Esta es la primera parte de un trabajo de revisión donde se reportan los conocimientos actuales del retinoblastoma (Rb) y sus implicaciones en México (incluyendo la experiencia de los autores en los principales centros de referencia), así como las brechas en la práctica y las posibles soluciones para mejorar el diagnóstico, tratamiento y referencia de pacientes. En esta parte se resumen los conocimientos generales del Rb, su diagnóstico y tratamiento. Se realizó una revisión de los avances más recientes en esta enfermedad publicados en PubMed y se resumieron los hallazgos más importantes.
La sospecha oportuna y la referencia adecuada de pacientes permiten que el tratamiento conservador del Rb sea curativo. En países en vías de desarrollo, el tratamiento primario es el salvamento ocular y la preservación de la visión. Las opciones de quimioterapia intraarterial o intravítrea permiten ofrecer opciones terapéuticas en estos pacientes.
Los avances en el tratamiento del Rb están generalmente limitados a países industrializados. Las implicaciones de los hallazgos de esta revisión serán discutidas en la segunda parte, la cual será un análisis de la situación de los programas hospitalarios del Rb en México, incluyendo la revisión de los datos demográficos disponibles de los centros de referencia más importantes.
This is the first of a two-part review that aims to report the current knowledge of retinoblastoma (Rb) and its implications in Mexico (including the authors’ experience at the leading Rb centers), identify the gaps in practice, and propose solutions to improve diagnosis, treatment, and patient uptake. In this first part, general knowledge of Rb diagnosis and management is summarized with a focus on the latest advances in chemotherapy. A general review of peer-reviewed literature of Rb was conducted on PubMed. Key findings were summarized.
Provided there is early detection and referral of patients followed by appropriate conservative management, Rb is curable. In developed countries, the primary treatment outcome is ocular salvage with sight preservation. Advanced chemotherapeutic options such as intra-arterial and intravitreal chemotherapy can now save even the most advanced tumors.
Advances in Rb therapy are generally limited to developed countries. The implications in Mexico, of the findings from this review will be discussed in Part 2, which will be a comprehensive situational analysis of the state of Rb programming in Mexico including a review of current demographic data available from hospitals that have Rb programs or treat Rb.
1. Introduction
Retinoblastoma (Rb) is the most common primary malignancy in children, most frequently occurring in children <5 years of age, with an annual incidence ranging worldwide from 36/1,000,000 live births to 67/1,000,000 live births.1–5 Accurate incident rates can be difficult to estimate, especially in developing countries that lack a national Rb registry. In fact, a recent study from the Asia-Pacific region would suggest that cases of Rb are being underreported by >50%.6
A curable cancer, Rb survival rates in the developed world range from 90-95%, mainly due to early diagnosis of the disease and to the advances made over the past few decades in conservative treatment.5,7,8 However, survival rates are significantly lower in developing countries; in Africa, they are estimated as low as 20%, and >3,000 annual childhood deaths are attributed worldwide to Rb.8–10 The poorer outcomes in developing countries have been associated with late diagnosis and treatment, lower educational levels of the mother, lack of access to health services, and treatment abandonment by families of the patient.11–13
This article is the first part of a two-part review with the objective to report the current situation of Rb in Mexico, including the authors’ own experience at the country’s leading Rb centers and a review of currently available demographic data of patients with Rb at hospitals with Rb programs or that treat for Rb. We will also identify gaps in practice and propose solutions to improve diagnosis, provide adequate treatment, and improve patient uptake. The situational analysis of Rb in Mexico will be performed within the context of the general universal knowledge of Rb diagnosis and management. In this first part, we will summarize the general knowledge of Rb diagnosis and management including the latest advances in chemotherapy options.
2. Methods
A general, unstructured literature search was performed using PubMed to search for peer-reviewed journal articles on the current knowledge of Rb diagnosis and management. No specific search parameters were applied. Key findings from the literature are summarized.
3. Results
3.1. Pathology, diagnosis, and clinical characteristics
There are two forms of Rb, hereditary or non-hereditary, both of which develop from the mutation of the Rb (RB1) gene.14–16 In the non-hereditary form, inactivation of the RB1 gene alleles causes a defect of the pRB protein, resulting in unilateral tumors.14,15 The presence of tumors in both eyes can occur with heritable Rb.15 A parent carrier of a single mutant allele of the RB1 gene is a hereditary risk factor that predisposes the child to the loss of the second copy by 1,000 times the rate of spontaneous mutation.14 There is a 50% chance that the parent passes the mutation to their child, who then has a 90% chance of developing Rb.17 The hereditary form increases the risk of patient susceptibility to other cancers and requires long-term follow-up, genetic counseling, and monitoring for second cancers.15 The genetic nature of Rb, therefore, is very important in predicting the risk of cancer and guiding treatment.
It has been recently discovered that amplification of the MYCN gene (found only in tumor cells) results in another genetic form of the disease.15,16 Patients with this form are not at risk for second cancers. However, their tumors tend to be larger, invasive, and aggressive; thus, ocular salvage risks high morbidity and treatment failure. For now, enucleation is the most optimal treatment for these cases.15,18
Rb is generally diagnosed based on clinical characteristics at presentation that are found using imaging modalities.5 Benign lesions and end-stage conditions can mimic the disease and lead to unnecessary treatment; thus, careful and accurate identification and staging of the disease is key to guiding patient-based treatment.19–23
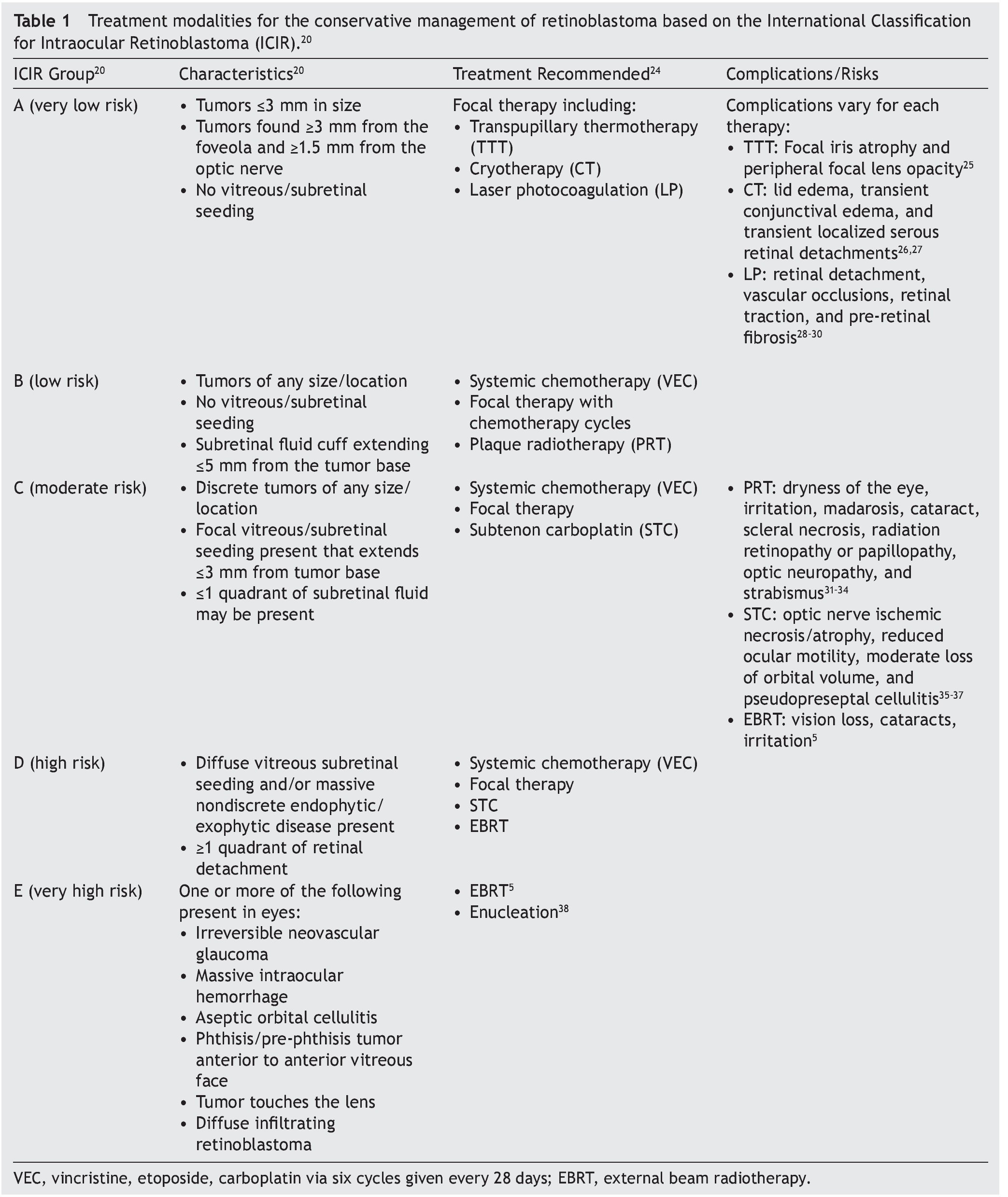
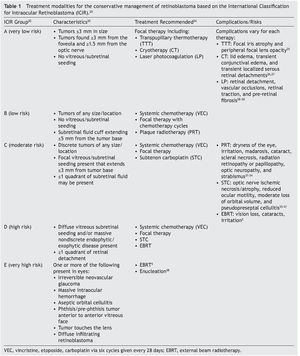
Although there is no universal staging system that considers all risk factors of the disease in the detection process, the International Classification for Intraocular Retinoblastoma (ICIR) has been validated to more accurately predict treatment outcomes.20–22 The ICIR stages Rb in five groups, A through E, with Group A representing more easily treatable eyes of very low risk, and risk progression and complexity of treatment increasing through Group E, the very high-risk eyes.20 The cancer characteristics of each ICIR group are summarized in Table 1 with the recommended treatments and their associated complications and risks. Group A eyes have small tumors ≤3 mm in size, lack vitreous/subretinal seeding, and are located ≥3 mm from the foveola and ≥1.5 mm from the optic nerve. Groups B, C, and D eyes have tumors of any size or location; vitreous/subretinal seeding becomes present in Group C eyes, whereas Group D eyes have massive seeds and retinal attachment can occur. Groups A, B, and C can be managed conservatively by chemotherapy with the objective to salvage the eye, whereas Group D requires intra-arterial chemotherapy (IAC) or intravitreal chemotherapy (IVC). For Group E tumors where co-morbidities such as irreversible glaucoma are present, the tumor approaches the lens of the eye, and massive intraocular hemorrhage is present. The standard of care is enucleation, although IAC may also be used.
Histopathologic evidence of high-risk, metastatic Rb has been found in 17% of Group D and 24% of Group E eyes, indicating that even after enucleation, adjunctive chemotherapy may still be necessary to manage the spread of cancer to other parts of the body.39
3.2. Rb treatment modalities: conservative management
Over the past few decades, advances in genetic technologies, improved staging and classification, and a multidisciplinary team approach to conservative management of Rb have transformed the treatment primary outcome to ocular salvage and preservation of vision in Group A, B, and C cases, in addition to many advanced cases (Group D).5,7,38,40–46 Systemic chemotherapy is commonly used to treat the tumor with ocular salvage rates of 30-90%.45,47 Other conservative modalities are focal consolidation and transpupillary thermotherapy (TTT), laser photocoagulation (LP), cryotherapy (CT), plaque brachytherapy, and local chemotherapy delivered by subconjunctival, subtenon, intravitreal, or intra-arterial routes (Table 1). Combined conservative treatment has been proven to be more effective, in which chemotherapy is initially applied to reduce the tumor size so that local therapies such as CT or TTT can then eliminate the disease.5 Choosing which modalities to use depends on the patient and tumor stage, and the complications and risks vary (Table 1).
In the more advanced cases (Groups E and D), external beam radiotherapy (EBRT) and enucleation are applied. A 10-year retrospective review of the use of systemic chemo-therapy in Sweden at the sole national Rb referral center found that 35% of all eyes required enucleation and EBRT, as well as 91% of eyes with Group C/D tumors.46 Enucleation is the standard of care for when sight preservation is very unlikely or where the tumor may spread to the optic nerve, choroid, or orbit.5,38
3.3. Advanced therapy for Rb: IAC and IVC
Vitreous seeding is the main barrier to successful conservative treatment of advanced Rb.48 Chemoresistant vitreous seeds form in tumor cells after the cells proliferate in the avascular vitreous environment. IAC and IVC are emerging primary therapies to prevent EBRT or enucleation for advanced cases with vitreous seeding in developed countries.48–52 IAC with melphalan/topotecan is now a first-line treatment for Group C and D eyes.52,53 The goal is to achieve tumor regression with minimal local and systemic toxicities. Evidence is, however, limited on the safety and efficacy of IAC.54
Multiple preclinical and clinical studies in Argentina have evaluated the safety and efficacy of intra-arterial melphalan and topotecan.55–57 Schaiquevich et al. reported the first pharmacokinetic study of melphalan after superselective ophthalmic artery infusion (SSOAI) in children with Rb, in addition to evaluating and validating the effect in pigs.55 The authors evaluated the cytotoxicity of melphalan administered with and without topotecan in Rb cell lines of 17 patients and five pigs. The authors previously found that topotecan permeated more efficiently by 5- to 10-fold into the vitreous cavity of the same animal model than melphalan.58 Plasma concentration vs. time profile was similar when corrected by weight in both patients and the animal model.55 There was thus a low systemic exposure to melphalan in the patients. At 4 h post-SSOAI, topotecan concentrations in the vitreous of the pigs remained greater than its IC50; a similar effect was not found in pigs treated solely with melphalan, which appears to permeate inefficiently through the blood-retinal barrier to the vitreous.55,58 The authors suggested that a SSOAI combined regimen of melphalan and topotecan might be a safer alternative to increasing melphalan dosage.55 These findings were further confirmed by the authors in a single-center, prospective study that evaluated the safety and efficacy of combined melphalan (3-6 mg) and topotecan (0.5-1) for 66 cycles SSOAI in 26 patients (27 eyes) with Rb.56 This regimen was administered as primary therapy in five eyes, which all responded favorably and were preserved. In 22 eyes with relapsed or resistant tumors, 16 responded well, whereas three were enucleated after a median of 8 months (range: 7.9-9.1 months). Grade III and IV neutropenia had respective incidence rates of 10.6% and 1.5%, without any fever. Blood transfusion was not required, which further demonstrated a hematologic toxicity profile comparable with single-agent melphalan.56
The same authors next evaluated the effect of SSOAI compared to a historical cohort of sequential periocular and systemic chemotherapy on ocular salvage for 18 patient eyes in 15 consecutive patients that failed chemoreduction and EBRT in a pilot program.57 Three eyes were treated with SSOAI using melphalan alone, four eyes were treated with combined topotecan, carboplatin, and melphalan, and one eye was treated with topotecan and carboplatin without melphalan; all eyes received a median of four cycles of SSOAI (range: 2-9). Periocular topotecan or carboplatin was administered, respectively, to nine and one eyes in a median of two cycles (range: 1-3) followed by intravenous topotecan and cyclophosphamide. All patients survived their treatment without extraocular dissemination or second malignancy and with similar, mild ocular toxicity. Enucleation-free eye survival at 12 months had a probability of 0.87 (95% confidence interval [CI]: 0.42-0.97) for the SSOAI group compared to 0.1 (95% CI: 0.06-0.35) for the periocular group (p <0.01). Systemic toxicity was low for both groups; however, patients treated with intravenous chemotherapy had five episodes of grade 4 neutropenia, three of which required hospitalizations, whereas no such complications occurred in the SSOAI group. Thus, SSOAI was significantly superior and less toxic when compared to periocular and systemic chemotherapy in eyes with relapsed Rb.57
Outside of Argentina, a systematic review on the complication of IAC found that significant complications were uncommon and supports that the risk may be minimized through careful injection and limiting the dosage.59 A more recent retrospective interventional case series found that IAC was an effective primary and secondary treatment with a mean globe salvage rate for Group A-D eyes of 95% and a salvage rate of 36% for Group E eyes.60 The authors observed that treatment failed in eyes with extensive recurrent vitreous seeds. It should be taken into consideration that vascular toxic effects in the eye and orbit have recently been demonstrated in primates after IAC.61 Further research is necessary to confirm and validate that IAC is safe and effective, including a multi-center, prospective trial that would analyze the globe salvage benefits of IAC,48,59 but IAC appears to be appropriate treatment for a select patient population with Rb.62
An alternative therapy for tumors with vitreous seeds, IVC is a regimen of high-dosage chemotherapy and has an ocular salvage rate of 71-95% and a vision salvage rate of 96% (among patients with ocular salvage).63 The presence of vitreous seeding reduces the prognosis of tumor control. IVC with melphalan offers an option for these patients. It has to be done by an experienced group because of eligibility criteria and the high risk of tumor spread, in addition to the risk of retinal detachment following repeated intravitreal applications.58 Francis et al. caution using a dosage of≥30 mg of intravitreal melphalan when vitreous seeds are present, as IVC using melphalan resulted in compromised retinal function in their combined clinical and animal study of 16 patients eye and 12 rabbit eyes.64 However, if the seeds are not injected with melphalan and cryotherapy is applied immediately at the site, risks can be minimal.65
For example, other recent animal and clinical studies in Argentina have evaluated the safety and efficacy of IVC using topotecan58,66,67 and digoxin68 with promising results. As an alternative to melphalan, intravitreal injections of 5 mg of topotecan were administered to rabbits.58 For up to 48 h following administration, high concentrations of topotecan were observed in the vitreous humor, with the respective median maximum vitreous, aqueous, and plasma total topotecan concentrations being 5.3, 0.68, and 0.21 mg/ml. There was evidence of low systemic exposure with total topotecan exposure in the vitreous 50 times greater than the total systemic exposure. Next, the authors tested two different doses of 5 mg vs. 0.5 mg of intravitreal topotecan administered to rabbits in 4 weekly injections to see if a lower dose had a potential therapeutic effect.66 Eyes injected with either dose demonstrated no significant differences in electroretinography wave amplitudes and implicit times in comparison in compared with a control group (p > 0.05). There was no significant histologic damage of the retinas in rabbits treated with topotecan and no other complications observed. Although 4 weekly intravitreal injections of 5 mg or 0.5 mg of topotecan were safe in the rabbits’ eye, lactone topotecan vitreous concentrations in rabbits injected with only 0.5 mg were potentially active only after 5 h.66 The same authors reviewed 42 animal and clinical studies for the ocular pharmacology and antitumor activity of topotecan and campothecins for Rb treatment via different administrative methods.67 Topotecan administered alone or combination via IAC and IVC was effective with minimal ocular toxicity. However, its clinical role and optimal dose and route of administration remain to be determined.67
4. Discussion
This first review of our two-part study has its limitations, as it is meant to be a general overview of the current advances in Rb diagnosis and management. It by no means employs a rigorous or systematic methodology. Part 1 serves as the back drop and context of the current situation of Rb knowledge and programming in Mexico, which will be explored in Part 2.
The key findings for this review of recent literature on the general knowledge and advances of Rb diagnosis and management are summarized in Table 2. Today, provided there is early detection and referral of patients with Rb, this most commonly occurring pediatric cancer is curable. In developed countries, the primary treatment outcome is ocular salvage with sight preservation. Advanced chemotherapeutic options such as IAC and IVC can now save even the most advanced tumors.
Unfortunately, there are gaps in practice and skill in conservative management in lesser developed countries where Rb is often diagnosed after metastasis has occurred.10,47,69–71 In upper-middle income countries, large urban centers may have the latest technology and skilled highly specialized medical professionals, but these services are often not accessible to the population living outside these urban areas.69,71 This is the case in Argentina, an upper-middle income country where more prolific, advanced research on Rb has been done in recent years.55–58,64,66–68 Yet, children living outside of the capital city of Buenos Aires have a significantly higher risk of having Rb.71
In Part 2 of this study, we will examine the literature related to Rb in another upper-middle income country, Mexico, and compare it to the general knowledge presented here in Part 1, analyze the state of Rb programming in the country, and report the patient data currently available at hospitals in Mexico that have formal Rb programs or treat patients with Rb.
Funding
Financing for the study was provided by the International Agency for the Prevention of Blindness/Orbis and the Hamilton Eye Institute of the University of Tennessee.
Conflicts of interest
VCL: paid employee of HelpMeSee, ad-honorem employee of the Instituto Mexicano de Oftalmología and the University of Tennessee, ex-employee of the International Agency for the Prevention of Blindness; KAE: independent consultant to International Agency for the Prevention of Blindness and Strategic Solutions, Inc; BGH: employed by University of Tennessee; BXP: employed by University of Tennessee; MARO: employed by Secretaría de Salud Pública (México); VBC: employed by Secretaría de Salud Pública (México).
Received 27 May 2015;
accepted 4 September 2015
* Corresponding author.
E-mail:marco@unam.mx (M.A. Ramírez-Ortiz).
☆ Please cite this article as: Lansingh VC, Eckert KA, Haik BG, Phillipps BX, Bosch-Canto V, Leal-Leal C, et al. Retinoblastoma in Mexico: part I. A review of general knowledge of the disease, diagnosis, and management. Bol Med Hosp Infant Mex. 2015. http://dx.doi.org/10.1016/j.bmhimx.2015.09.001