Background. In the management of chronic hepatitis C (CHC) patients, liver biopsy is the gold standard for liver fibrosis assessment despite some technical limits and risks. Non-invasive approaches have been proposed as alternative methods to evaluate structural liver damage.
Aim. To investigate the diagnostic accuracy of transient elastography, 13C-aminopyrine breath test (13C-ABT), serum hyaluronic acid (HA) and cytokeratin 18 Asp396 fragment (CK-18) as non-invasive methods of liver fibrosis assessment ad their correlation to METAVIR score.
Material and methods. In a cohort of 57 CHC patients, liver stiffness, cumulative percentage of administered dose of 13C-aminopyrine at 120 min, serum HA and serum CK-18 concentration were determined. Diagnostic accuracy in detecting significant fibrosis (F ≥ 2), severe fibrosis (F ≥ 3) and cirrhosis (F = 4) was assessed by the area under the receiver operating characteristic curve.
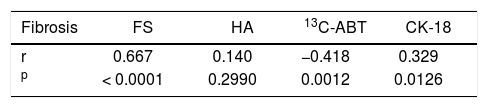
Results. Liver fibrosis score showed a strong correlation with liver stiffness (r = 0.667; p < 0.0001) and a significant inverse correlation with 13C-ABT results (r = -0.418; p = 0.0012). A weaker correlation was found with CK18 (r = 0.329; p = 0.0126) and no correlation with HA. Areas under the curve of elastography, 13C-ABT, HA and CK18 were: 0.98, 0.75, 0.69, 0.64, respectively, for F ≥ 2; 0.97, 0.69, 0.80, 0.66, respectively, for F ≥ 3; 0.95, 0.64, 0.70, 0.56, respectively, for F = 4.
Conclusion. Elastography has the best diagnostic accuracy for the assessment of the degree of liver fibrosis in CHC patients. Its application can provide an alternative useful tool for monitoring the disease evolution.
Prognosis and management of chronic hepatitis C (CHC) patients is strongly influenced by the degree of liver fibrosis, in fact a precise definition of the hepatic fibrosis stage is an important parameter to asses the risk of disease progression and to prompt immediate antiviral therapy.1,2
Liver biopsy (LB) is the gold standard for the evaluation of hepatic fibrosis and provides useful information on many parameters like inflammation, necrosis, steatosis and hepatic iron, allowing to identify suspected or unexpected cofactors and comorbidities.3 Unfortunately, LB has some limitations and risks that make it unsuitable for patients tight monitoring: it is an invasive procedure and it can lead to complications such as bleeding, pain at biopsy site, infection and accidental injury to a nearby organ.4 Other limitations include sampling error and pathologist’s inter-observer variability.5 Therefore, there is a need for accurate non-invasive methods for measuring the degree of liver fibrosis. Non-invasive approaches can be distinguished in direct markers of fibrogenesis, as expression of either deposition or removal of extra cellular matrix (ECM) in the liver,3 or indirect markers of fibrosis, wich reflect liver changes induced by fibrosis without a direct link with fibrogenesis mechanisms,6 and imaging methods.7 Direct markers include hyaluronic acid (HA), laminin, type IV collagen, type III collagen N-terminal peptide, matrix metalloproteinases and cytokines, such as tumor necrosis factor-α and trasforming growth factor-β.4,7 Indirect markers are obtained by combining together routinely performed blood tests to calculate scores to identify alterations in liver function. They include aspartate aminotransferase (AST) to platelet ratio index (APRI), FibroTest™ and Forns’ index.6 Imaging methods refer to ultrasonography, magnetic resonance imaging and transient elastography.7
Hyaluronic acid is a glycosaminoglican component of ECM, syntetized by hepatic stellate cells.8 Elevated levels of HA are due to increased production, decreased hepatic removal or both.9 High concentrations of serum HA have been detected in CHC patients and have been related to different degrees of liver fibrosis.10
Cytokeratin 18 Asp 396 fragment (CK-18) is a serological marker of apoptosis that has been widely associated with fibrosis and steatosis in nonalcoholic steatohepatitis (NASH).11,12 Nevertheless, a recent study found higher levels of CK-18 in CHC patients compared to healthy controls.13 Additionally, the same study found a significant correlation of serum CK-18 titer and degree of fibrosis.13
13C-aminopyrine breath test (13C-ABT) is a dynamic function test based on the use of a labelled substrate selectively metabolized within the liver. It has been shown to be useful in the evaluation of hepatocyte microsomial function providing a quantitative information on liver function activity.14 A recent study compared 13C-ABT measurements of CHC and Child-Pugh class A cirrhosis patients to those of normal subjects and found that 13C-ABT was able to distinguish different degrees of liver function impairment with a partial correlation to histology.15
Transient elastography (Fibroscan™) (FS) is an ultrasonographic method for assessing liver fibrosis. It evaluates liver stiffness measuring the velocity of propagation of an elastic wave through the hepatic tissues. The speed of transmission of the elastic wave is directly related to tissue stiffness and consequently to fibrosis amount.1,5,6 Several large-scale prospectives studies already tested the effectiveness of this method in patients with chronic liver diseases, especially in CHC.16,17
Since data are few or conflicting, the reliability of these non-invasive methods as alternative to LB is not fully known, particularly for CK-18, 13C-ABT and HA.We examined the hypothesis whether noninvasive tests adequately assess liver fibrosis. If our hypothesis is true this may decrease the need for liver biopsies.
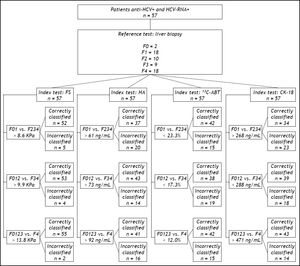
Material and MethodsThis study included 57 consecutive CHC patients (32M, 25F; mean age 52.5 ± 11.9) tested positive for anti-HCV (Ortho HCV SAVe 3.0, Raritan, NJ, USA) and HCV RNA (TaqMan, Roche, detection limit 15 IU/mL). Patients with other etiologies of chronic hepatitis, such as chronic hepatitis B, NASH, autoimmune hepatitis, primary biliary cirrhosis, alcoholic liver disease and hemochromatosis were excluded. The study protocol was conformed to the principles of the 1975 Declaration of Helsinki. All patients gave their written informed consent prior to recruitment.
Liver histologyAll patients underwent LB in the year preceding non-invasive liver fibrosis assessment (from 6 to 12 months).18 All biopsy specimens were analyzed by an experienced pathologist blinded to the clinical results of the patients. Liver specimens shorter than 20 mm were excluded from the analysis. Liver fibrosis was staged according to METAVIR score:
- •
F0: absence of fibrosis.
- •
F1: portal fibrosis without septa.
- •
F2: portal fibrosis with few septa.
- •
F3: numerous septa without cirrhosis.
- •
F4: cirrhosis.
A blood sample was obtained on the same day of liver stiffness assessment by transient elastograpy and breath test. After centrifugation, serum samples were stored -80°C for further analysis. Measurements of serum HA and CK-18 were performed according to manufacturer’s recommendations by ELISA assays: Hyaluronic Acid Test Kit (Corgenix, Westminster, Colorado, USA) and M30-Apoptosense ELISA (PEVIVA, Sweden). Concentrations were given in ng/mL.
Transient elastographyLiver stiffness measurements were performed by FS (Echosens, Paris, France) on the right lobe of the liver through intercostal spaces on patients lying in the dorsal decubitus position with the right arm in maximal abduction. Details have been described in previous studies.1 Measurement depth was between 25 mm and 65 mm below the skin surface. The success rate was calculated as the ratio of the number of the successful measurements over the total number of acquisitions. Liver stiffness was expressed in kilopascals (KPa) as the median value of the successful measurements. Only liver stiffness data with at least 10 successful measurements, success rate higher than 60% and inter quartile ratio (IQR) inferior to 30%, were considered reliable.
Breath test13C-ABT was performed as previously described.19 A basal breath sample was collected after an overnight fast; then a dose of 2 mg/kg of 13C-aminopyrine (EXPIROLiver, Sofar, Italy) was dissolved in 200 mL of water and administered orally. The percentage of the exhaled dose per hour and the cumulative percentage of metabolized dose in 2 h were dinamically monitored. Results obtained from 13C-ABT were expressed as cumulative percentage dose of 13C recovered (%) over time (at 120 min after ingestion of labelled aminopyrine).
Statistical analysisCorrelation among studied parameters and the degree of liver fibrosis was performed using Spearman’s nonparametric correlation. A multiple regression analysis was used to assess the association between the results obtained by the different non-invasive methods tested and liver fibrosis, while controlling for patients age and gender. Diagnostic performance, sensitivity and specificity for all possible cut-off values of FS, HA, 13C-ABT, CK-18 and their combination was assessed by using receiver operating characteristic (ROC) curves. A P-value < 0.05 was considered statistically significant. All statistical procedures were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL).
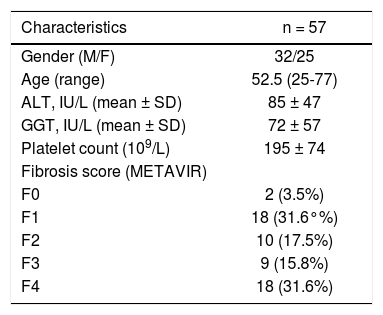
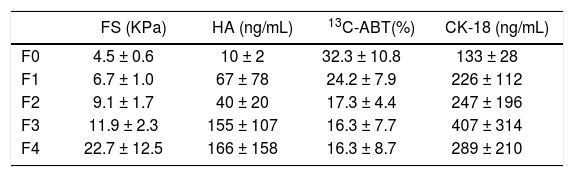
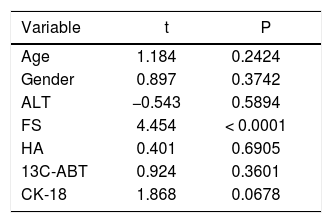
ResultsThe main demographic characteristics of the studied patients are reported in table 1. Mean values of liver stiffness, HA, 13C-ABT and CK-18 categorized according to fibrosis METAVIR score are reported in table 2. At univariate analysis, liver fibrosis showed a strong correlation with liver stiffness (r = 0.667; p < 0.0001) and a significant inverse correlation with 13C-ABT results (r = −0.418; p = 0.0012). A weaker correlation was found with CK18 (r = 0.329; p = 0.0126) and no correlation with HA (Table 3). In a multiple regression model including age, gender and ALT, liver stiffness (p < 0.0001) remained the only factor associated with fibrosis degree (Table 4).
Demographic, biochemical and histological characteristics of patients
| Characteristics | n = 57 |
|---|---|
| Gender (M/F) | 32/25 |
| Age (range) | 52.5 (25-77) |
| ALT, IU/L (mean ± SD) | 85 ± 47 |
| GGT, IU/L (mean ± SD) | 72 ± 57 |
| Platelet count (109/L) | 195 ± 74 |
| Fibrosis score (METAVIR) | |
| F0 | 2 (3.5%) |
| F1 | 18 (31.6°%) |
| F2 | 10 (17.5%) |
| F3 | 9 (15.8%) |
| F4 | 18 (31.6%) |
M: male. F: female. ALT: alanine aminotransferase. GGT: γ-glutamyltransferase.
Mean values and standard deviations of liver stiffness, HA, 13C-ABT and CK-18 according to fibrosis METAVIR score.
| FS (KPa) | HA (ng/mL) | 13C-ABT(%) | CK-18 (ng/mL) | |
|---|---|---|---|---|
| F0 | 4.5 ± 0.6 | 10 ± 2 | 32.3 ± 10.8 | 133 ± 28 |
| F1 | 6.7 ± 1.0 | 67 ± 78 | 24.2 ± 7.9 | 226 ± 112 |
| F2 | 9.1 ± 1.7 | 40 ± 20 | 17.3 ± 4.4 | 247 ± 196 |
| F3 | 11.9 ± 2.3 | 155 ± 107 | 16.3 ± 7.7 | 407 ± 314 |
| F4 | 22.7 ± 12.5 | 166 ± 158 | 16.3 ± 8.7 | 289 ± 210 |
FS: fibroscan. HA: hyaluronic acid. 13C-ABT: 13C-aminopyrine breath test. CK18: cytokeratin 18 asp 396 fragment.
Correlation coefficient between the parameters studied and the degree of fibrosis according to METAVIR score.
| Fibrosis | FS | HA | 13C-ABT | CK-18 |
|---|---|---|---|---|
| r | 0.667 | 0.140 | −0.418 | 0.329 |
| p | < 0.0001 | 0.2990 | 0.0012 | 0.0126 |
FS: fibroscan. HA: hyaluronic acid. 13C-ABT: 13C-aminopyrine breath test. CK-18: cytokeratin 18 asp 396 fragment.
Multiple regression analysis of the parameters studied and the degree of hepatic fibrosis according to METAVIR score.
| Variable | t | P |
|---|---|---|
| Age | 1.184 | 0.2424 |
| Gender | 0.897 | 0.3742 |
| ALT | −0.543 | 0.5894 |
| FS | 4.454 | < 0.0001 |
| HA | 0.401 | 0.6905 |
| 13C-ABT | 0.924 | 0.3601 |
| CK-18 | 1.868 | 0.0678 |
ALT: alanine aminotransferase. FS: fibroscan. HA: hyaluronic acid. 13C-ABT: 13C-aminopyrine breath test. CK-18: cytokeratin 18 asp 396 fragment.
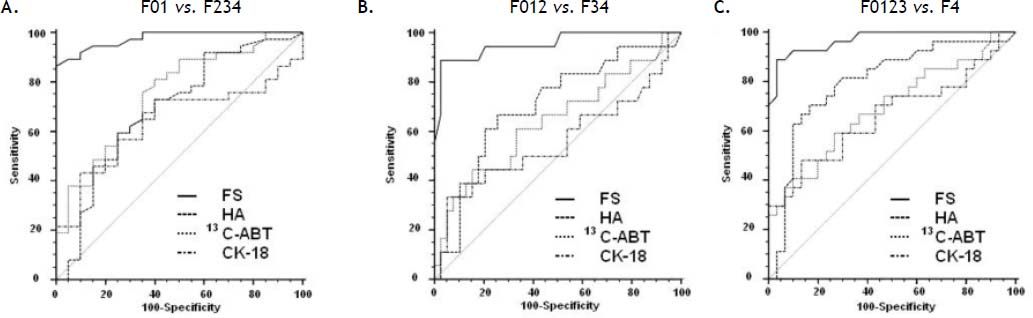
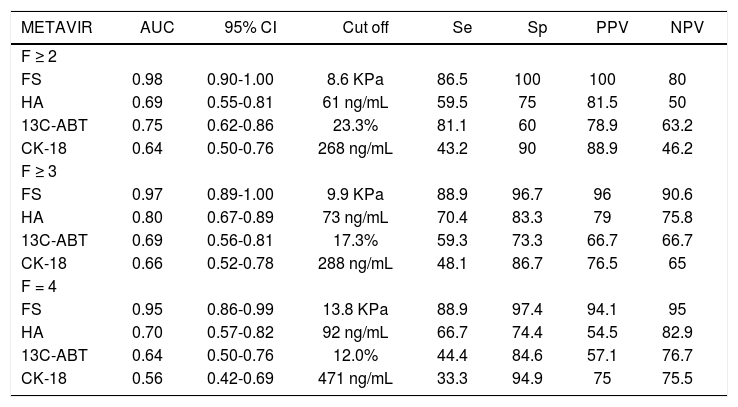
Figure 1 shows the areas under the curve (AUCs) calculated by ROC analysis of FS, HA, 13C-ABT and CK18 for the diagnosis of significant fibrosis (A), severe fibrosis (B) and cirrhosis (C). Corresponding AUCs, cut off values that maximized sensitivity and specificity, positive predictive values (PPV) and negative predictive values (NPV) are shown in table 5. All possible combinations of FS, HA, 13C-ABT and CK-18 for the diagnosis of fibrosis stage compared to histology were evaluated. No statistically significant improvement in AUC values compared to liver stiffness alone was found (data not shown).
Sensitivity, specificity and area under the curve of the non-invasive methods studied for the diagnosis of significant fibrosis (F ≥ 2), severe fibrosis (F ≥ 3) and cirrhosis (F = 4).
| METAVIR | AUC | 95% CI | Cut off | Se | Sp | PPV | NPV |
|---|---|---|---|---|---|---|---|
| F ≥ 2 | |||||||
| FS | 0.98 | 0.90-1.00 | 8.6 KPa | 86.5 | 100 | 100 | 80 |
| HA | 0.69 | 0.55-0.81 | 61 ng/mL | 59.5 | 75 | 81.5 | 50 |
| 13C-ABT | 0.75 | 0.62-0.86 | 23.3% | 81.1 | 60 | 78.9 | 63.2 |
| CK-18 | 0.64 | 0.50-0.76 | 268 ng/mL | 43.2 | 90 | 88.9 | 46.2 |
| F ≥ 3 | |||||||
| FS | 0.97 | 0.89-1.00 | 9.9 KPa | 88.9 | 96.7 | 96 | 90.6 |
| HA | 0.80 | 0.67-0.89 | 73 ng/mL | 70.4 | 83.3 | 79 | 75.8 |
| 13C-ABT | 0.69 | 0.56-0.81 | 17.3% | 59.3 | 73.3 | 66.7 | 66.7 |
| CK-18 | 0.66 | 0.52-0.78 | 288 ng/mL | 48.1 | 86.7 | 76.5 | 65 |
| F = 4 | |||||||
| FS | 0.95 | 0.86-0.99 | 13.8 KPa | 88.9 | 97.4 | 94.1 | 95 |
| HA | 0.70 | 0.57-0.82 | 92 ng/mL | 66.7 | 74.4 | 54.5 | 82.9 |
| 13C-ABT | 0.64 | 0.50-0.76 | 12.0% | 44.4 | 84.6 | 57.1 | 76.7 |
| CK-18 | 0.56 | 0.42-0.69 | 471 ng/mL | 33.3 | 94.9 | 75 | 75.5 |
FS: fibroscan. HA: hyaluronic acid. 13C-ABT: 13C-aminopyrine breath test. CK-18: cytokeratin 18 asp396 fragment. AUC: area under the curve. 95% CI: 95% confidence interval. Se: sensitivity. Sp: specificity. PPV: positive predictive value. NPV: negative predictive value.
In recent years, transient elastography has become a widely used method for the assessment of liver fibrosis degree.1,5 Among the non-invasive approaches evaluated in this study (Figure 2), FS obtained an excellent diagnostic accuracy in predicting significant fibrosis (AUC = 0.98), severe fibrosis (AUC = 0.97) and cirrhosis (AUC = 0.95) compared to LB. Using 8.6 KPa, 9.9 KPa and 13.8 KPa as cut off values for significant fibrosis, severe fibrosis and cirrhosis respectively, we obtained PPV of 100%, 96% and 94.1% and NPV of 80%, 90.6% and 95%. Sensitivity and specificity values were 86.5% and 100% respectively, for F ≥ 2; 88.9% and 96.7% respectively, for F ≥ 3; 88.9% and 97.4% respectively, for F = 4. In contrast to previous reports1,20 and meta-analysis,21–23 where FS showed to perform slightly better in the diagnosis of cirrhosis than in detecting significant fibrosis, our data shows FS usefulness for early detection of significant fibrosis also. However, this discrepancy could be due to the small number of patients enrolled in our study. Infact, Castéra, et al.1 reported a FS AUC value of 83% in a cohort of 183 patients with CHC, and similarly, in a study population of 251 subject with CHC, Ziol, et al.20 reported a FS diagnostic accuracy of 79% in detecting significant fibrosis.
Regarding serum markers, previous reports showed a good performance in the diagnosis of both cirrhosis and significant fibrosis, emphasizing an elevated sensivity and specificity for HA.10,24 Another study suggested that the simple non-invasive measurement of serum HA could be used to exclude the presence of cirrhosis in CHC patients at least.25 In agreement with other reports,26 we did not find a correlation of HA to the degree of liver fibrosis and, moreover, AUC values indicated only a moderate diagnostic accuracy for cirrhosis detection.
A recent study showed that serum CK18 levels are higher in CHC patients compared to healthy controls and are associated with significant hepatic fibrosis, despite of a considerable overlap in serum CK-18 concentrations distribution.13 Given that the mechanisms leading to caspases activation and cytokeratin 18 Asp 396 fragment release in the blood are not well understood, we evaluated this marker in our cohort. As expected, we found a weak correlation with histological fibrosis at the univariate analysis and the AUC values revealed a low diagnostic accuracy (AUC < 0.7).
We performed the 13C-ABT to evaluate the correlation between liver function and hepatic fibrosis. A significant inverse correlation between 13C-ABT results (r = −0.418; p = 0.0012) and liver fibrosis degree was found. However, as for CK18, when multiple regression analysis including age and gender was performed, 13C-ABT lost statistical significance. In the evaluation of this method to discriminate patients on the basis of significant fibrosis, severe fibrosis and cirrhosis, we found AUC values of 0.75, 0.69 and 0.64 respectively, hence 13C-ABT did not reliably distinguish between any fibrosis stages.
The present study is limited by the rather small number of patients included, however, the proportion of patients with different degrees of liver fibrosis was well balanced. Another limitation of our analysis could be the different timing between liver biopsy sampling and non-invasive assessments. However, in the majority of patients with chronic hepatitis C the progression of fibrosis is linear and slow,27 therefore a liver biopsy taken from 6 to 12 months before non-invasive assessments is unlikely to be a bias.
Despite the small size of the study cohort, our results show that transient elastography performs significantly better than other tested methods. Althought liver biopsy is still necessary to assess the presence of comorbidity and metabolic disorders, the present data suggest that transient elastography is a useful tool for monitoring the disease evolution in CHC patients and a suitable non-invasive procedure that limits the need to perform a liver biopsy for fibrosis assessment.
Abbreviations- •
AST: aspartate aminotransferase.
- •
AUC: area under the curve.
- •
CHC: chronic hepatitis C.
- •
CK-18: cytokeratin 18 asp 396 fragment.
- •
ECM: extra cellular matrix.
- •
HA: hyaluronic acid.
- •
LB: liver biopsy.
- •
NASH: nonalcoholic steatohepatitis.
- •
NPV: negative predictive value.
- •
PPV: positive predictive value.
- •
13C-ABT:13C-aminopyrine breathtest.
None to declare.
Conflict of InterestNone to declare.