The objective of these study is to know the characteristics of COVID-19 in patients with uveitis associated with Systemic Autoimmune Disease (SAD) through telematic survey.
Material and methodsInternal Medicine Society and Group of Systemic Autoimmune disease conducted a telematic survey of patients with SAD to learn about the characteristics of COVID-19 in this population.
ResultsA total of 2,789 patients answered the survey, of which 28 had a diagnosis of uveitis associated with SAE. The majority (82%) were female and caucasian (82%), with a mean age of 48 years. The most frequent SAEs were Behçet’s disease followed by sarcoidosis and systemic lupus erythematosus. 46% of the patients were receiving corticosteroid treatment at a mean prednisone dose of 11 mg/day. Regarding infection, 14 (50%) patients reported symptoms compatible with SARS-CoV-2 infection. RT-PCR was performed on the nasopharyngeal smear in two patients and in one of them (4%) it was positive.
ConclusionsBoth asymptomatic and symptomatic COVID-19 patients with ASD-associated UNI had received similar immunosuppressive treatment.
El objetivo de este estudio es conocer las características de la COVID-19 en pacientes con uveítis asociada a enfermedades autoinmunes sistémicas (EAS) mediante una encuesta telemática.
Material y métodosLa Sociedad Española de Medicina Interna por medio del Grupo de Trabajo de Enfermedades Autoinmunes realizó una encuesta telemática a pacientes con EAS para conocer las características de la COVID-19 en esta población.
ResultadosUn total de 2.789 pacientes contestaron la encuesta, de los que 28 tenían un diagnóstico de uveítis asociada a una EAS. La mayoría (82%) eran mujeres y caucásicas (82%), con una media de 48 años. Las EAS más frecuentes fueron la enfermedad de Behçet seguida de la sarcoidosis y del lupus eritematoso sistémico. El 46% de los pacientes estaban recibiendo tratamiento con corticoides a una dosis media de prednisona de 11 mg/día. Respecto a la infección, 14 (50%) pacientes referían síntomas compatibles con infección por SARS-CoV-2. Se realizó RT-PCR en el frotis nasofaríngeo en dos pacientes y en uno de ellos (4%) fue positivo.
ConclusionesLos pacientes con UNI asociada a EAS tanto los asintomáticos como los sintomáticos de COVID-19 habían recibido de forma similar tratamiento inmunosupresor.
Coronavirus disease 2019 or COronaVIrus Disease 2019 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) or adult respiratory distress syndrome (ARDS) coronavirus 2 (SARS-CoV-2). It originated in Wuhan City, Hubei Province, China, in December 2019 and has caused the largest coronavirus outbreak described to date after SARS in 2002 and MERS in 2012.
After a variable incubation phase, ranging from 3 to 10 days, it initially manifests with respiratory symptoms. About 80% of patients develop a paucisymptomatic, or even asymptomatic, condition. The remaining 20% develop a clinical picture characterised by bilateral interstitial pneumonia that is sometimes complicated by ARDS leading to respiratory failure and an increased systemic inflammatory response with elevated inflammatory markers.1 Mortality in patients who develop this inflammatory condition ranges from 10% to 20%.2 COVID-19 is associated with other less frequent and somewhat later clinical manifestations, such as thrombotic phenomena (arterial or venous), skin inflammation, vasculitis and the development of pulmonary fibrosis.
Data from observational studies and case reports published in China3,4 and Europe5,6 that have included transplant recipients as well as patients with systemic autoimmune diseases (SAD) under immunosuppressive treatment have not demonstrated to date that immunosuppressed patients are at higher risk of SARS-COV-2 infection than the general population, nor that the clinical presentation, disease course, laboratory and imaging findings are different or more severe than those of non-immunosuppressed patients.
Some scientific societies such as the International Uveitis Study Group, the International Ocular Inflammation Society and the Foster Ocular Inflammation Society have published a consensus with recommendations to be followed in patients with uveitis in times of COVID-197 infection. Similar to other scientific societies,8 it has been recommended to maintain immunosuppressive treatment in patients without symptoms of COVID-19 and that social isolation measures should be strictly followed and follow-up consultations should be carried out via telemedicine as far as possible. In addition, the use of the lowest possible doses of corticosteroids (ideally below 20 mg per day of oral prednisone or equivalent)9 is recommended.
There are no data at this time on the impact of COVID-19 in patients with SAD-associated uveitis. Some of these patients are treated with corticosteroids and disease-modifying drugs such as antimetabolites, calcineurin inhibitors, alkylating agents or biologics7 that may increase the risk of infection.
The aim of this study is to determine the number of patients with SAD-associated uveitis who may have been infected with SARS-COV-2 virus during the first wave of the pandemic, the characteristics of COVID-19 in these patients, the degree of immunosuppression and the performance of diagnostic tests as well as the need for hospitalisation, by means of a telematic survey addressed to different associations of patients with systemic autoimmune diseases and uveitis.
Material and methodsThe Spanish Society of Internal Medicine (SEMI), through the Working Group on Autoimmune Diseases (GEAS), conducted a telematic survey of patients with SAD to find out the characteristics of COVID-19 in this population. This study, carried out in collaboration with the Spanish Society of Ocular Inflammation, presents the results corresponding to patients with uveitis associated with SAD.
This is a descriptive, observational, cross-sectional study with information collected through a structured survey. The survey was elaborated by 3 internists (FP, LSC and BGT), with extensive experience in the field of multidisciplinary management of SAD. The survey was conducted using the online survey platform Survey Monkey (Survey Monkey Inc. San Mateo, California, USA). Informed consent was attached along with the survey link. When the patient accessed the survey, the first item to be filled in was whether the patient agreed to take the survey; if the answer was affirmative, the informed consent was accepted.
The following variables were collected in the survey: underlying systemic autoimmune disease and whether the patient had previously been diagnosed with uveitis, date of birth, sex, ethnicity, treatment with hydroxychloroquine or other antimalaria drugs in the last 3 months and daily dose, treatment with oral corticosteroids in the last month and average doses of corticosteroids in the last month, immunosuppressive or biological treatment received in the last 6 months, symptoms of suspected COVID-19 in the last 14 days, previous contact in the last 14 days with a patient with symptoms, and real-time polymerase chain reaction (RT-PCR) for SARS-COV-2 in nasopharyngeal swab in the last 14 days (Annex).
The Department of Medicines for Human Use of the Spanish Agency for Medicines and Health Products classified the study as a “non-post-authorisation observational study” (non-PAE). Subsequently, the survey on COVID-19 infection and SAD was approved by the Clinical Research Ethics Committee of Aragon.
The survey was sent through the patient associations and disseminated to their members from 15 April to 15 May 2020. The patient associations that distributed the survey among their members and participated were the following: Association of Uveitis Patients (AUVEA), Retina Association of Navarra, Spanish Federation of Lupus, Galician Association of Lupus, Association of Lupus Patients of Asturias, Lupus Association of Cantabria, Association of Lupus Patient Care of Vizcaya, Lupus and Antiphospholipid Association of León, Lupus Association of Salamanca, Lupus Association of Aragón, Lupus and Phospholipid Syndrome Patients Association of Madrid, Lupus Association of Badajoz, Lupus Association of Castilla-La Mancha, Generalized Erithematous Lupus Association of Catalonia, Lupus Patients Association of Valencia, Lupus Association of Baleares, Lupus Association of Almería, Lupus Patient Association of Cádiz, Lupus Association of Granada, Associated Lupus Patients of Huelva, Lupus Association of Jaén, Lupus Association of Málaga, Seville Lupus Association, Spanish Sjögren's Syndrome Association, Spanish Scleroderma Association, Scleroderma Association of Castellón, Spanish Systemic Vasculitis Association, Spanish Association of Behçet’s Disease, and the National Association of Sarcoidosis Patients.
Statistical analysisData were collected and analysed using an Excel database (Microsoft Office, Redmond, WA 98052, USA). Quantitative variables were expressed as means and qualitative variables are summarised in their frequency distribution.
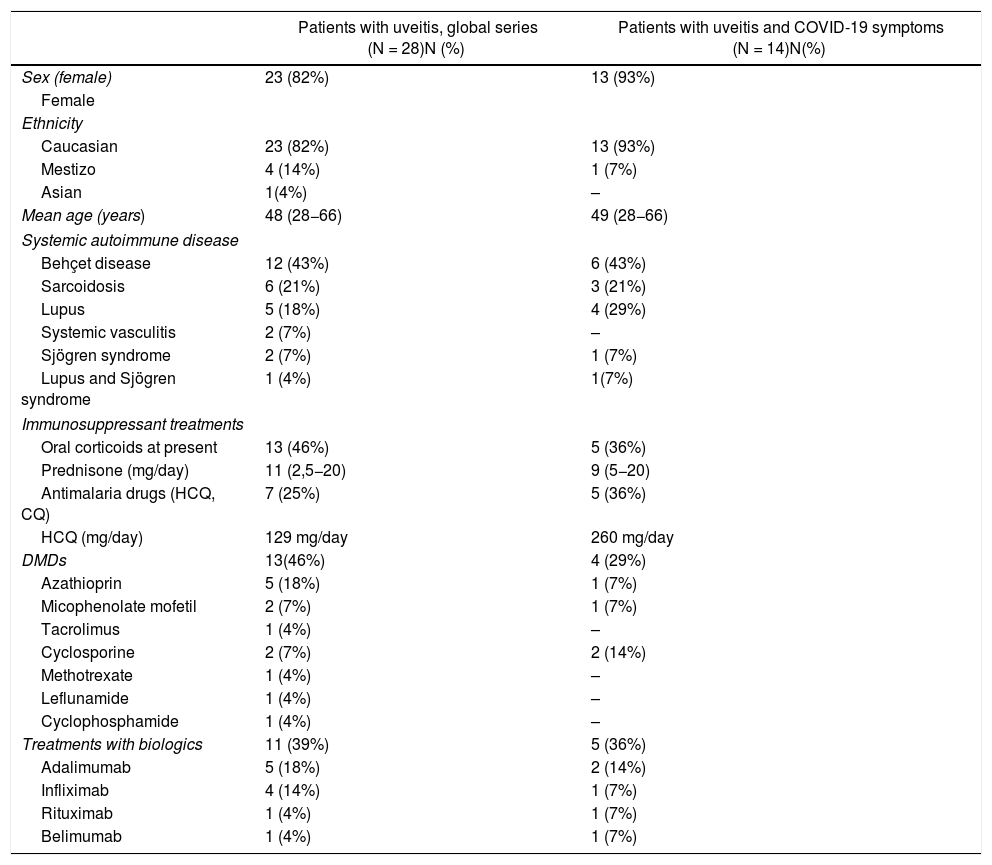
ResultsOverall, 2,789 patients responded to the survey, of whom 28 had a diagnosis of uveitis associated with SAD (Table 1). There was no response to the survey from any patient with a diagnosis of isolated uveitis without associated systemic autoimmune disease despite referral to the Retina Association of Navarra and AUVEA.
Demographic and clinical characteristics of patients with uveitis.
| Patients with uveitis, global series (N = 28)N (%) | Patients with uveitis and COVID-19 symptoms (N = 14)N(%) | |
|---|---|---|
| Sex (female) | 23 (82%) | 13 (93%) |
| Female | ||
| Ethnicity | ||
| Caucasian | 23 (82%) | 13 (93%) |
| Mestizo | 4 (14%) | 1 (7%) |
| Asian | 1(4%) | – |
| Mean age (years) | 48 (28−66) | 49 (28−66) |
| Systemic autoimmune disease | ||
| Behçet disease | 12 (43%) | 6 (43%) |
| Sarcoidosis | 6 (21%) | 3 (21%) |
| Lupus | 5 (18%) | 4 (29%) |
| Systemic vasculitis | 2 (7%) | – |
| Sjögren syndrome | 2 (7%) | 1 (7%) |
| Lupus and Sjögren syndrome | 1 (4%) | 1(7%) |
| Immunosuppressant treatments | ||
| Oral corticoids at present | 13 (46%) | 5 (36%) |
| Prednisone (mg/day) | 11 (2,5−20) | 9 (5−20) |
| Antimalaria drugs (HCQ, CQ) | 7 (25%) | 5 (36%) |
| HCQ (mg/day) | 129 mg/day | 260 mg/day |
| DMDs | 13(46%) | 4 (29%) |
| Azathioprin | 5 (18%) | 1 (7%) |
| Micophenolate mofetil | 2 (7%) | 1 (7%) |
| Tacrolimus | 1 (4%) | – |
| Cyclosporine | 2 (7%) | 2 (14%) |
| Methotrexate | 1 (4%) | – |
| Leflunamide | 1 (4%) | – |
| Cyclophosphamide | 1 (4%) | – |
| Treatments with biologics | 11 (39%) | 5 (36%) |
| Adalimumab | 5 (18%) | 2 (14%) |
| Infliximab | 4 (14%) | 1 (7%) |
| Rituximab | 1 (4%) | 1 (7%) |
| Belimumab | 1 (4%) | 1 (7%) |
The majority were women (82%) and of Caucasian ethnicity (82%), with a mean age of 48 years. The most frequent SAIDs were Behçet’s disease followed by sarcoidosis and systemic lupus erythematosus.
Forty-six per cent of the patients were receiving corticosteroid treatment at a mean prednisone dose of 11 mg/day. Twenty-five per cent of patients had received hydroxychloroquine or another antimalaria drug in the last 3 months and the mean dose was 129 mg/day. Regarding immunosuppressants, 18% had received azathioprine followed by 7% mycophenolate mofetil, 7% cyclosporine, 4% tacrolimus, 4% methotrexate, 4% leflunomide and 4% cyclophosphamide. Eleven (39%) patients had received biologic therapy in the last 6 months (Table 1).
Regarding infection, 14 (50%) patients reported symptoms compatible with SARS-CoV-2 infection. Among the most frequent symptoms reported by patients were cough (9 patients), diarrhoea (7 patients), dysgeusia (4 patients), fever or febrile (3 patients), dyspnoea (3 patients) and anosmia (3 patients).
Fourteen (50%) patients were unaware of previous contact with patients with symptoms in the previous 14 days, 12 (43%) patients had no previous contact and 2 (7%) patients had previous contact. RT-PCR was performed on the nasopharyngeal swab in two patients and one (4%) was positive. The patient was a 50-year-old Caucasian woman with uveitis associated with sarcoidosis in treatment with hydroxychloroquine 100 mg/day, prednisone 20 mg/day and mycophenolate mofetil. The patient had no previous contact in the previous 14 days and exhibited fever of >38 C, cough, dyspnoea, dysgeusia, anosmia, diarrhoea and asthenia requiring hospital admission.
DiscussionThis study shows that after surveying different associations of patients with SAD, 28 out of 2,789 (1%) patients had a diagnosis of uveitis associated with SAD. Possible COVID-19 cases (clinically compatible without diagnostic confirmation) were half and only one patient was confirmed to have SARS-COV-2 infection (by RT-PCR gold test on nasopharyngeal swab) and required hospital admission.
This is the first study to publish results of possible SARS-COV-2 infection in a group of patients with SAD-associated uveitis on immunosuppressive therapy.
A study in Hubei showed that up to 1/3 of patients with COVID-19 had ocular disturbances and that these were more frequently found in patients with more severe symptoms of COVID-19.10 To date, there are published cases of conjunctivitis, keratitis, anterior uveitis, retinitis and optic neuritis as part of the inflammatory syndrome produced by COVID-19.11 Some of these patients responded to topical steroid treatment and it has been postulated that ocular inflammation may be part of the cytokine release syndrome.12
Paradoxically, however, immunosuppression may be seen as a protective factor or even as part of the treatment of uveitis secondary to COVID-19 infection. Wu et al. studied 201 patients with ARDS and showed that those who had received corticosteroid treatment were associated with a lower risk of mortality (46% mortality rate with corticosteroids vs. 62% without corticosteroid treatment).13 More recently, based on the findings published in the Randomised Evaluation of COVID-19 therapy trial by Horby et al. of a total of 2,104 patients who received treatment with dexamethasone 6 mg per day for 10 days compared to 4,321 who did not receive steroids, it was observed that dexamethasone reduced mortality in 35% of patients requiring mechanical ventilation and in 20% of patients requiring oxygen therapy.14 In Spain, two studies have demonstrated the usefulness of methylprednisolone pulses in the treatment of pneumonia secondary to systemic inflammatory response syndrome generated by COVID-19.15,16
Numerous clinical trials and studies are currently underway with biological therapies and small molecule inhibitors for the treatment of COVID-19 pneumonia. In the era of precision medicine, these therapies allow us to block cytokines of the inflammatory cascade triggered by the SARS-CoV-2 coronavirus without the need to use them in the treatment of pneumonia SARS-CoV-2 without impairing the host’s protective immune response.
Given the key role of IL-6 in the inflammatory cascade, in the systemic hyperinflammation phase and in the late phase of COVID-19, tocilizumab, a humanised monoclonal antibody inhibitor of IL-6, is one of the most widely used and studied biological agents during the COVID-1917 pandemic. A recent Spanish study published by Rodríguez-Baño et al. has demonstrated the usefulness of using tocilizumab both alone and in combination with steroids for the treatment of hyperinflammatory response.18 Similarly, anakinra, an IL-1 receptor inhibitor, has also been studied in several prospective and retrospective cohorts with satisfactory results,19,20 demonstrating clinical improvement in 72% of patients and reducing the need for mechanical ventilation and mortality with COVID-19 and ARDS.
It has been shown that patients with inflammatory bowel disease treated with adalimumab or infliximab did not have a higher risk of infection than the general population. Based on the hypothesis of a possible protective effect of these treatments, as TNF alpha is one of the main cytokines that underlies the cytokine storm syndrome, there is currently only one clinical trial with infliximab in COVID 19, which is in the recruitment phase.21
As for adalimumab, the only biologic therapy licensed for the treatment of non-infectious uveitis, there are no published studies or ongoing clinical trials.
In the present study, patients with possible COVID-19 symptoms as well as asymptomatic patients were treated with steroids, DMDs and biologics, with adalimumab being the most frequently used in both groups.
The limitations of this study include the fact that it is a survey and therefore its validity depends primarily on the willingness of the patients and their knowledge of their SAD and the immunosuppressive treatments they are receiving. The fact that the survey was not addressed to patients with uveitis alone and that the spondyloarthropathy group was not explicitly included among the associated systemic autoimmune diseases leads us to think that there may be a selection bias and that, for this reason, more patients than usual appear with the diagnosis associated with lupus uveitis or other SAD. In addition, the survey is sent from SEMI and from the Systemic Autoimmune Diseases Working Group, which possibly also implies a different patient profile to that which can be managed from the rheumatology consultation.
In addition, another possible selection bias that may have occurred is the fact that the majority of patients who answered the survey had presented symptoms suggestive of COVID-19. Another limitation is that we do not know the type of uveitis according to location (anterior, intermediate, posterior or panuveitis), nor how many of the patients with uveitis were in an outbreak at the time the survey was carried out. Moreover, the study does not have a control group of patients without SAD and RT-PCR of the nasopharyngeal swab was performed in only two patients.
This telematic survey identified a confirmed case of COVID-19 in a group of patients with SAD-associated uveitis, when half of the patients reported symptoms suspicious for coronavirus infection. This study highlights the need for PCR and diagnostic tests in patients with suspected symptoms of coronavirus infection and in at-risk populations such as patients on immunosuppressive therapies. Patients with uveitis and systemic autoimmune diseases who, despite the guidelines and recommendations of scientific societies22,23 may not have received the necessary care with appropriate diagnostic tests, face-to-face consultations and follow-up care that they required during confinement. While the coronavirus pandemic persists, these patients with chronic and autoimmune pathology should continue their regular consultations and undergo the relevant diagnostic tests for screening for coronavirus infection, in the event of the onset of symptoms or contact with a confirmed or suspected case.
Further studies with a larger number of patients and with a control group are needed to determine the real incidence of this infection and its characteristics in this population.
ConclusionsPatients with UNI associated with SAD, both asymptomatic and symptomatic COVID-19 patients, had similarly received immunosuppressive treatment. Patients with UNI with symptoms suspicious for COVID-19 need to undergo diagnostic testing to rule out coronavirus infection.
Further studies in UNI patients on immunosuppressive therapy are needed to assess the risk of COVID-19 infection in this population.
Conflict of interestNo conflict of interest was declared by the authors.
To all patient associations and their patients for their collaboration in conducting the GEAS-COVID survey during the COVID-19 pandemic.
Please cite this article as: Fanlo P, Espinosa G, Adán A, Arnáez R, Fonollosa A, Heras H, et al. Impacto de la infección por el nuevo coronavirus en los pacientes con uveítis asociada a una enfermedad autoinmune: resultado de la encuesta COVID-19-GEAS pacientes. Arch Soc Esp Oftalmol. 2021;96:347–352.