Hemospray® is a new device for endoscopic hemostasis used for non-variceal gastrointestinal bleeding. It enables hemostasis and platelet activation by increasing the concentration of clotting factors and forming a mechanical barrier on the wall of a bleeding vessel creating a mechanical plug at the site of bleeding. Within the main indications for use are injuries with difficult endoscopic access, massive gastrointestinal bleeding, multiple bleeding sites, modification of the anatomy by previous endoscopic therapy, presence of coagulopathy, difficulty in having direct visualization or when it is impossible to have contact with the bleeding lesion. However, its use in children has not been approved yet by the FDA. There is a case report of an 11-month-old patient successfully treated with Hemospray® for non-variceal gastrointestinal bleeding.
Clinical caseWe report the case of a 2-year-old female with acute liver failure and primary biliary cirrhosis with portal hypertension and bleeding after sclerotherapy. We analyzed the case to support new therapies for massive bleeding control in post-sclerotherapy esophageal ulcers.
ConclusionsThe application showed to be safe without side effects. Using Hemospray® is an effective alternative in controlling gastrointestinal bleeding.
Hemospray® es un nuevo dispositivo para hemostasia endoscópica utilizado para el sangrado gastrointestinal no variceal. Permite la hemostasia mediante la activación plaquetaria y el aumento de la concentración de factores de coagulación, así como la formación de una barrera mecánica sobre la pared de un vaso sangrante creando un tapón mecánico en el sitio de sangrado. Dentro de las principales indicaciones para su uso se encuentran lesiones de difícil acceso endoscópico, hemorragia gastrointestinal masiva, múltiples sitios de sangrado, modificación de la anatomía por terapia endoscópica previa, presencia de coagulopatía, dificultad para tener visualización directa o cuando es imposible tener contacto con la lesión sangrante. Sin embargo, su uso en niños aún no ha sido aprobado por la FDA. Existe un caso publicado de un paciente de 11 meses tratado exitosamente con Hemospray® por hemorragia gastrointestinal no variceal.
Caso clínicoSe reporta el caso de una paciente de 2 años con falla hepática aguda y cirrosis biliar primaria con hipertensión portal y sangrado post-escleroterapia. Se analizó el caso para sustentar las nuevas terapias para el control del sangrado masivo en úlceras post-escleroterapia.
ConclusionesLa aplicación demostró ser segura y sin efectos adversos. El uso de Hemospray® es una alternativa efectiva en el control del sangrado gastrointestinal.
TC-325 (trade name Hemospray®) is a new device for endoscopic hemostasis that belongs to the family of the so-called hemostatic powders, which consist mainly of small mineral granules that stimulate hemostasis by activating platelets and increasing the concentration of coagulation factors. Also, they form a mechanical barrier on the wall of the bleeding vessel creating a fibrin plug in the bleeding site.1,2 They are biologically inert and readily washable in a period of 12 to 24h and do not present a risk for toxicity since they are not absorbed or metabolized by the tissue mucosa. In Europe and Canada, they are used mainly to stop non-variceal gastrointestinal bleeding.
Among the main indications for its use are lesions with difficult endoscopic access, malignant gastrointestinal bleeding, massive gastrointestinal bleeding and unexperienced endoscopists.2,3
Hemospray® use is ideal when it is not feasible to perform an endoscopy due to multiple bleeding sites, particularly in those patients whose anatomy is modified by previous endoscopic therapy or those who present a coagulopathy that complicates direct visualization or prevents direct contact with the endoscope since it does not require direct contact with the bleeding lesion, and its application may cover large areas of the bleeding site.4–7 However, its use in children has not yet been approved by the FDA. A case series reported hemostasis in 85% of patients treated with Hemospray® as monotherapy, with re-bleeding in 15% within 7 days of application. A case of an 11-month patient successfully treated for non-variceal gastrointestinal hemorrhage with Hemospray® has been reported.8
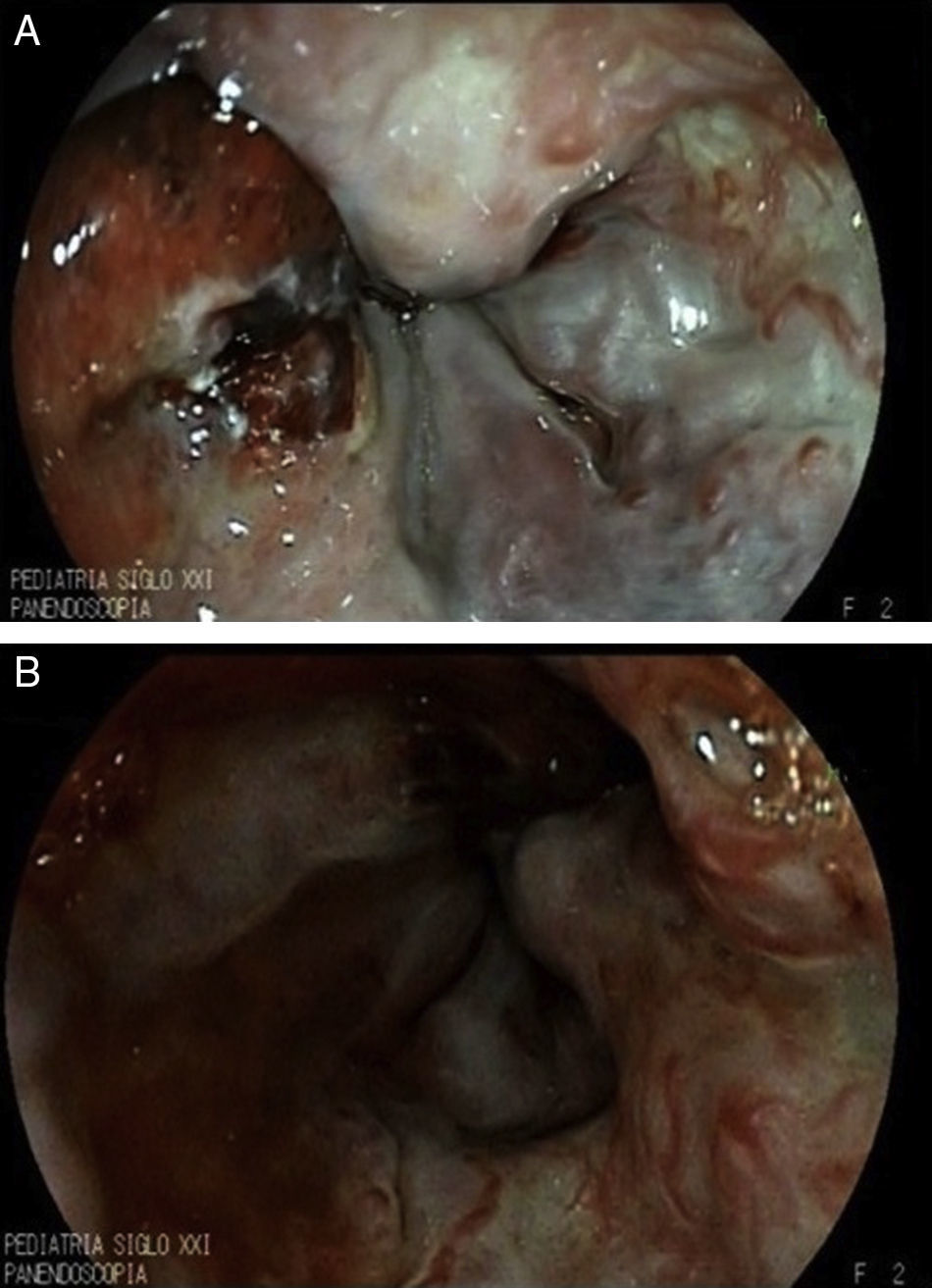
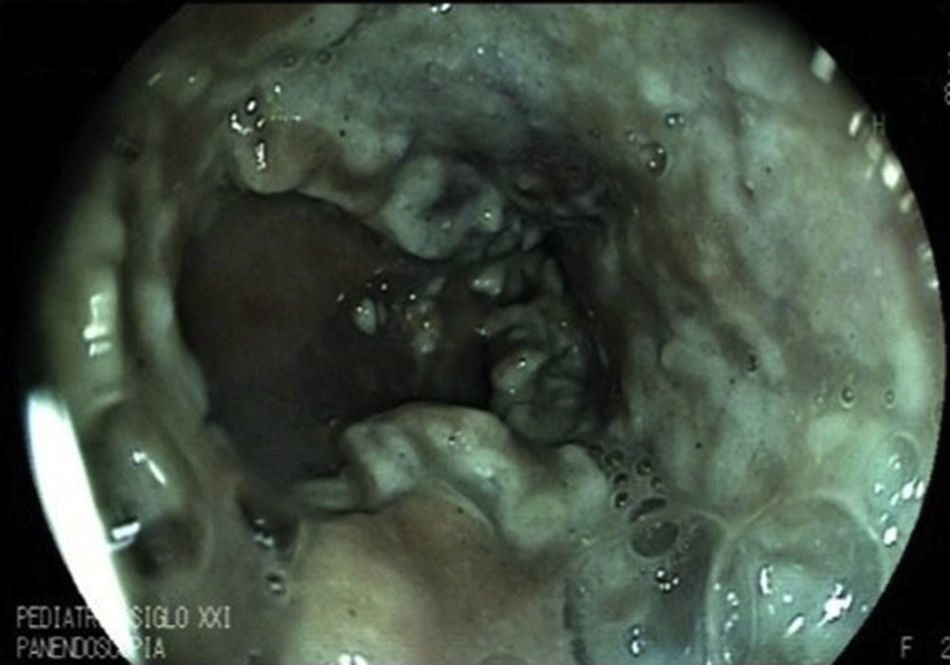
2Clinical caseWe present the case of a 2-year-old patient referred to the Gastroenterology service with liver failure secondary to primary biliary cirrhosis and severe portal hypertension. A prophylactic panendoscopy was performed and large esophageal varices were found. Sclerotherapy was performed with 1.5% polidocanol, and secondary prophylaxis was initiated. Thirty days after the endoscopy, the patient presented with hematemesis and melena accompanied by hemodynamic alterations and a 4g decrease in hemoglobin (Hb) for which a second endoscopy was performed. Three large esophageal varices were found and treated with sclerotherapy, as well as gastric varices GOV1. She entered the transplant list (PELD 40) due to progressive deterioration of liver function. However, 16 days after the second endoscopy, another bleeding event with a decrease of 5g of Hb presented. Despite the use of an octreotide infusion for 72h, bleeding did not decrease, so an emergency panendoscopy was performed and active bleeding of previous post-sclerotherapy ulcer (Figure 1A) that complicated full visualization (Figure 1B) was found. Hemospray® (20g) was administered in a single application achieving bleeding control without adverse effects (Figure 2), allowing an enteral diet. The patient remained stable for 40 days. Subsequently, massive hematemesis caused her death.
3DiscussionUpper gastrointestinal bleeding in children with small and large esophageal varices occurs in 60% and 100%, respectively. Some randomized controlled trials show the efficacy of prophylaxis with sclerotherapy or ligation to prevent variceal bleeding.9 However, ligation is impossible in infants and toddlers with malnutrition since the introduction of the band ligator through the esophagus is technically difficult, which makes sclerotherapy the only possible alternative treatment.9,10
It should be remembered that multiple sessions of variceal ligation or sclerotherapy modify the anatomy of patients. For this reason, Hemospray® has been useful in the management of acute bleeding emergencies in patients who develop post-sclerotherapy ulcers as a bridge to definitive therapy since great endoscopic dexterity is not required. A successful Hemospray® treatment without any adverse events has been reported in adult population with variceal and non-variceal massive bleeding refractory to conventional treatment.11,12 In this case, Hemospray® proved to be effective and safe.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe authors’ own resources. Children's Hospital, SXXI National Medical Center, Mexican Institute of Social Security, Mexico City, Mexico.
Conflict of interestThe authors declare no conflict of interest of any nature.
Please cite this article as: González Ortiz B, Tapia Monge DM, Reyes Cerecedo A, Hernández Mondragón O. Uso de Hemospray® en sangrado post-escleroterapia. Bol Med Hosp Infant Mex. 2016;73:335–337.