Hydroxychloroquine sulfate (HCQ) is utilized as an anti-inflammatory agent in rheumatic diseases. Despite its safety it could produce severe retinal toxicity when taken for extended periods of time or in high dosages. The mechanism causing said toxicity is not fully known.
The literature describes a characteristic perifoveal retinopathy with loss of photoreceptors and ring-shaped retinal pigment epithelium (RPE) alterations. Recent studies have demonstrated that this retinopathy could evolve even after terminating the use of the drug, establishing an association between the integrity of the external limiting membrane (ELM) and the final visual prognostic. Diminished choroidal thickness has also been related with said retinopathy.1
The guides of the American Academy of Ophthalmology (AAO) recommend a baseline examination to discard maculopathy followed by annual examinations after 5 years. Said examination comprises best corrected visual acuity (BCVA), funduscopy, central campimetry (CV) (10-2) and spectral domain optical coherence tomography (SD-OCT).2 Additional tests include autofluorescence (AF) and multifocal electroretinogram.
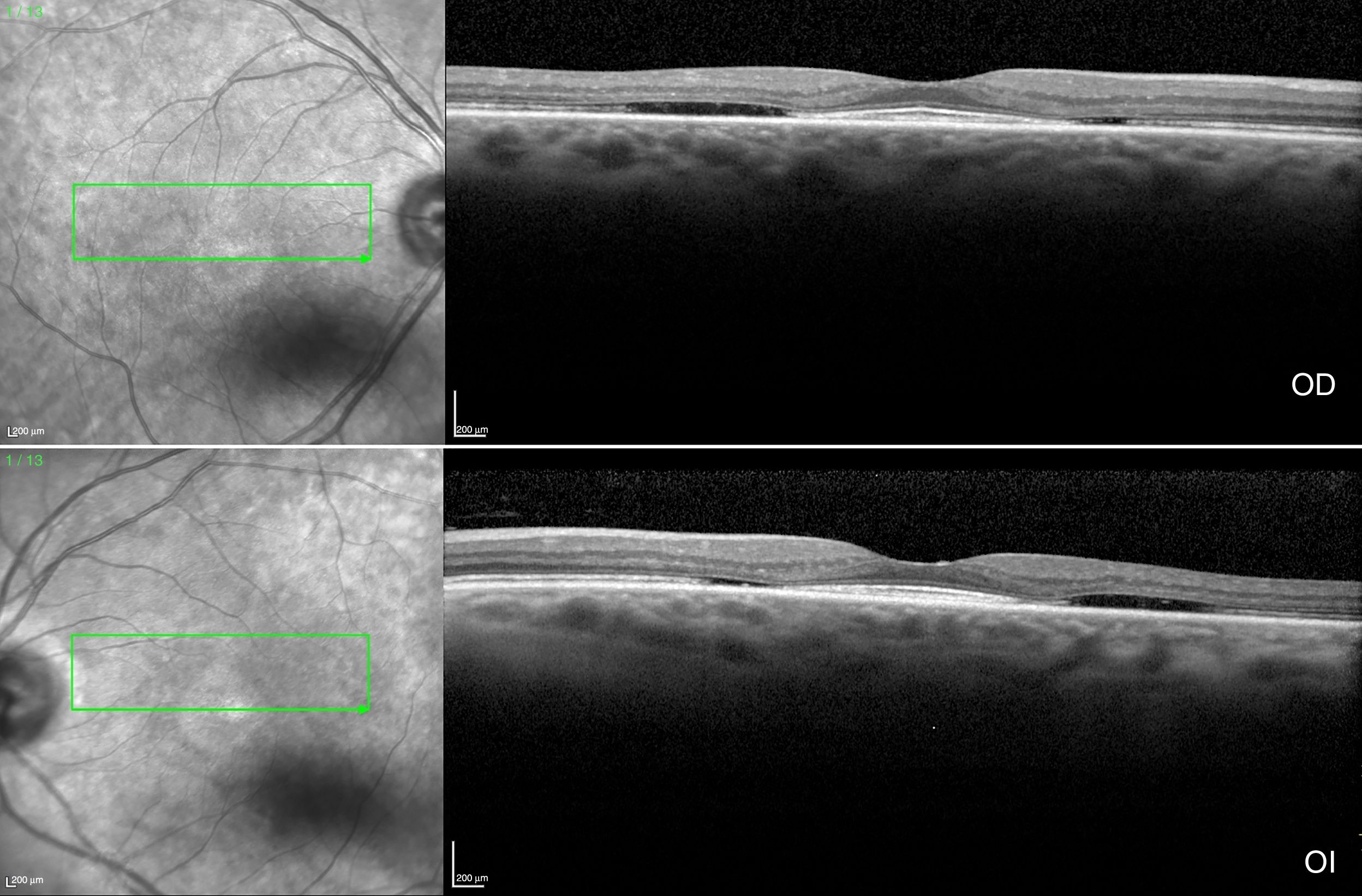
The case of a 60-year-old female with rheumatoid arthritis diagnosed 12 years ago is presented. The patient is in treatment with methotrexate (MTX). She visited in compliance with the screening program to discard maculopathy prior to beginning treatment, although due to delays in the checkup visit she had been taking HCQ 200mg/day during 2 months. No other relevant antecedents were identified. The patient exhibited BCVA of 20/20 in both eyes (BE), without evident alterations in biomicroscopy and funduscopy. SD-OCT (Fig. 1) showed the flying saucer sign secondary to the loss of the perifoveal ellipsoid line with foveal preservation thereof, perifoveal thinning of the external nuclear layer and posterior displacement of the external retina towards the RPE. No ELM alterations or choroidal thinning were found. AF was normal and CV 10-2 showed central scotomae in BE. After discarding other ophthalmic disorders included in the differential diagnostic of bulls-eye retinopathy, treatment was terminated and substituted by a biological medicament.
A range of pre-existing conditions and dosage factors facilitate said retinopathy, including an aggregate dose of >1.000g, use >5 years and liver or kidney damage that impairs drug metabolism or excretion.1 The risk of HCQ toxicity is of 1% in the first 5 years and 2% up to 10 years. In this series of cases, Yam et al. only included 2 patients with toxicity during the first year of treatment.3 The mechanism is unknown but it has been postulated that it is due to a genetic predisposition or acquired susceptibility, probably related to the use of nonsteroid anti-inflammatory drugs (NSAIDs) that inhibit cytochrome P450, increasing bioavailable HCQ levels. The present patient took a dose of HCQ within recommended limits but associated to the occasional use of NSAIDs and concomitant treatment with MTX, which could involve pharmacological interaction due to liver and kidney metabolization.
The early development of HCQ toxicity in the present patient indicates the multifactorial etiology of said maculopathy. Accordingly, more studies are needed in order to prevent the development thereof.
Please cite this article as: Hernández Bel L, Monferrer Adsuara C, Hernández Garfella M, Cervera Taulet E. Toxicidad macular precoz tras 2 meses de tratamiento con hidroxicloroquina. Arch Soc Esp Oftalmol. 2018;93:e20–e21.