cholangitis. Cholestatic disorders are identified by a predominant elevation in alkaline phosphatase (ALP) level rather than AST and ALT levels. The R ratio (calculated as [ALT/ALT ULN]/[ALP/ALP ULN] is valuable indicator for identifying cholestatic injury when R ≤ 2 [5].
3.7Other hepatic disordersAbnormal transaminase levels may also be observed in vascular or genetic liver disorders. Porto-sinusoidal vascular disorder (PSVD) is characterized by histological lesions in portal venules and sinusoids, distinct from cirrhosis but often leading to significant microcirculatory obstruction irrespective of the presence/absence of portal hypertension. While many patients remain asymptomatic with mild liver enzyme elevations (notably ALT up to two times the ULN), these anomalies can be an early indicator of underlying vascular issues in the liver. In a recent paper persistent and unexplained GGT elevations usually lower than 200 IU/l has been demonstrated to be common in PSVD patients [70]. Diagnosis typically requires a liver biopsy reviewed by an experienced pathologist, as liver enzymes alone may not reflect the extent of vascular damage present in PSVD [71].
Persistently elevated ALT/AST levels, rather than isolated elevations, may raise suspicion of underlying genetic liver disorders such as hereditary hemochromatosis, Wilson's disease, or alpha-1 antitrypsin deficiency. This is particularly relevant in childhood, where unexplained liver enzyme abnormalities warrant further investigation, including genetic testing. Although ALT elevation reflects ongoing liver damage, its utility as a prognostic marker in genetic liver disorders is variable and requires to be complemented by disease-specific diagnostic tests [5].
4ALT and liver injury biomarkers alterations in vulnerable subpopulationsCases of hypertransaminasemia in the most vulnerable subpopulations, including paediatric patients, pregnant women, patients with renal disease, or recipients of liver transplantation, should prompt primary care providers to perform a proper clinical investigation. Early recognition between transiently benign conditions and underlying liver diseases is crucial for timely referral to specialised clinical care.
4.1PaediatricsDuring childhood, a plethora of diseases can cause a remarkable ALT elevation. The most common disorder, with a prevalence of nearly 10 %, is MASLD. The burden of this disorder is rapidly increasing due to the excessive intake of industrial products that increase the risk of obesity, insulin resistance, metabolic syndrome and cardiovascular diseases [72,73]. Moreover, persistently elevated transaminase levels in asymptomatic children may be associated with viral infections, autoimmune diseases, drug toxicity, and other rare, often genetic, hepatobiliary conditions [74].
Previous research showed that ALT cut-offs in use in paediatric clinical care have poor sensitivity and could mislead the detection of chronic liver injury in this subpopulation [75]. Indeed, the peculiar developmental and biological dynamics of the paediatric population reflect the need to use specific ranges of transaminase to best interpret the context of enzyme elevations, taking into account anthropometric characteristics such as age, sex, and BMI [76,77]. The investigations conducted by England and coworkers set more reliable gender- and age-specific reference ranges [78]. The analysed data derive from a healthy cohort of European children, suggesting that the ALT cut-off for adolescents should be 25.8 U/L for boys and 22.1 U/L for girls.
In clinical practice, cases of protracted hypertransaminasemia should be investigated by adopting a step-by-step approach. In paediatric primary care, it is recommended to collect the medical history, and perform a comprehensive physical examination and liver test work-up, encompassing serology, biochemical tests and ultrasonography [79]. In asymptomatic children, physicians should distinguish between mild and moderate/severe liver enzyme elevation and closely re-assess transaminase levels at follow-up [74]. Re-assessment of transaminase values should be performed every 1–2 weeks in mild cases or within 3 days in moderate/severe cases of liver enzyme elevation. Whenever abnormalities of the liver enzyme panel persist, a specialised referral is unavoidable.
4.2PregnancyIt is estimated that 3 % of pregnancies are complicated by liver diseases, and early detection in primary care is lifesaving [80]. Transaminase elevations remain a good indicator to consider in the initial diagnosis workup. Usually, mildly elevated transaminase levels can be reversible and lead to favourable maternal and foetal outcomes, while severe liver enzyme alterations indicate hepatobiliary injury with potentially fatal consequences.
Guidance on the management of liver diseases recommend clinicians to discriminate between liver diseases specific to pregnancy and those that are unrelated [81,82]. For instance, exacerbation of pre-existing liver conditions characterised by alcohol-, viral- or autoimmune-related causes are not specific but may coincide with the onset of pregnancy. Although these cases are rare, a thorough clinical history and physical examination are essential to rule out such conditions and focus on liver diseases specific to pregnancy. The latter are de novo liver diseases that are typically dependent on the gestational period. Hyperemesis gravidarum (HG), affects about 3.6 % of pregnancies. Hypertransaminasemia is common with ALT/AST levels reaching up to 3 times the ULNs, but higher values has have been documented [83]. Other disorders include intrahepatic cholestasis of pregnancy (ICP), pre-eclampsia/eclampsia (PE) with haemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome, and acute fatty liver of pregnancy (AFLP) [84]. ICP is a reversible cholestatic condition and the most common pregnancy-related condition, with a prevalence of 1.5 %. Despite being a benign condition, it is associated with severe outcomes, including preterm labour, foetal distress and stillbirth, and it is associated with aminotransferase rise by 2- to 10-fold the ULN, which may reach up to 20-fold. However, altered transaminase values are not considered the primary diagnostic criterion, nor are they used to evaluate the clinical outcome [84]. In contrast, transaminases play a crucial role in the clinical management of PE, a severe condition significantly contributing to maternal and perinatal mortality [80]. In cases of PE, alterations in AST and ALT levels typically range from 40 to 100 IU/L. Together with other serum biochemical parameters, these liver enzymes have been integrated into novel risk assessment tools, such as fullPIERS or PREP-S models, that are found to effectively predict maternal outcomes in these patients [85,86]. Approximately 10 % of women with PE progress to HELLP syndrome, a condition characterized by haemolysis, elevated liver enzymes, and low platelet count, where AST and ALT levels can rise up to 500 IU/L, next to high serum unconjugated bilirubin and LDH>600 IU/l [87]. Lastly, AFLP is a rare but life-threatening condition requiring medical emergency to prevent maternal and foetal mortalities. Important elevations in AST and ALT levels reaching as high as 1000 IU/l, concomitant with hyperbilirubinemia and kidney dysfunction, are among the biochemical abnormalities associated to this condition [87].
4.3Chronic kidney diseaseSeveral studies point out that patients living with chronic kidney disease (CKD) exhibit lower transaminase levels compared to the general population [88–91]. Evidence shows that the progression of renal impairment in patients undergoing dialysis is accompanied by a proportional decrease in ALT levels, in addition to a higher AST/ALT ratio associated with elevated cardiac mortality [92,93]. Hemodilution, loss of transaminases by filtration during hemodialysis, and high lactate serum levels with subsequent consumption of nicotinamide adenine dinucleotide phosphate have been reported as possible causes of subnormal transaminases serum levels in hemodyalized patients [94]. Moreover, CKD patients undergoing dialysis are at a higher risk of developing viral hepatitis, yet exhibiting near-to-normal transaminase values [95]. These circumstances pose substantial challenges to clinicians because CKD patients with active liver damage may be underdiagnosed and undertreated. Given that liver enzymes decrease as CKD worsens, adequate cut-off values specific to varying stages of renal disease may be necessary. Research indicates that it may be beneficial to revise current cut-off values by adopting a lower ULN to increase detection test sensitivity and improve the management of liver diseases in CKD [96].
4.4Liver transplantationTransaminase activities are considerably useful in the early postoperative settings of liver transplantation, as abnormal elevations are associated with poor graft outcome [97]. Their elevation is typically transient in the post-operative period, with a 5- to 20-fold increase above the ULN occurring over a week [98]. However, there is a borderline situation between recognizing a physiological postoperative scenario and identifying liver injury complications that requires timely interventions to prevent organ rejection. Abnormal elevation of liver enzymes protracting beyond a week usually suggests an immune-mediated response or viral and bacterial infections that may indicate grafting complications.
One-year post-transplant transaminase levels stabilize. Similarly to the general population, transaminase levels are regularly used as a surveillance routine panel to monitor the overall liver health status in liver transplant recipients (LTRs). Proposed reference values of ALT and AST in LTRs are higher than in the healthy population primarily due to post-transplantation clinical sequelae, including chronic exposure to immunosuppressant medications, insulin resistance and bone impairment [99]. A report from Siddiqui and colleagues suggests LTR-specific thresholds for normal ALT and AST, independent of gender, comorbidities and medications, to be 57 IU/l for ALT and 54 IU/l for AST. Moreover, the cut-off values showed high sensitivity in identifying cases of recurrent hepatitis C but not of recurrent NAFLD after OLT [100]. Clinicians should critically interpret the causes of liver enzyme elevations and promptly initiate appropriate evaluation, diagnosis, and treatment to ensure long-term survival of LTRs.
5The added value of performing aminotransferase determination for the management of liver disease5.1Guidance in general practiceDespite substantial clinical evidence supporting the role of transaminases as a crucial prognostic and diagnostic markers for MASLD and liver diseases [41], cumulative studies indicate that unchanged ALT levels may not accurately reflect histological assessments in the presence of advanced chronic liver disease. Notably, patients with severe hepatic steatosis can maintain normal ALT levels, potentially obscuring severe liver conditions [47,101]. This dual role of ALT underscores the need for an integrated and personalized diagnostic approach to effectively recognise a liver disease.
This expert panel suggests pragmatic recommendations for general practice to streamline the early identification of cases at risk of potentially progressive liver diseases. In particular, the integration of data derived from anamnesis, physical examination and non-invasive parameters provide valuable indication for an initial diagnosis.
According to Agrawal et al., patients should be considered for referral to a specialist in the following situations: (i) unexplained liver abnormalities greater than 1.5 times the normal level, detected on two occasions at least 6 months apart; (ii) unexplained liver disease with evidence of hepatic dysfunction (hypoalbuminemia, hyperbilirubinemia, prolonged prothrombin time, or INR), or (iii) known liver disease where treatment beyond the withdrawal of the implicating agent is required [102]. Another approach is to consider for investigation in the primary care setting all patients with abnormal liver blood tests irrespective of level and duration of abnormality with a careful clinical history and a liver aetiology screen. The patients should then be referred to the specialist for treatment if needed or for further investigation in case of negative etiology screen and no risk factors for MASLD [23].
Screening and risk stratification of liver conditions should include the use of non-invasive clinical algorithms, such as the aspartate aminotransferase to APRI, NAFLD fibrosis score (NFS), ELF™ test, and FIB-4 score. These are useful predictive and prognostic tools with sensitive performances for the identification and staging of advanced fibrosis and cirrhosis [1].
All primary care points should have access to first-line tests. The FIB-4 score, which incorporates transaminases, platelet count, AST, ALT, and age, is extensively utilized in local clinical workflows due to its reliability as a prognostic biomarker. In addition to predicting liver-related events, FIB-4 has proven utility in monitoring all-cause mortality, further reinforcing its role in patient management and risk stratification [103]. The adoption of automatic calculation of FIB-4 score and combined use of with ELF™ test in general practice have underscored effectiveness for timely intervention alongside the reduction of unnecessary referrals by 80 % [4,104].
To obtain unequivocal confirmation of fibrosis staging and initiate intervention, non-invasive imaging techniques as second-line testing are recommended, including vibration-controlled transient elastography (VTCE or Fibroscan®), shear wave elastography, and magnetic resonance elastography. These techniques may be combined with haematological data, such as the FibroScan-aspartate aminotransferase (FAST) score [105]. However, in cases where diagnostic uncertainties persist or a more detailed histological evaluation is required, liver biopsy remains indispensable for accurate staging and diagnosis.
5.2Medical education and technology for prevention and timely interventionA significant gap exists in the clinical referral pathways within primary care, with only one-third of patients with suspected liver disease being timely referred [106]. Delay in diagnosis results in a significant increase in liver-disease mortality [107]. Approximately 40 % of patients presenting to emergency departments with decompensated end-stage liver disease have no prior diagnosis, while many others are unnecessarily referred to hospitals due to abnormal liver blood tests. This stark contrast highlights a significant gap in the active management of liver conditions [108]. In this scenario, both patient and healthcare professionals represent key players for active interventions to modulate the future of liver disease management.
Efficient screening, risk stratification and case identification in primary care would lower the risk of hospitalisation and complications. Thus, continuing medical education among primary care providers is crucial to guide on the management of liver biochemistry results and to take prompt interventions. General practitioners require high-quality education on the importance of fibrosis screening to stratify patients and optimize the referral. Standardised protocols and frameworks, plain language communication competences, integration into a coordinated multidisciplinary care system are some key solutions that are proposed as essential parts of medical education programs [109,110].
Despite the growing understanding that liver diseases significantly impact multiple organ systems, liver diseases remain disregarded. Both general practitioners and specialists in fields indirectly involved in liver care, such as diabetologists, often lack sufficient knowledge of chronic liver diseases [111], highlighting the need for better multidisciplinary coordination between primary and secondary care [112].
One of the most promising solutions to address this discrepancy lies in the integration of digital medicine, computational tools, and AI. These technologies can expedite prevention, screening, diagnosis, and monitoring, offering multilevel and targeted care [113]. Electronic health records (EHRs) represent a useful tool to continuously collect and update patient information, that can facilitate interdisciplinary care plans and attenuates the communication breakdown among care departments [114]. Several reports have successfully proven that embedding HER with advanced algorithm and machine learning models allows for automated interpretation of massive information including clinical data and imaging [115–117]. These models enable for a longitudinal monitoring of patient health status, ultimately developing sustainable and cost saving healthcare systems [118]. Validated AI-based systems demonstrated the discriminatory ability to early identify patients at significant risk of MASLD and cirrhosis [116,119]. Such tools may greatly support healthcare professionals in the decision-making process in the clinical routine, reducing diagnostic errors, and enabling customized treatment plans [120]. On the other hand, although integration of medical AI outpaces the traditional clinical framework, paving the way towards a personalised care, need for robust evidence in terms of clinical validation and ethical challenges such as patient safety, privacy, security remain to be addressed [121]. Legal concerns arising from algorithm inaccuracy leading to patient harm, question medical liability and require transparent governance and recommendations to standardize AI medical practice.
In addition, digital therapeutic technologies are an emergent and useful engagement strategy to induce proactive behavioural changes, monitor remote disease management and improve adherence to therapies [122]. An increasing number of mobile apps, wearable devices, telemedicine platforms, have been developed for chronic liver diseases research purposes, suggesting their role as adjunct proxy to patient self-management and routine medical care [123,124]. For example, implementation of a mobile application helped in addiction management, by reducing alcohol intake and hospitalisation rates in patients with alcohol-related liver disease [125].
5.3Patient engagementIncreasing patient engagement and health education can also help the management of chronic liver diseases. By promoting health awareness and preventive measures, we can enhance patients’ understanding of the risks associated with neglecting liver diseases and the consequences of late diagnosis. Moreover, implementing lifestyle interventions can significantly reduce the incidence of liver diseases, potentially preventing up to 300,000 deaths annually [126].
Healthcare professionals should implement patient-oriented education strategies to bridge patients’ knowledge gaps and to transition towards a more patient-centred clinical approach [127,128]. For example, actively involving patients in reporting their symptom severity and health-related quality-of-life indices through patient-reported outcome measures (PROMs) is a valuable way to incorporate patient preferences into health policy. Yet, PROMs may not fully consider individual differences and contextual factors, potentially limiting their effectiveness in accurately reflecting patient needs and preferences.
Direct cooperation with patients is becoming vital to align with unmet patient priorities and improve satisfaction and responsiveness to therapies. Clinicians should encourage the active involvement of patients into care procedures by allocating the time needed to provide accessible and understandable information. Undoubtedly, health literacy enables individuals to use information and healthcare services to maintain a good health status, filter misinformation, and guide health-related decisions while maturing a critical appraisal [129,130].
Providing accessible and plain-language materials such as brochures, videos, workshops and online resources that explain liver disease, treatment options, and lifestyle changes can help patients understand their condition and engage in their care actively [131–133].
Moreover, the medical community should consider that patients with liver diseases encounter massive symptom burden, physical, psychological and existential complexities. Thus, stigmatisation may represent another barrier that hinders communication between patients and healthcare professionals, reducing clinical adherence and delaying treatment. Negative perceptions and blame associated with liver disease may deter these patients from seeking medical care. Language in healthcare significantly influences patient motivation and engagement [129]. Terms with negative connotations are to be replaced with more supportive and non-judgmental language to foster better communication and encourage patients to pursue treatment.
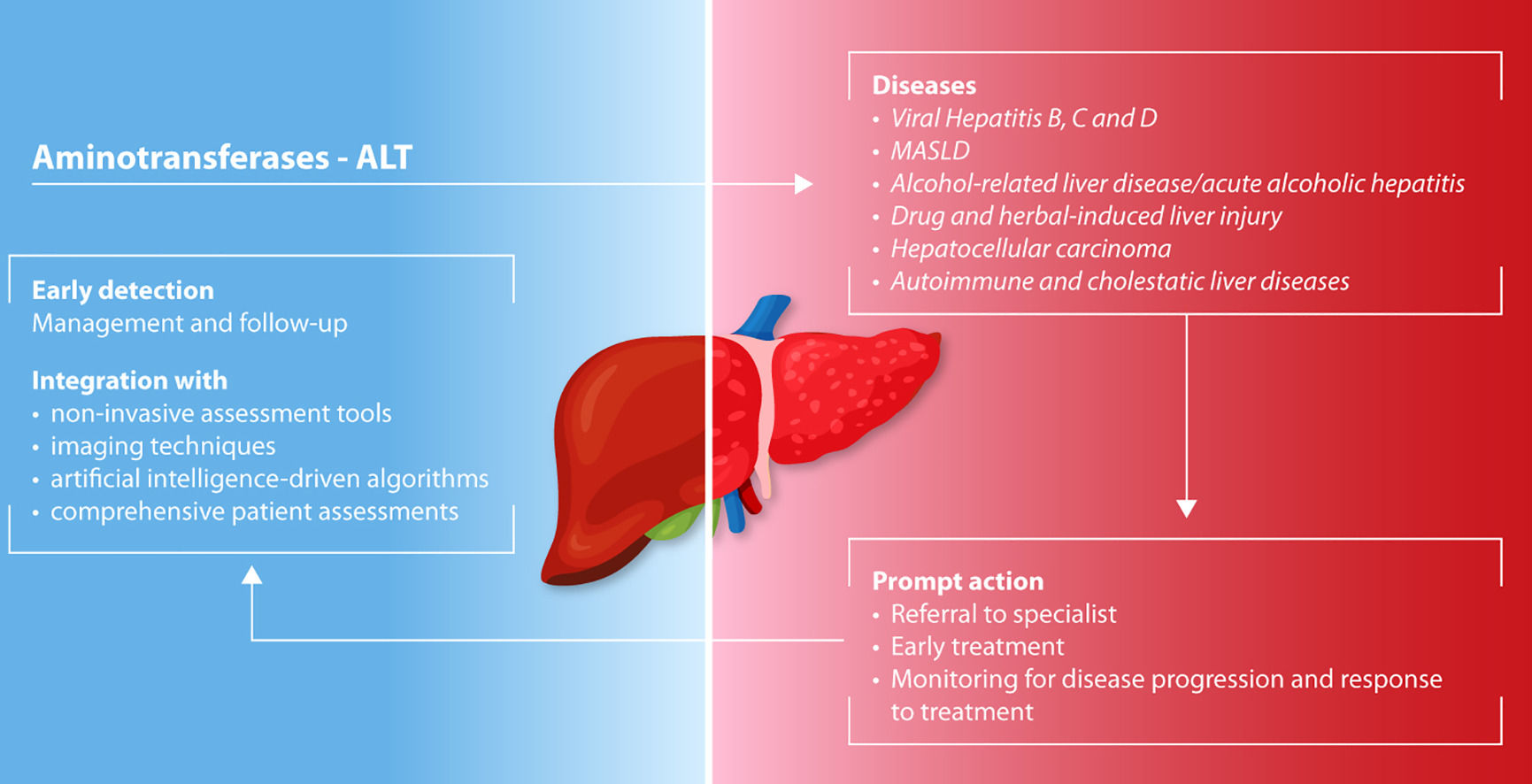
6ConclusionsALT remains a pivotal biomarker in the early detection and management of liver diseases. Its integration with additional liver function biomarkers, non-invasive multiparametric and imaging techniques, and emerging AI-driven tools can further enhance its use to tailor diagnostic approaches in primary and specialized care to effectively address the global burden of liver diseases.
When elevated ALT is detected, prompt action is essential. For general practitioners, this necessitates referral to a specialist after collecting an accurate clinical history and performing a preliminary liver etiology screen to ensure timely diagnosis and intervention. Specialists, on the other hand, should address the underlying cause of liver dysfunction and start appropriate management strategies. These may include behavioral and dietary modifications, or pharmacological treatment, depending on the identified etiology.
Regular monitoring of ALT levels allows for assessment of disease progression and response to treatment. Understanding the implications of ALT fluctuations can guide clinicians in adjusting therapeutic approaches, ultimately aiming to prevent further liver damage and improve patient outcomes, for ensuring comprehensive and effective liver health management.
Authors contributionAll authors conceived the work, analysed the material, wrote and edited the manuscript. All authors approved the final version for the submission.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Editorial assistance was provided by Alessandra Natale, PhD, on behalf of Health Publishing and Services Srl.
This assistance was supported by an unrestricted support by Gilead.