El hipotiroidismo congénito (HC) es una causa prevenible de retraso mental, por lo que es de suma importancia que el diagnóstico y tratamiento oportunos sean realizados por el médico de primer contacto. El tamizaje para HC se debe realizar entre el segundo y quinto días de vida, con sangre capilar mediante la punción del talón. Debe confirmarse mediante el perfil tiroideo en sangre venosa.
La etiología más frecuente es la disgenesia tiroidea, la cual se identifica con gamagrafía tiroidea antes de iniciar el tratamiento. El tratamiento es con levotiroxina (10-15 μg/kg/día) y no debe ser retrasado ni suspendido durante los tres primeros años de vida debido al efecto deletéreo en el neurodesarrollo en el caso de deficiencia de hormonas tiroideas durante esta etapa. Los recién nacidos prematuros, enfermos o con síndrome de Down requieren una valoración especial. En este artículo se describen los algoritmos diagnósticos y terapéuticos del HC.
Congenital hypothyroidism (CH) is a cause of preventable mental retardation; therefore, timely diagnosis and treatment by the primary care physician is very important. CH screening must be performed between the second and fifth days of life with capillary blood done with a heel prick and must be confirmed by measurement of thyroid hormones in venous blood.
The most common cause of CH is thyroid dysgenesis, which may be identified by a thyroid scan carried out before initiating treatment. Treatment should be with levothyroxine (10-15 μg/kg/day) and should not be delayed or suspended during the first 3 years of life due to the deleterious effect on neurodevelopment in case of low thyroid hormones during this time. Preterm or sick infants or those with Down syndrome require special consideration. This article provides diagnostic and therapeutic algorithms for CH.
Pagina nueva 1
1. Introduction
Congenital hypothyroidism (CH) is a thyroid hormone deficiency present at birth. CH is one of the causes of preventable mental delay. There are generally no obvious signs or symptoms present at the time of birth; however, neurological prognosis depends on timely diagnosis and accurate treatment initiation. These considerations establish the importance of early diagnosis with neonatal screening.
2. Epidemiology
The frequency of CH varies according to different factors: 1) geographic area; 2) frequency of iodine deficiency in the population; 3) period of study; 4) methodology used for the screening; and 5) concentrations of hormones selected as cut-off points for diagnosis. Worldwide, the prevalence has been estimated to be between 1:800 and 1:10,000; in Mexico it is estimated at 1:2,400.1 CH is more common in Asians, Hispanic and Native Americans compared with the Caucasian or Afro-American population. It has a female predominance with a ratio of 2:1-3:1.2
3. Etiology
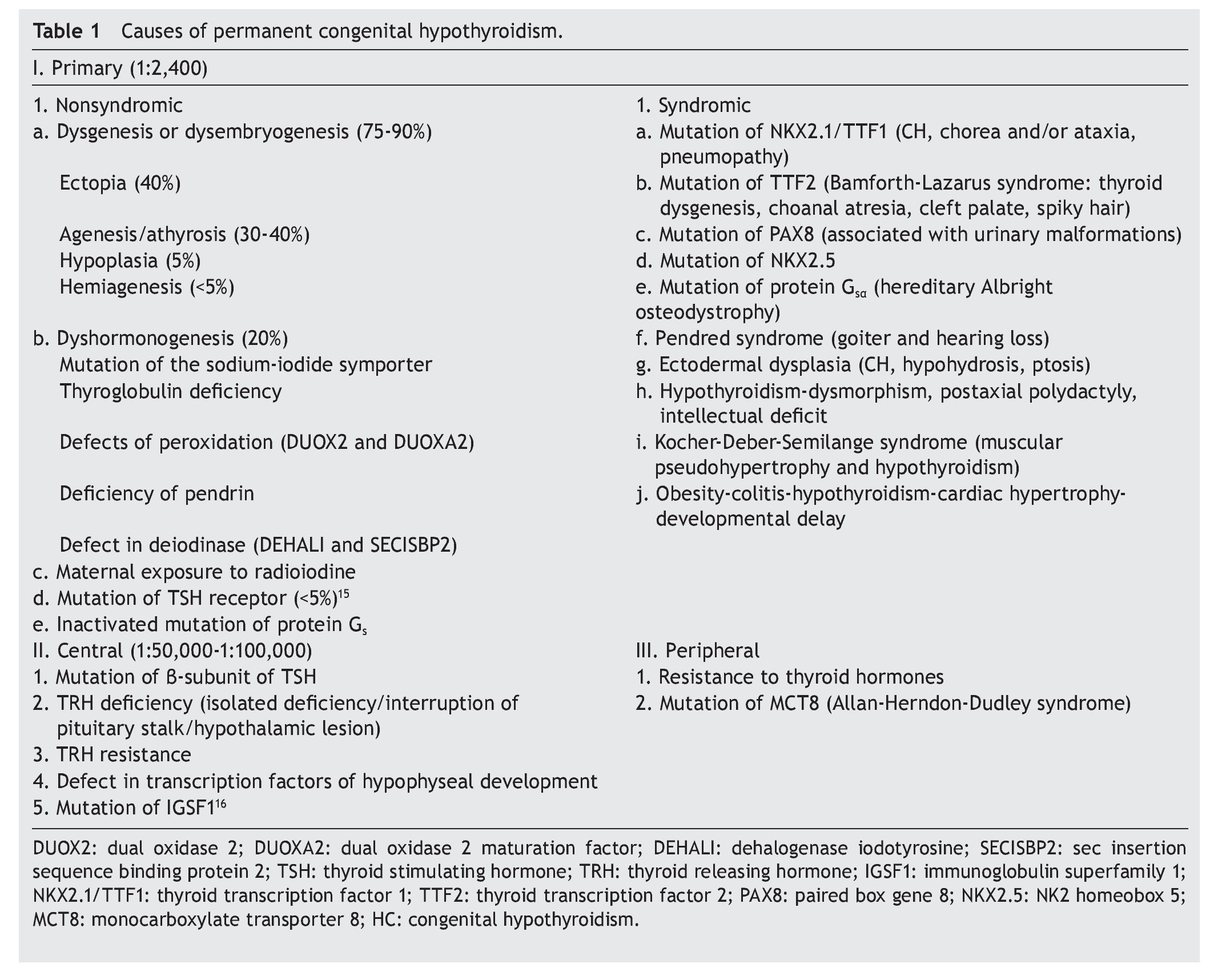
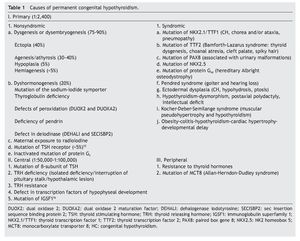
The principal cause of CH is iodine deficiency.3 In regions with iodine sufficiency the majority of CH cases are sporadic. Most of these, in turn, are due to thyroid dysgenesis, i.e., alterations in the morphogenesis of the thyroid. In only 2% of the cases is there familial association.4 In Mexico it has been reported that 57% of the cases detected by screening are due to ectopic thyroid, 36% to thyroid agenesis and 7% to dyshormogenesis.5 Table 1 shows the causes of permanent CH.6
When thyroid hormone concentrations normalize during follow-up, it is referred to as transitory CH, whose frequency is between 1:11,000 and 12,000. It may be secondary to maternal intake of anti-thyroid drugs, transplacental passage of anti-thyroid antibodies, iodine deficiency or excess, heterozygous mutations of DUOX2 or DUOXA2 or congenital hepatic hemangiomas that express elevated levels of type 3 deiodinase. It is also frequently present in newborns with Down syndrome. Transitory hypothyroidism of the central type (normal or low levels of thyroid-stimulating hormone) can be present in cases of maternal hyperthyroidism, prematurity or in newborns who are in critical state, particularly if they receive dopamine, steroids, aminophylline or caffeine.7
4. Clinical manifestations
The majority of patients with CH do not present clinical symptoms at birth. A wide posterior fontanel (diameter >0.5 cm) is one of the common findings. Other data presented if treatment is not started in a timely manner are macroglossia, edema, hoarse cry, rough fascies, umbilical hernia, hypotonia, mottled skin, hypothermia, lethargy, prolonged icterus (>2 weeks), bradycardia, difficulty feeding and constipation. Occasionally the birth is post-term. The presence of clinical data at birth and a distal femoral ossification nucleus, absent or <3 mm in diameter, suggests that the hypothyroidism is very severe and both maternal as well as fetal. It is important to examine the thyroid because in case of dysgenesis it is not palpable and in case of dyshormonogenesis, usually there is goiter.
Patients with CH have a higher prevalence of hypoacusia and of extrathyroid congenital malformations than the general population (8.4-10% vs. 3% in the general population)8,9, especially cardiac anomalies (1.5-5.8%), cleft palate and hip dysplasia (1-1-3.8%) as well as neurological, genitourinary, digestive and ophthalmological malformations.
5. Diagnosis
5.1. Neonatal screening
Due to the scant clinical data at birth and the need for early treatment initiation to avoid sequelae, CH is a disease that should be searched for with neonatal screening. There are various strategies listed below:
1. Primary measurement of tetraiodothyronine (T4) and confirmation with the measurement of thyroid stimulating hormone (TSH)
2. Primary measurement of TSH with T4 confirmation
3. Simultaneous primary measurement of T4 and TSH
Mexico uses the second strategy (primary measurement of TSH) because it has the advantages of being inexpensive, highly sensitive and detects subclinical hypothyroidism. The disadvantage that it presents is not detecting hypothyroidism of central origin or cases of delayed elevation of TSH.
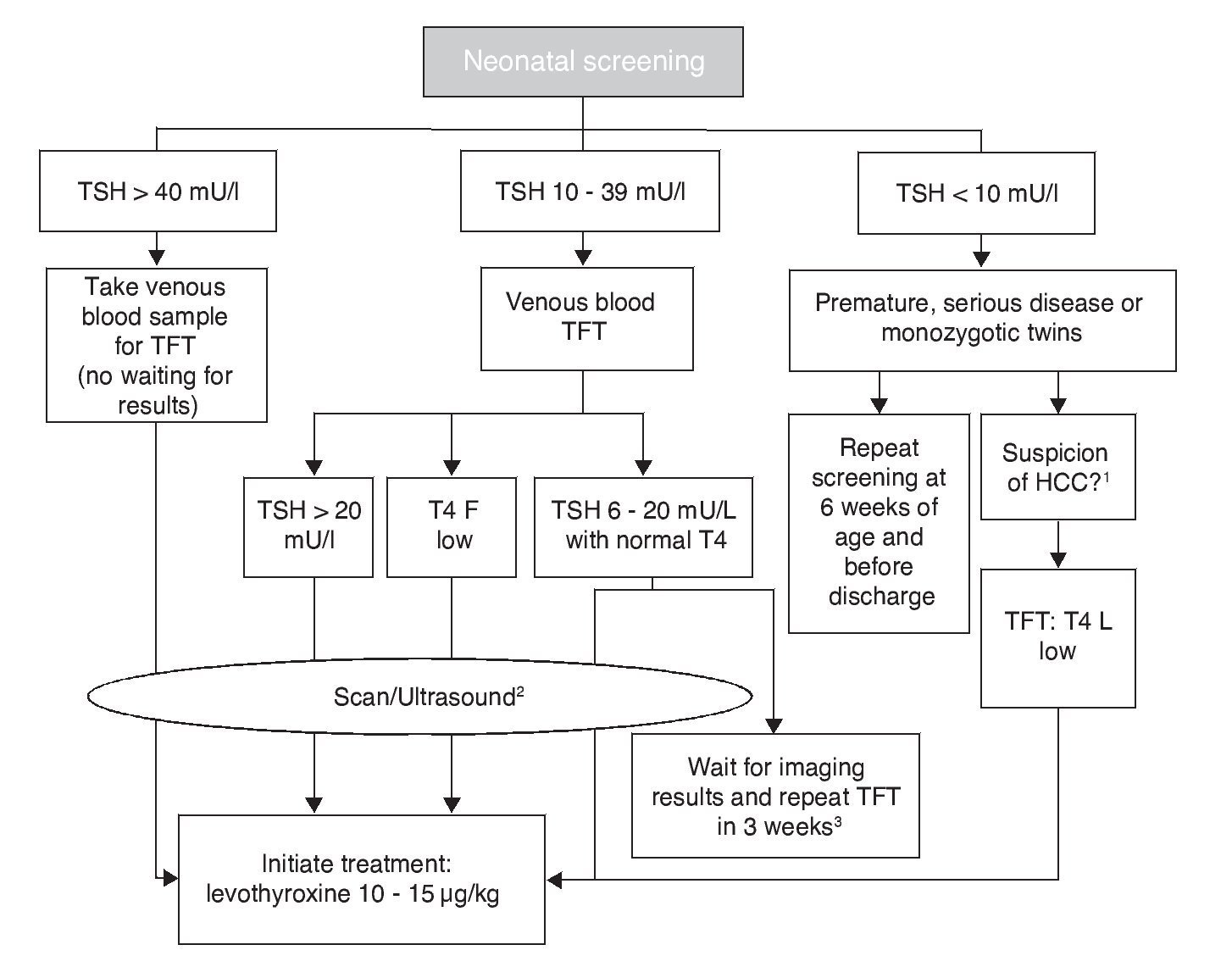
Each screening program for CH adjusts the cut-off points of TSH concentration to maximize the sensitivity and minimize the “recall” rate. Levels as low as 6 mU/l and up to 20 mU/l have been proposed as cut-off points.10 In Mexico, the screening sample is obtained by puncture of the heel to soak a filter paper, between the second and fifth day of life. Measurements before 48 h increase the frequency of false positives and late measurements increase the risk of delay in initiating treatment. The result of neonatal screening should be reported before 15 days of life. Some false negatives occur due to the late elevation of TSH. To decrease the risk of underdiagnosis in case of CH with late elevation of TSH, some programs, including those in Mexico, indicate repeating the screening between 2 and 6 weeks of life in premature infants, in patients admitted to the neonatal intensive care unit (NICU), in patients with cardiovascular anomalies, and in homozygous twins. Measurements of T4 diagnose from 12 to 15% more cases that are of central origin. TSH concentration >40 mU/l or >20 mU/l accompanied by T4 <5 μg/dl are 100% specific for diagnosis of permanent CH and warrant urgent initiation of treatment. Of the cases with TSH with screening results between 20 and 39 mU/l, 75% are false positives or cases of transitory hypothyroidism. Cases of CH not detected with neonatal screening programs could reach up to 5%, which is generally due to human error in the management of the sample, the analysis or report of the results.
5.2. Confirmatory diagnosis
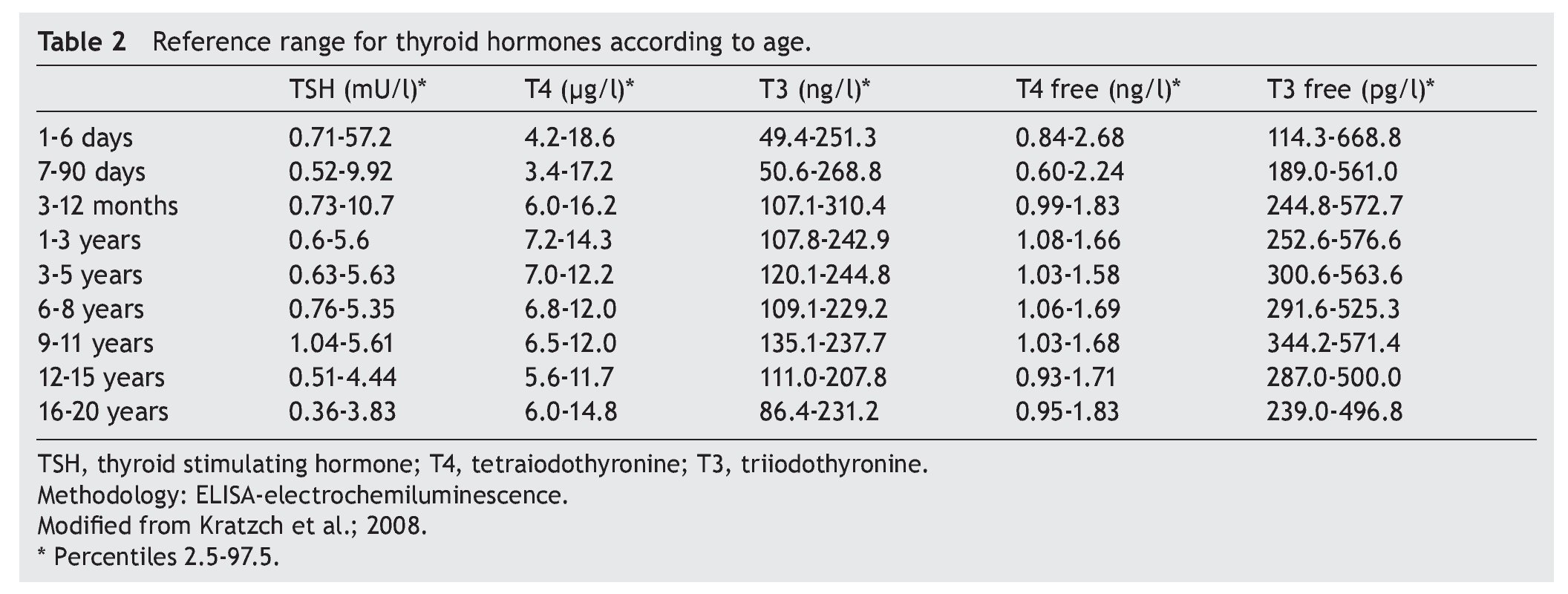
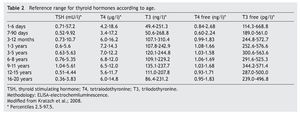
The cut-off point for requesting confirmatory testing is a TSH concentration of 10 mU/l determined by fluoroimmunoassay or by ELISA.11 To confirm the diagnosis, it is necessary to measure TSH and total and/or free T4 total in venous blood in the following 24 h after results of the screening. TSH >40 mU/l with low T4 is indicative of CH, which is generally permanent. Patients with milder anomalies can be followed with repeated sampling because the disorder is frequently transitory (Fig. 1). It is important to consider the reference values of thyroid hormones specific for each age (Table 2).
Figure 1 Flow chart for neonatal screening. TFT, thyroid function tests; T4 F, free T4; CCH, central congenital hypothyroidism. Based on the Consensus of the European Society of Pediatric Endocrinology for Screening, Diagnosis and Treatment of Congenital Hypothyroidism (Léger et al., 2014). 1 Micropenis, cryptorchidism, hypoglycemia, midline defects. 2 Scan should be carried out 5 days after treatment initiation, without any delays waiting for imaging studies. 3 In these cases the clinician should decide to start treatment immediately and reassess after 3 years or postpone the decision for 3 weeks with new measurements of thyroid hormones and imaging studies.
5.3. Imaging studies
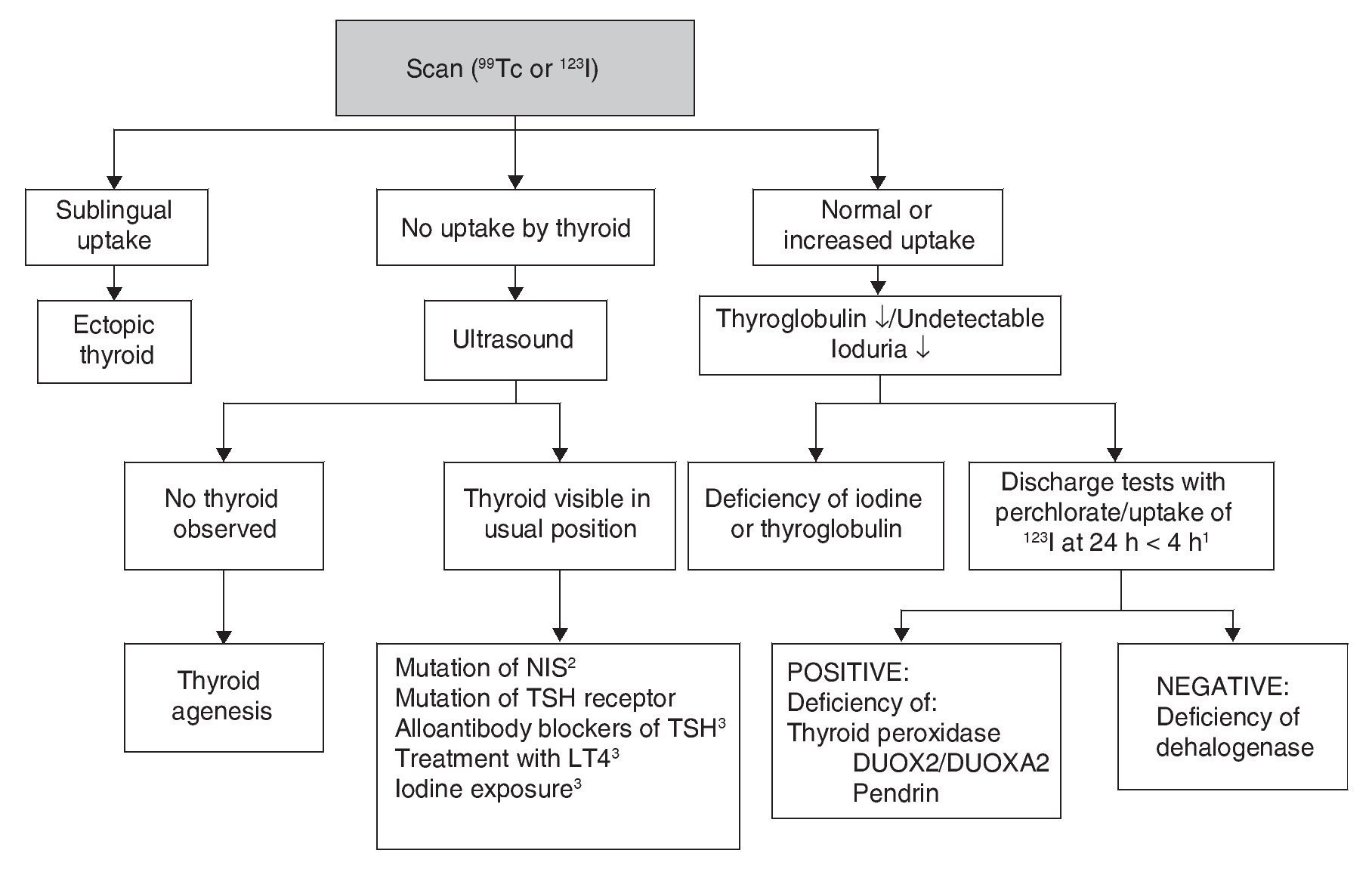
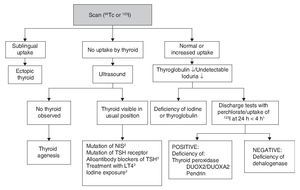
Although CH treatment should not be delayed until imaging studies are done, the scan and the ultrasound are useful to determine its etiology (Fig. 2). 99Tc is the radionuclide of choice to identify the location of the thyroid because its depuration is faster and the radiation exposure is less than with 123I. 123I scan is necessary when one wishes to quantify the thyroid uptake of iodine in cases where there is suspicion of dyshormonogenesis. Lack of uptake of the radionuclide by the thyroid almost always indicates aplasia (athyrosis). If the thyroid is not seen in the scan but is seen in the ultrasound, the possible causes are a defect of the TSH receptor, a defect in the transport of iodine, blocking antibodies of the TSH receptor or low TSH due to treatment with levothyroxine. The scan can also show an ectopic localization of the thyroid. Increased 123I uptake, which decreases at 24 h with respect to the 4-h uptake, suggests dyshormonogenesis. Scan is not useful if >5 days have passed after initiation of treatment because the radionuclide up-take will be low due to the low TSH levels. Ultrasound has little sensitivity for identifying thyroid dysgenesis. It is possible to perform molecular diagnosis of the causes of dyshormonogenesis and of syndromatic hypothyroidism when they are due to known mutations.
Figure 2 Etiologic diagnosis of primary congenital hypothyroidism. 1 Perchlorate is not available in our environment, but uptake of 123I that decreases at 24 h compared to 4-h uptake leads to diagnosis of dyshormogenesis. 2 NIS, sodium/iodide symporter. 3 Low uptake can be present. TSH, thyroid-stimulating hormone; LT4, levothyroxine.
Other diagnostic studies that could provide information are measurement of thyroglobulin, antithyroid antibodies, urinary iodine and bone age. The high concentration of thyroglobulin suggests dyshormonogenesis, whereas a very low or undetectable concentration suggests thyroid agenesis or some type of disorder in the synthesis of this protein although the concentrations found in these patients overlap with normal intervals. The finding of antithyroid antibody in plasma generally suggests a transient alloimmune process. It is possible to diagnose iodine deficiency or excess with a 24-h urine iodine measurement which, in neonates, should be from 50-100 μg. Absence of the distal femoral ossification nucleus (“empty knee” sign) or a diameter <3 mm in a term newborn suggests severe hypothyroidism of intrauterine origin.
The usefulness of the etiological diagnosis lies in the prognosis. If thyroid dysgenesis is confirmed (ectopic thyroid or agenesis) or dyshormonogenesis, hypothyroidism is without a doubt permanent. If its etiology is not established, whether it is due to not being able to perform a scan before initiating treatment or because of inconclusive results, particularly when there is moderate TSH elevation (<40 mU/l), one should wait until 3 years of age to suspend treatment and re-evaluate because suspending treatment before this age in a child with hypothyroidism would jeopardize neurological development.
Central-type hypothyroidism (normal or low TSH with low T4) is not identified with the screening based on primary measurement of the TSH. It should be suspected whenever there are midline defects or data of deficiency in any other pituitary hormone such as hypoglycemia, small penis, cryptorchidism or prolonged jaundice. Hypoacusia should be ruled out in patients with CH with audiological tests appropriate for age and evaluate the need for imaging studies for ruling out congenital malformations.
6. Treatment
Levothyroxine (LT4) is the treatment of choice for CH. The objective is to attain a neurological development and growth corresponding to the genetic potential of the child. For this, an adequate dose of LT4 should be initiated within the first 2 weeks of life. If screening TSH concentration is >40 mU/l, the primary care physician should initiate LT4 as soon as possible and without waiting for examination by a specialist or confirmatory test results (although whenever possible a venous sample should be obtained for TSH and free T4 determination before beginning treatment.) If the TSH concentration is high but <40 mU/l, it is possible to wait 1-2 days for the venous blood analysis to start treatment. If analysis reveals low free T4 for age or TSH >20 mU/l, even if the free T4 is normal, treatment should still be initiated. If free T4 is normal and TSH is between 6 and 20 mU/l after 21 days of life, it can be opted to wait for the results of the imaging studies with strict monitoring and repeating a thyroid profile 2 weeks later or initiate treatment immediately and re-evaluate until 3 years of age (Fig. 1).12
The biochemical goals of treatment with levothyroxine are the following:
1. Normalize the free T4 concentration as soon as possible, i.e., in the first week of initiating the replacement.
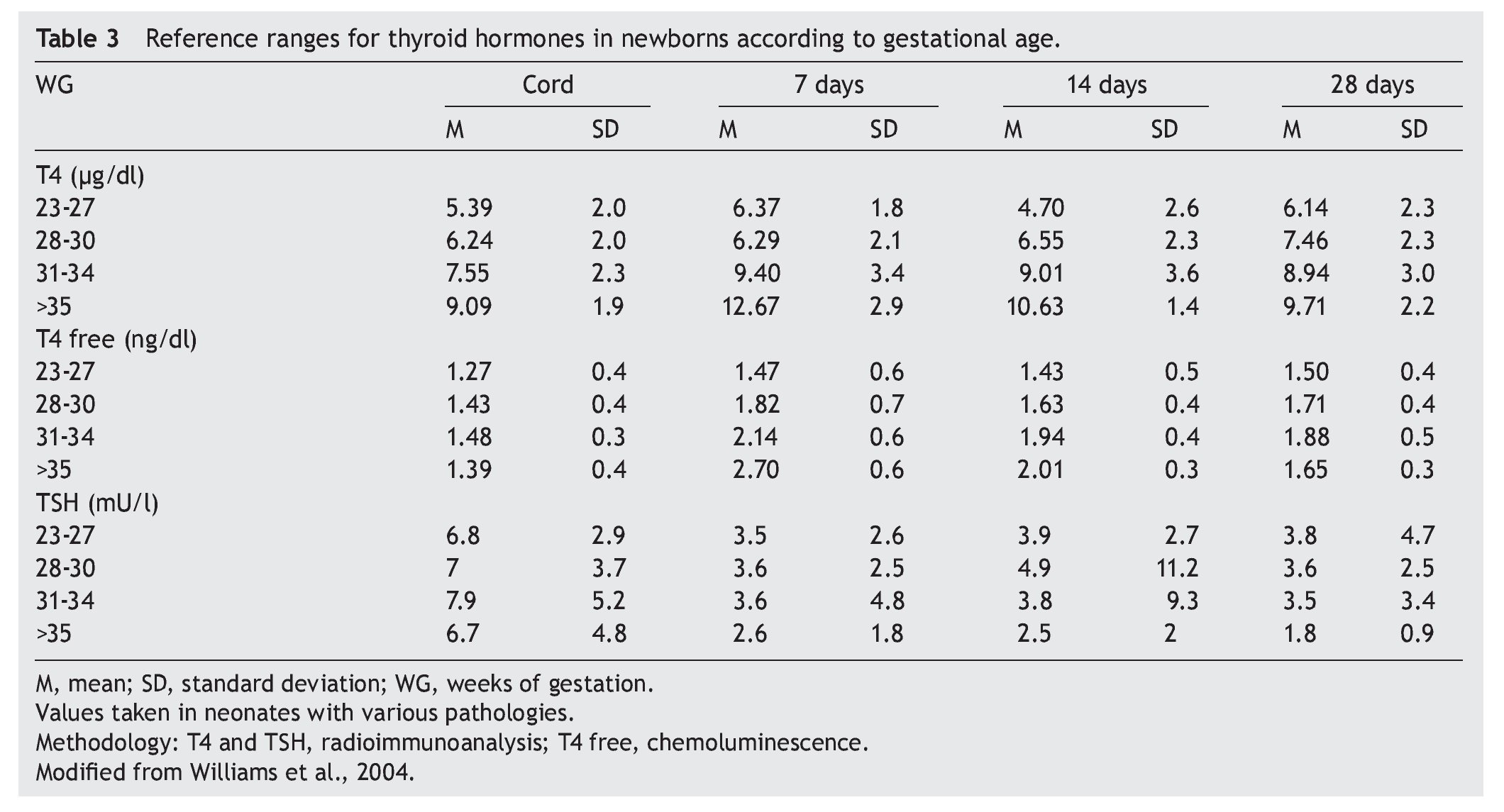
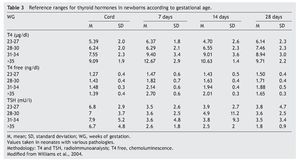
2. Maintain free T4 in the upper half of the reference ranges consistent with the age (Table 3).13
3. Maintain TSH concentration between 0.5 and 2 mU/l from the first month of treatment.14
To achieve these goals, the daily dose of levothyroxine should be from 10 to 15 μg/kg.13,15 Generally, a dose of 50 μg daily is started initially, although it may be necessary to decrease it later on. It is necessary to verify the serum levels of total T4 and/or free T4 and of TSH between 1 and 2 weeks after initiating treatment.
Levothyroxine should be administered in crushed tablets and suspended in water with a small metal spoon. It should not be diluted or given in a plastic container such as a syringe or bottle because it is not water soluble and adheres to the surface of the plastic container.
Iron, soybean, fiber, sucralfate, cholestyramine, calcium and aluminum hydroxide interfere with the absorption of levothyroxine. It should be given fasting (at least 30 min before the first meal) or with breast milk, but always in the same manner. It is necessary to raise awareness among parents and caregivers of the importance of strict adherence to treatment and to provide them with detailed written instructions. The first dose should be administered at the clinic. Recently, in some European countries, it has become available in a liquid formulation easier to administer to infants than tablets (Tirosint drops, IBSA Farmaceutici, Italy). Preliminary studies demonstrate an adequate absorption, apparently without differences in neurodevelopment or growth with respect to conventional treatment. However, there are some reservations about the alcohol content and stability of this formulation.16 An unauthorized liquid formation of LT4 should not be given whose pharmacokinetics have not been appropriately assessed. Simultaneous administration of triiodothyronine (T3) is not useful because most T3 in the central nervous system is produced by local monodeiodination of T4.
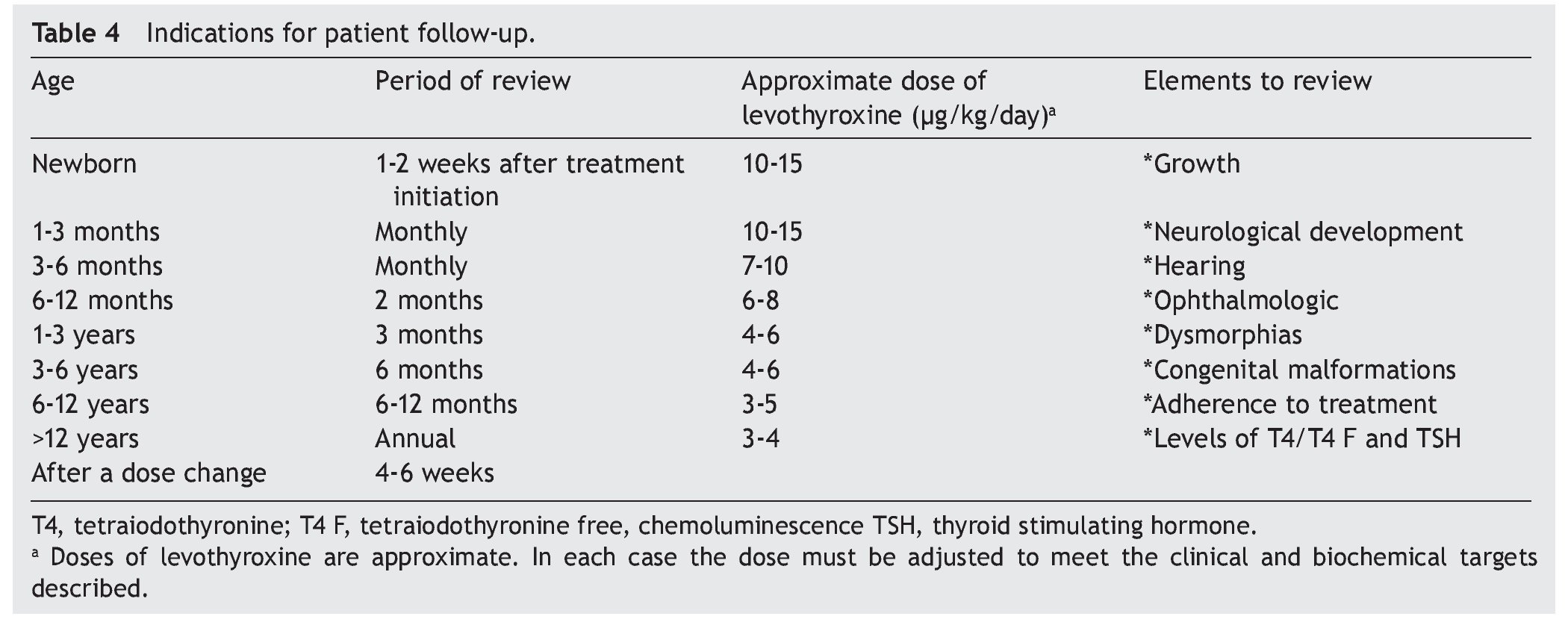
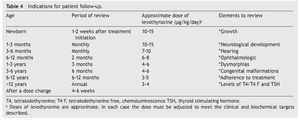
Recomendations for patient follow-up are listed in Table 4. In addition to verifying the serum concentrations of thyroid hormones, it is important to rule out hypoacusia, visual changes and congenital malformations during follow-up and to monitor growth and neurodevelopment. Early initiation with neurorehabilitation therapy is important. It is necessary to confirm thyroid hormone levels 4 weeks after a change in dosage. In some cases the TSH does not normalize even with adequate T4 levels. In these cases it is important to rule out poor compliance with treatment or poor technique of administration, although in some patients it may be necessary to have supraphysiological levels of T4 to maintain the TSH <2 mU/l. Overtreatment has been associated with hyperactivity, aggressiveness and craniosynostosis.17
7. Prognosis
The deleterious effect of hypothyroidism on the neurodevelopment of children <3 years of age is widely studied. Delay of even 1 week in the initiation of replacement therapy has negative effects on cognition.18 Athyrosis, delayed bone age (distal nucleus of the femur <0.5 cm2 in a term newborn), T4 levels <2 μg/l at the time of diagnosis, prolonged time for TSH normalization, treatment initiation after the second week of life and four or more episodes of TSH >5 mU/l in the first 3 years of life are prognostic factors for a suboptimal neurodevelopment.13,19-21It has recently been questioned if children who receive the recommended treatment have an optimal neurodevelopment. It has been reported that these children have mild neurocognitive changes. Some studies report an intellectual coefficient of 5 to 25 points lower than healthy children,22 whereas others have found no differences or only identify them in specific areas such as attention and hearing.17,18 In terms of transitory hypothyroidism, the effect of antithyroid medications persist up to 2 or 3 weeks of life and generally do not require treatment. CH due to the presence of antithyroid antibodies is usually resolved at 2-3 months of age and usually do require thyroid hormone replacement during this period.23
8. Special cases
8.1. Isolated hyperthyrotropinemia (high TSH and normal T4)
The etiology is heterogeneous and includes mutation in the TSH receptor, iodine deficiency and Down syndrome, although it is frequently idiopathic. Sometimes the TSH elevation is transitory. There are controversies with respect to treatment. It is generally recommended to repeat the measurements 2 weeks later and to administer treatment if the TSH persists >10 mU/l or the free T4 concentration decreases.13
8.2. Isolated hypothyroxinemia (low T4 and normal TSH)
This pattern is often seen in premature, sick newborns or in those who are given dopamine or glucocorticoids. Although an association has been described between hypothyroxinemia and an adverse neurological prognosis, it is not clear if the association is causal, and there is no consensus on the treatment of these patients. If the T4 is low, but the free T4 and the TSH are normal, the most probable diagnosis is protein deficiency of thyroxin binding globulin (TBG), and no treatment is required. Secondary or tertiary hypothyroidism, i.e., of central origin, can also present as isolated hypothyroxinemia. Finally, there are patients with primary hypothyroidism with late increase in TSH, i.e., after the date of screening, and this pattern is more common in premature infants and critically ill newborns.24,25
8.3. Thyroid dysfunction in patients with Down syndrome
Patients with Down syndrome have an increased prevalence up to 30 times greater than the general population, both for CH (1.5-6.1%) as well as for acquired thyroid dysfunction.26 There is also a higher frequency of isolated hyperthyrotropinemia or subclinical hypothyroidism (25.3-60%) and of thyroid autoimmunity (7.5-34%). Between 40 and 80% have isolated high and mild elevations of TSH,27 although apparently in most cases this elevation is transient or does not evolve to frank hypothyroidism.28 Different mechanisms have been proposed to explain thyroid dysfunction in patients with Down syndrome. These include the existence of dysfunctions of the hypothalamusthyroid axis, mild insensitivity to TSH and decreased bioactivity of TSH, with none of these having been corroborated.28 Hypothyroidism could aggravate the cognitive deficit, growth, and cardiometabolic risk factors present in patients with Down syndrome.29 However, it is unclear if isolated hyperthyrotropnemia or subclinical hypothyroidism has a harmful effect on these patients.30 It has been reported that patients with Down syndrome and hyperthyrotropinemia or free T4 below the median have lower levels of hemoglobin, higher prevalence for anemia and are found to be more hypotonic than those with normal TSH and free T4 above the mean.31 It is recommended that a thyroid profile be done at birth, at 6 and 12 months of life and then once a year in subjects with Down syndrome. However, it is unclear when a patient with isolated hyper-thyrotropinemia should receive treatment because on many occasions it is transient.32 It has been suggested that treatment be initiated if TSH is >10 mU/l, if there are symptoms or if there are antiperoxidase antibodies.33
8.4. Thyroid dysfunction in premature infants
Thyroid function in the newborn with prematurity is different with respect to that of a term newborn. Frequently, premature newborns have changes in thyroid function that are difficult to interpret. Thyroid hormone concentrations vary according to the age after conception and with the days of extrauterine life34 (Table 2). However, it is difficult to establish “normal” values because premature newborns almost always have multiple co-morbidities and receive multiple drugs that interfere with thyroid function. CH in premature infants is more common than in term infants. Despite this, it may not be diagnosed in the first neonatal screening because generally in <1% of those newborns <1,500 g35 a “late TSH elevation” is found and because the peak TSH in premature infants is not as accentuated as in term children.23 Up to 30% of the cases of late TSH elevation could correspond to permanent hypothyroidism. For this reason it is important to repeat the neonatal screening at 4 to 6 weeks of life and prior to discharge to avoid false negatives in these patients.
Premature infants are more sensitive to excess iodine because of the exposure to medications, contrast media or antiseptics as well as because skin absorption is greater and the clearance is lower than in term children. Premature infants are also more susceptible to iodine deficiency because their reserves are very low and their requirements higher than in term children and are not covered by breast milk, infant formulas or parenteral nutrition. Up to 50% of newborns <28 weeks of gestation have “transitory hypothyroxinemia”,36 i.e., low free T4 with normal TSH. It is possible that this phenomenon is due to an immaturity of the pituitary-hypothalamic-thyroid axis and/or having mechanisms similar to thyroid dysfunction of the critically ill patient or patient with euthyroid syndrome. Hypothyroxinemia is associated with adverse outcomes in premature infants,37 but it is not known if replacement therapy provides some benefit.25,38
Funding
None.
Conflict of interest
The author declares no conflicts of interest of any nature.
Received 14 April 2015;
accepted 30 April 2015
http://dx.doi.org/10.1016/j.bmhimx.2015.05.001
E-mail:fernandabmhim@gmail.com.