Bariatric surgery continues to be the best treatment for weight loss and control of obesity related comorbidities. Gastric bypass and sleeve gastrectomy have demonstrated to be the most effective surgeries, but this has not been established in a Mexican (non-American) population.
ObjectiveTo analyse the improvement in type 2 diabetes mellitus and carbohydrate intolerance in obese patients after bariatric surgery.
Material and methodsA retrospective analysis was performed on the data collected prospectively between 2013 and 2015 on every obese patient with diabetes and carbohydrate intolerance submitted for bariatric surgery. Analysis was performed at baseline, and at 1, 3, 6, 9 and 12 months, and included metabolic, clinical, lipid, and anthropometrical parameters. A peri-operative and morbidity and mortality analysis was also performed. Remission rates for patients with diabetes were also established.
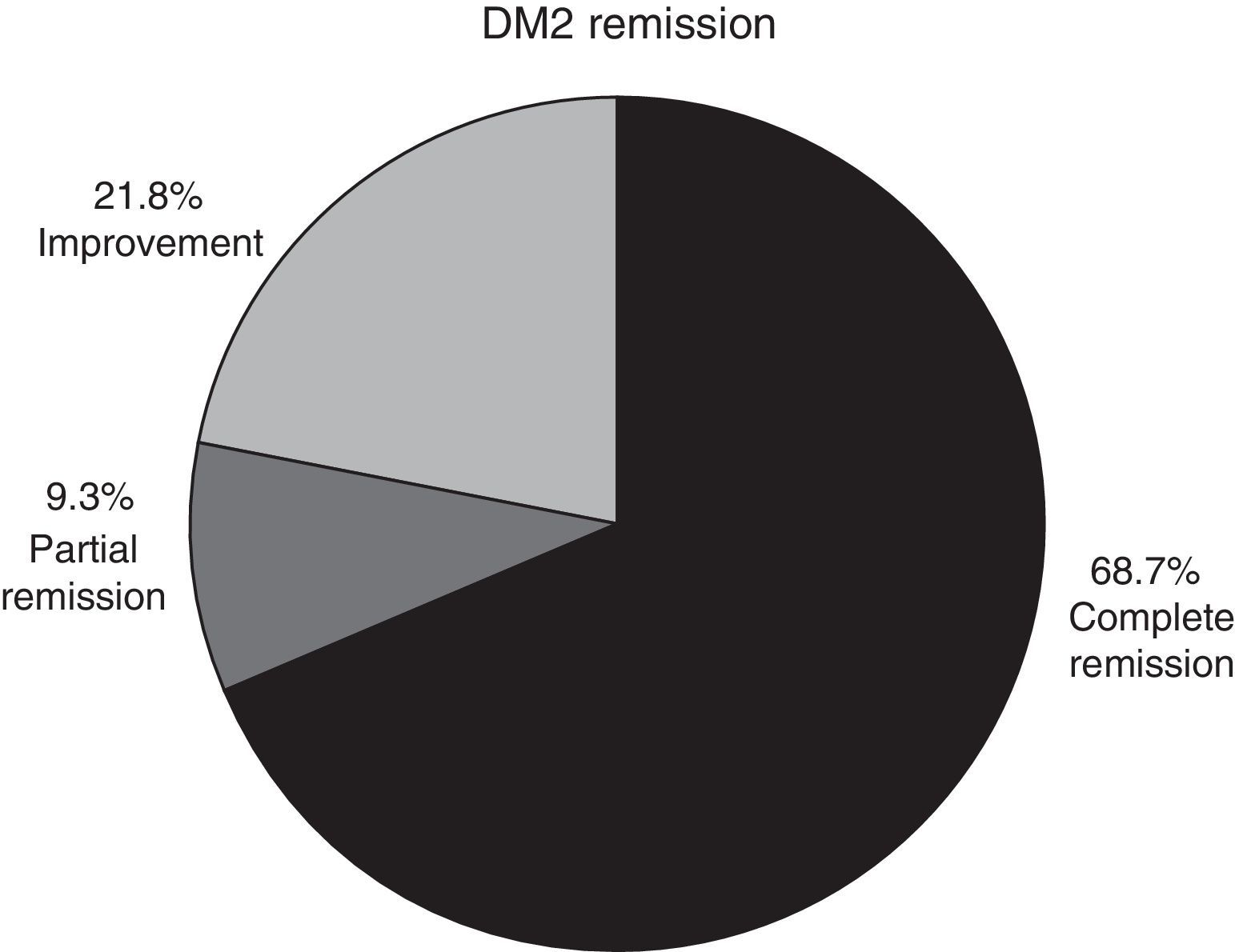
ResultsThe analysis included 73 patients, 46 with diabetes and 27 with carbohydrate intolerance. Sixty-two patients were female with a mean age of 42 years. Baseline glucose and glycosylated haemoglobin were 123±34mg/dl and 6.8±1.6%, and at 12 months they were 90.1±8mg/dl and 5.4±0.3%, respectively. Diabetes remission was observed in 68.7% of patients, including 9.3% with partial remission and 21.8% with an improvement. There was also a significant improvement in all metabolic and non-metabolic parameters.
ConclusionsBariatric surgery safely improves the metabolic status of patients with diabetes mellitus or carbohydrate intolerance during the first year, inducing high rates of complete remission. It has also shown a significant improvement on blood pressure, lipid, and anthropometric parameters during the first year of follow-up.
La cirugía bariátrica es el mejor método para la pérdida de peso y control de comorbilidades asociadas a la obesidad. El bypass y la manga gástrica han demostrado la mayor efectividad; sin embargo, no existen estudios de alto impacto en la población mexicana.
ObjetivoAnalizar la mejora de la diabetes mellitus 2 y la intolerancia a los carbohidratos en pacientes con obesidad sometidos a cirugía bariátrica.
Material y métodosEstudio retrospectivo en el que se analizaron los expedientes de pacientes con diabetes e intolerancia a los carbohidratos asociados a obesidad y que fueron sometidos a cirugía entre 2013-2015. Se realizó un análisis (0, 1, 3, 6, 9 y 12 meses) de los parámetros metabólicos, clínicos, lipídicos y de peso, así como un análisis perioperatorio y de morbimortalidad. Se establecieron cifras de remisión de diabetes.
ResultadosSe analizaron 73 pacientes (46 con diabetes y 27 con intolerancia a los carbohidratos). Sesenta y dos fueron mujeres, con un promedio de edad de 42 años. La glucosa y hemoglobina glucosilada iniciales fueron de 123±34mg/dl y de 6.8±1.6%, y a los 12 meses de 90.1±8mg/dl y de 5.4±0.3%, respectivamente. El 68.7% de los pacientes presentó una remisión completa, el 9.3% una remisión parcial y el 21.8% una mejoría de la diabetes. Durante todo el seguimiento mejoraron significativamente todos los parámetros metabólicos y no metabólicos.
ConclusionesLa cirugía bariátrica mejora eficazmente el estado metabólico de los pacientes con diabetes o con intolerancia a los carbohidratos en el transcurso del primer año, lo que induce a altas tasas de remisión completa. La cirugía incide en una mejoría de la presión arterial, los parámetros lipídicos y los antropométricos.
The prevalence of obesity and overweight has increased progressively and alarmingly in the worldwide population, and constitutes a serious national health problem. The relationship between obesity and type 2 diabetes mellitus is well known and affects between 50% and 65% of the worldwide population, with a projected annual increase of 1.5%–2% in Latin American countries.1,2 In Mexico overweight and obesity has been increasing to such an extent that in 2012 it was reported that both conditions affected 71.2% of the population. The prevalence of type 2 diabetes mellitus has increased similarly and continues to rise. It rose from 5.8% in 2000 to 9.4% in 2012.3
Treatment of type 2 diabetes mellitus consists of blood sugar goals, i.e. the patient would have to obtain a glycosylated haemoglobin value under 7% or of 6.5%, depending on different authors’ interpretations.1,2 Current guidelines established by different national and international associations are based on healthcare management and changes in lifestyle, with acknowledgement in recent years of bariatric surgery as a therapeutic option.4 Several studies (randomised, observational, meta-analysis and systematic reviews)5–9 have demonstrated that the gastric bypass and laparoscopic sleeve gastrectomy procedures significantly improve glycaemic control and reduce cardiovascular risks by a greater margin than medical care treatment.6,10
It has also been reported that the prevalence of type 2 diabetes mellitus after bariatric surgery remains stable for a period of 8 years (10.7% initial and 10.5% final) in contrast to an increases in prevalence during the same period for patients receiving medical care management (7.8% initial and 24.9% final).11 The impact of this type of surgery in the Mexican population has not been reported, since it has only been reported for Hispano American population.
ObjectiveThe aim of this study was to analyse the impact of bariatric surgery (gastric bypass and sleeve gastrectomy), in patients diagnosed with type 2 diabetes mellitus or carbohydrate intolerance, to establish remission or improvement in the Mexican population.
Material and methodsA retrospective, observational and cohort study was conducted, where analysis was performed on the data collected for all patients who underwent bariatric surgery, in a single hospital, from 1st January 2013 to 1st January 2015, and who had been diagnosed with type 2 diabetes mellitus or carbohydrate intolerance. Data collection was performed prospectively and patients whose record was incomplete at any time of the analysis period were excluded.
The impact of the bariatric surgery in this study was analysed according to the following metabolic parameters: glucose, glycosylated haemoglobin, C-peptide, insulin and the use of drugs preoperatively and at 1, 3, 6, 9, and 12 follow-up months postoperatively in both groups (type 2 diabetes mellitus and carbohydrate intolerance). The percentage of remission or improvement in the patients with 12 months follow-up was also analysed. The following types of analysis were performed: demographic, regarding comorbidities, perioperative and early morbidity mortality (<30 days). Finally, the evolution of weight was also reported, as was body mass index (BMI), percentage of excess weight loss (EWL %) and lipid parameters such as total cholesterol, triglycerides, high density lipoproteins and low density lipoproteins.
No comparative analysis was performed between the different types of surgery due to the low number of patients who underwent sleeve gastrectomy.
DefinitionsRemission definitions and improvement of type 2 diabetes mellitus were based on criteria from the Americana Diabetes Association, ADA) from 2009.
Partial remission. Hyperglycaemia below the threshold of type 2 diabetes mellitus (100–125mg/dl), glycosylated haemoglobin <6.5%, and absence of any pharmacological treatment, for at least one year.
Complete remission. Normal glycaemia (<100mg/dl), with glycosylated haemoglobin <5.7% and without any pharmacological treatment, for at least one year.
Improvement. Reduction in the number of drugs and/or dose (including insulin), associated with glucose level control.
Bariatric surgery was performed on the patients in accordance with established international guidelines12 and the NOM-008-SSA3-2010: patients between 18 and 60, with a BMI>35kg/m2, with associated comorbidity, or with a BMI>40kg/m2 with no comorbidity. The patients had been previously assessed by a multidisciplinary team which evaluated: attachment to treatment, psychological assessment and preoperative weight loss. This was to decide whether they should undergo surgery or not and what the best option would be.
Surgical techniqueThe “simplified” Roux-en-y laparoscopic gastric bypass was performed,”13 with an antecolic loop and a mechanical calibrated 2cm anastomosis; the biliary and alimentary loops measured 50 and 150cm respectively. A division was made between the greater omentum and both mesenteric spaces were closed. The methylene blue dye test was performed systematically and a drain was inserted.
Laparoscopic sleeve gastrectomy was performed at 5–6cm distance from the pylorus, with a 36Fr calibration. Oversew was used along the line of staples in all cases with non absorbable (2–0 polypropylene) suture. The methylene blue dye test was performed systematically and a drain was inserted.
Statistical analysisFindings were expressed as a mean±standard deviation (SD) or in percentage, depending on the variable. The Student's t-test was performed both for the dependent and independent samples, depending on the case. The categorical variables were compared with the χ2 test for distribution. A p-value of 0.05 was considered statistically significant. Statistical analysis was performed with the NCSS 2007 (NCSS, Kaysville, Utah, U.S.A.).
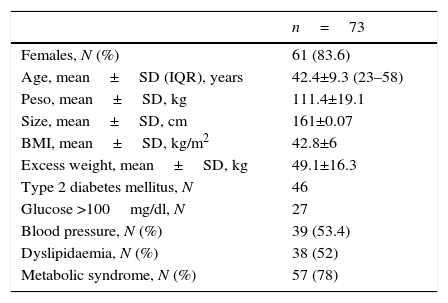
ResultsDuring the 2-year analysis period, 243 bariatric operations were performed. 46 patients (either with previous diagnosis or new diagnosis) were diagnosed with type 2 diabetes mellitus, and 27 patients with carbohydrate intolerance, accounting for a prevalence of 18.9% and 10.6% in our sample, respectively. Out of the total patients who underwent bariatric surgery, 61 were female (83.6%) and 12 were male (16.4%), with a median age of 42 (range between 25 and 48). The average preoperative weight was 111.4±19.1kg/m2 and with a BMI of 42.8±6kg/m2. Baseline parameters analysed are listed in Table 1.
Initial demographic, metabolic and comorbidity analysis.
| n=73 | |
|---|---|
| Females, N (%) | 61 (83.6) |
| Age, mean±SD (IQR), years | 42.4±9.3 (23–58) |
| Peso, mean±SD, kg | 111.4±19.1 |
| Size, mean±SD, cm | 161±0.07 |
| BMI, mean±SD, kg/m2 | 42.8±6 |
| Excess weight, mean±SD, kg | 49.1±16.3 |
| Type 2 diabetes mellitus, N | 46 |
| Glucose >100mg/dl, N | 27 |
| Blood pressure, N (%) | 39 (53.4) |
| Dyslipidaemia, N (%) | 38 (52) |
| Metabolic syndrome, N (%) | 57 (78) |
DE: standard deviation; BMI: body mass index; N: number; IQR: interquartile range.
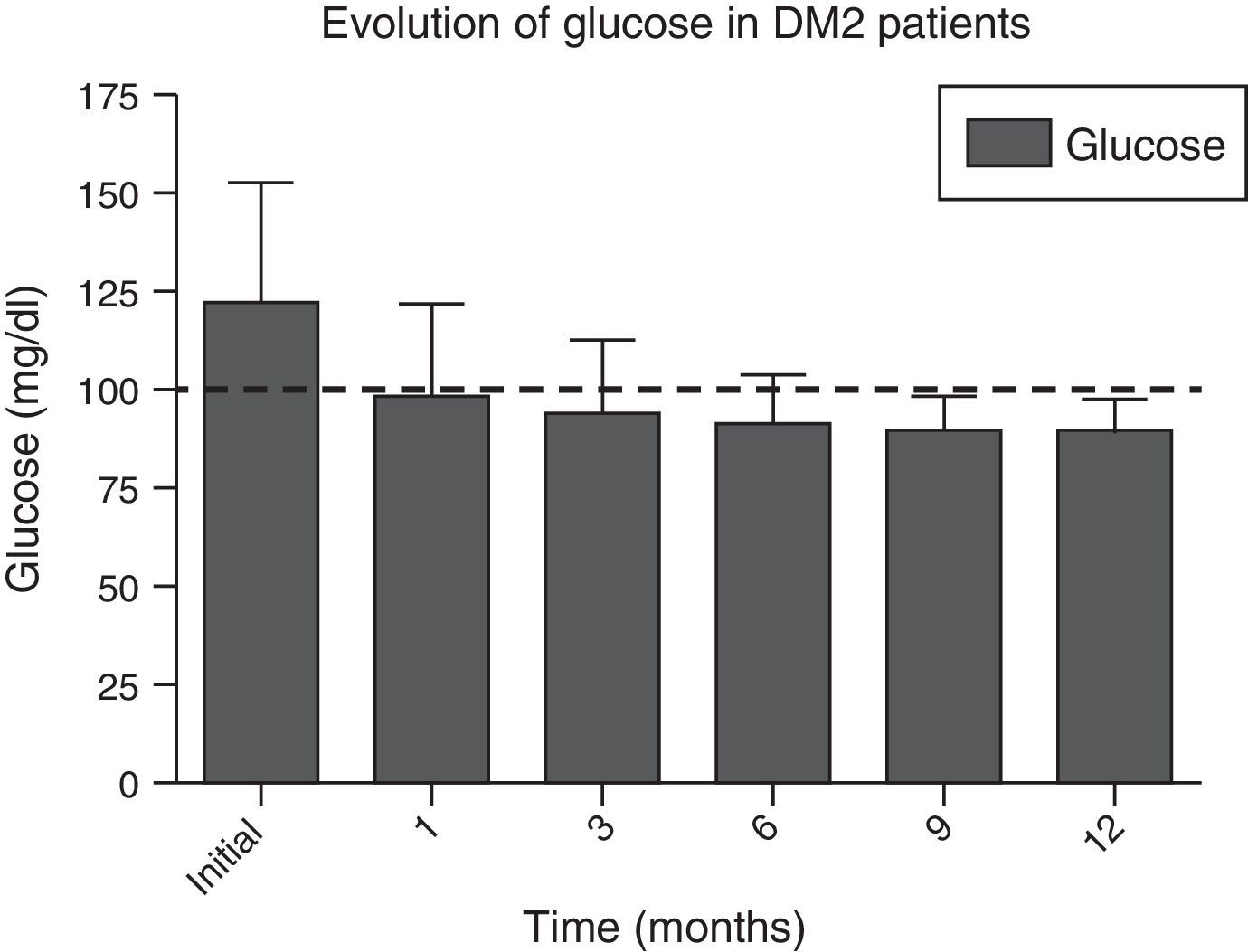
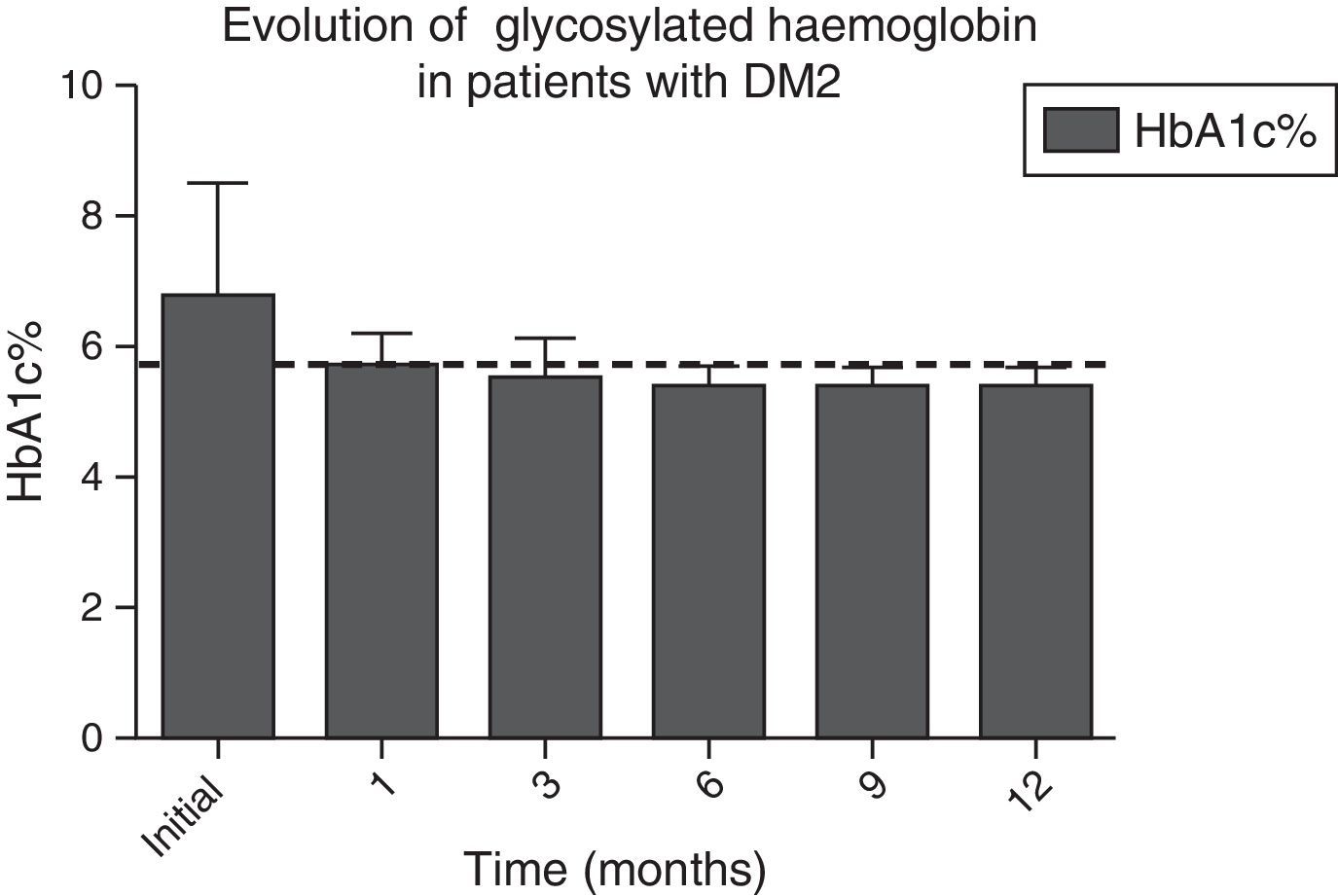
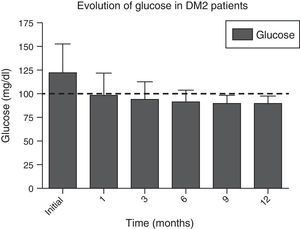
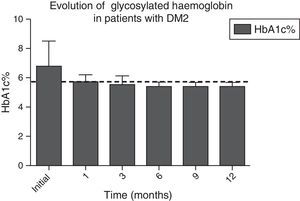
In Figs. 1 and 2, a significant improvement was observed for fasting blood glucose levels and glycosylated haemoglobin levels in all patients, from the first month after surgery, with levels staying below the ADA criteria during the whole of the follow-up period, in the majority of cases, The average preoperative glucose levels were 123±34mg/dl; after 6 months they were 91±11.9mg/dl, after 12 months they were 90.1±8mg/dl. The initial average glycosylated haemoglobin level was 6.8±1.6%; at the sixth month it was 5.5±0.3%, and at 12 months it was 5.4±0.3%.
Evolution of HbA1c% levels during the whole follow-up period. The dotted line indicates the limit established by the ADA for the remission of the diagnosis of type 2 diabetes mellitus.
ADA: American Diabetes Association; DM2: type 2 diabetes mellitus; HbA1c%: percentage of glycosylated haemoglobin.
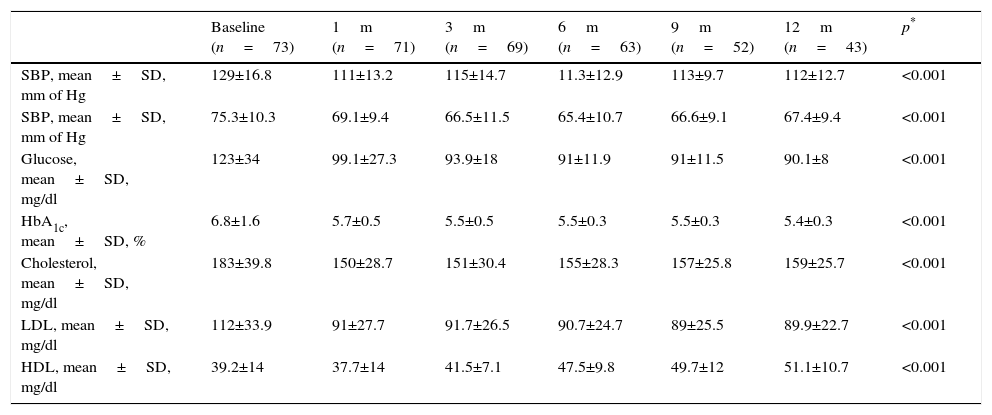
Overall analysis of the evolution of clinical and biochemical parameters are contained in Table 2, where a significant improvement in all of them at 12 months may be found.
Clinical and biochemical evolution of all patients.
| Baseline (n=73) | 1m (n=71) | 3m (n=69) | 6m (n=63) | 9m (n=52) | 12m (n=43) | p* | |
|---|---|---|---|---|---|---|---|
| SBP, mean±SD, mm of Hg | 129±16.8 | 111±13.2 | 115±14.7 | 11.3±12.9 | 113±9.7 | 112±12.7 | <0.001 |
| SBP, mean±SD, mm of Hg | 75.3±10.3 | 69.1±9.4 | 66.5±11.5 | 65.4±10.7 | 66.6±9.1 | 67.4±9.4 | <0.001 |
| Glucose, mean±SD, mg/dl | 123±34 | 99.1±27.3 | 93.9±18 | 91±11.9 | 91±11.5 | 90.1±8 | <0.001 |
| HbA1c, mean±SD, % | 6.8±1.6 | 5.7±0.5 | 5.5±0.5 | 5.5±0.3 | 5.5±0.3 | 5.4±0.3 | <0.001 |
| Cholesterol, mean±SD, mg/dl | 183±39.8 | 150±28.7 | 151±30.4 | 155±28.3 | 157±25.8 | 159±25.7 | <0.001 |
| LDL, mean±SD, mg/dl | 112±33.9 | 91±27.7 | 91.7±26.5 | 90.7±24.7 | 89±25.5 | 89.9±22.7 | <0.001 |
| HDL, mean±SD, mg/dl | 39.2±14 | 37.7±14 | 41.5±7.1 | 47.5±9.8 | 49.7±12 | 51.1±10.7 | <0.001 |
SD: standard deviation; HbA1c: glycosylated haemoglobin; HDL: high density lipoproteins; LDL: low density lipoproteins; SDP: diastolic blood pressure; SBP: systolic blood pressure.
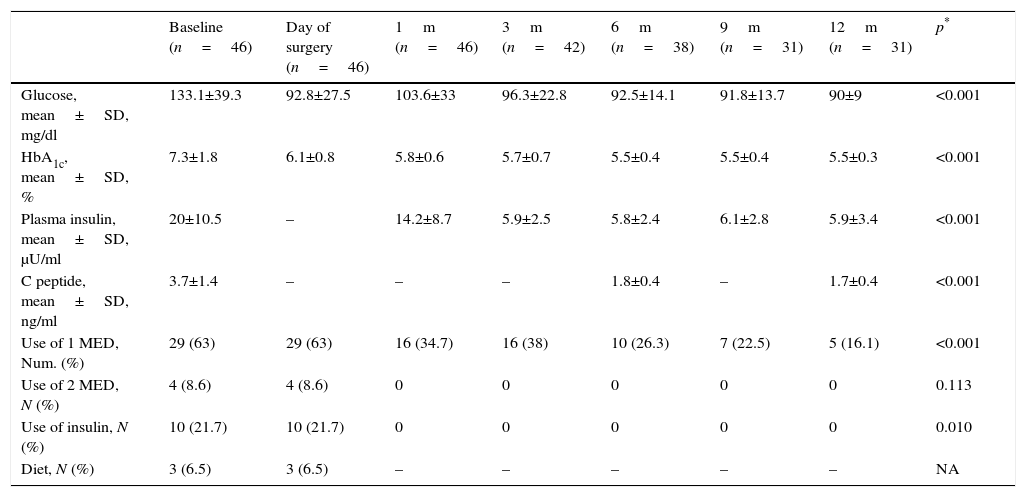
Independent analysis was made of the patients’ evolution with type 2 diabetes and carbohydrate intolerance (Tables 3 and 4). Patients diagnosed with type 2 diabetes mellitus presented with an initial average level of glucose of 133.1±39.3mg/dl and of glycosylated haemoglobin of 7.3±1.8%, with the latter falling significantly during the first few months and remaining up to 12 months with glucose values being recorded as 90±90mg/dl and glycosylated haemoglobin as 5.5±0.3%. A statistically significant reduction of the other metabolic parameters analysed (Table 3) was also observed. In this group in over half of the patients (63%) a hypoglycaemic therapeutic drug was used initially, which was reduced after 6 months to 26.3%, and after one year only 16.1% continued taking the prescribed hypoglycaemic drug. Similarly, 21.7% of the diabetic patients had been administered insulin, which was interrupted from the first postoperative months in all cases, and persisted as such during the whole follow-up period.
Metabolic evolution and use of drugs of patients diagnosed with type 2 diabetes mellitus.
| Baseline (n=46) | Day of surgery (n=46) | 1m (n=46) | 3m (n=42) | 6m (n=38) | 9m (n=31) | 12m (n=31) | p* | |
|---|---|---|---|---|---|---|---|---|
| Glucose, mean±SD, mg/dl | 133.1±39.3 | 92.8±27.5 | 103.6±33 | 96.3±22.8 | 92.5±14.1 | 91.8±13.7 | 90±9 | <0.001 |
| HbA1c, mean±SD, % | 7.3±1.8 | 6.1±0.8 | 5.8±0.6 | 5.7±0.7 | 5.5±0.4 | 5.5±0.4 | 5.5±0.3 | <0.001 |
| Plasma insulin, mean±SD, μU/ml | 20±10.5 | – | 14.2±8.7 | 5.9±2.5 | 5.8±2.4 | 6.1±2.8 | 5.9±3.4 | <0.001 |
| C peptide, mean±SD, ng/ml | 3.7±1.4 | – | – | – | 1.8±0.4 | – | 1.7±0.4 | <0.001 |
| Use of 1 MED, Num. (%) | 29 (63) | 29 (63) | 16 (34.7) | 16 (38) | 10 (26.3) | 7 (22.5) | 5 (16.1) | <0.001 |
| Use of 2 MED, N (%) | 4 (8.6) | 4 (8.6) | 0 | 0 | 0 | 0 | 0 | 0.113 |
| Use of insulin, N (%) | 10 (21.7) | 10 (21.7) | 0 | 0 | 0 | 0 | 0 | 0.010 |
| Diet, N (%) | 3 (6.5) | 3 (6.5) | – | – | – | – | – | NA |
SD: standard deviation; HbA1c: glycosylated haemoglobin; MED: medication; N: number.
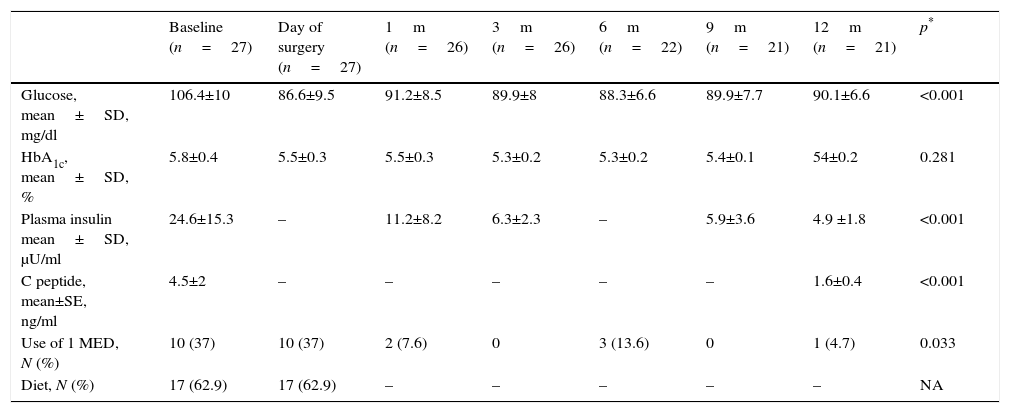
Metabolic evolution and use of drugs of patients diagnosed with HC intolerance.
| Baseline (n=27) | Day of surgery (n=27) | 1m (n=26) | 3m (n=26) | 6m (n=22) | 9m (n=21) | 12m (n=21) | p* | |
|---|---|---|---|---|---|---|---|---|
| Glucose, mean±SD, mg/dl | 106.4±10 | 86.6±9.5 | 91.2±8.5 | 89.9±8 | 88.3±6.6 | 89.9±7.7 | 90.1±6.6 | <0.001 |
| HbA1c, mean±SD, % | 5.8±0.4 | 5.5±0.3 | 5.5±0.3 | 5.3±0.2 | 5.3±0.2 | 5.4±0.1 | 54±0.2 | 0.281 |
| Plasma insulin mean±SD, μU/ml | 24.6±15.3 | – | 11.2±8.2 | 6.3±2.3 | – | 5.9±3.6 | 4.9 ±1.8 | <0.001 |
| C peptide, mean±SE, ng/ml | 4.5±2 | – | – | – | – | – | 1.6±0.4 | <0.001 |
| Use of 1 MED, N (%) | 10 (37) | 10 (37) | 2 (7.6) | 0 | 3 (13.6) | 0 | 1 (4.7) | 0.033 |
| Diet, N (%) | 17 (62.9) | 17 (62.9) | – | – | – | – | – | NA |
SD: standard deviation; HbA1c: glycosylated haemoglobin; HC: carbohydrates, MED: drug; N: number.
With regard to the group of patients with carbohydrate intolerance (Table 4), an improvement in metabolic values from the first month after surgery was observed, which continued during the whole of the follow-up period. After 12 months there had been an 80.9% improvement in patients who had completed said follow-up. Only 4 patients continued with pharmacological and/or glucose treatment >100mg/dl.
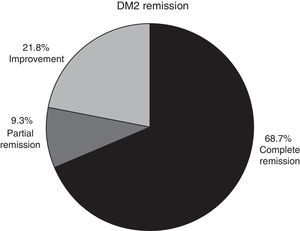
In patients with type 2 diabetes mellitus who concluded a whole year of follow-up and whose medical record was intact, complete remission was observed in 68.7% of cases, partial remission in 9.3% and improvement in 21.8% (Fig. 3).
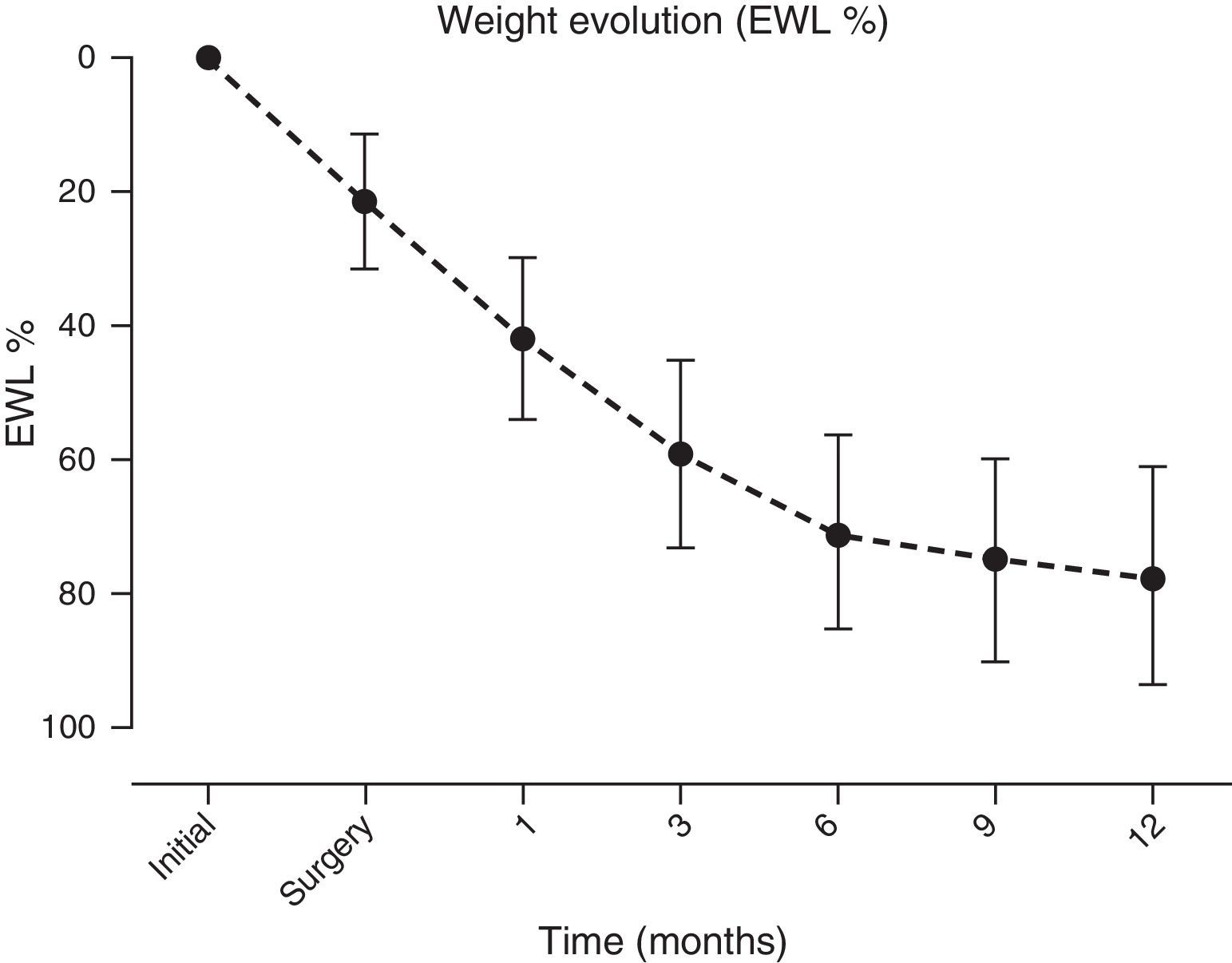
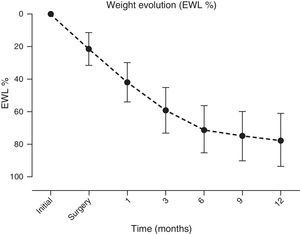
A significant and sustained weight loss was present in patients throughout the follow-up period, with an EWL % at year end of 77.5±16.2 (initial BMI of 42.8±6kg/m2vs. 12 months of 28.9±4.3kg/m2, p≤0.001) (Fig. 4).
On analysis of biochemical parameters corresponding to lipid profile, a significant and sustained improvement was also observed in each patient (Table 3).
Analysis of the evolution of albumin and protein levels as nutritional parameters showed no change in albumin. However, a significant reduction in protein levels was detected (7.3 vs. 7.1 at 6 months; p<0.001), within the normal range. As a result there was no clinical significance.
Perioperative and morbidity mortality analysis revealed 70 gastric bypasses (95%) and 3 gastric sleeve surgical interventions (5%). Time in surgery was 167.5±39min, and there was no conversion to open surgery. There were 10 (13.6%) early (<30 days) complications. Out of the total complications, 4 were minor and 6 major. In the case of major complications the following was observed: 1 paroxysmal auricular fibrillation; 1 incarcerated umbilical hernia which was resolved with surgery; 2 moderate stenosis of gastrojejunal anastomosis which each required endoscopic dilatation; 1 case of melaena with a transfusion of two red blood units, and 1 laparascopic reintervention for infected residual haematoma. There was no leakage, fistula or mortality. The average hospital stay was from 3.1±0.6 days, taking into account that patients were hospitalised 1 day prior to surgical intervention.
DiscussionIn this retrospective study, based on 73 obese patients submitted to bariatric surgery with a diagnosis of type diabetes mellitus or carbohydrate intolerance, we observed a significant improvement after surgery in all metabolic parameters and non metabolic parameters analysed. Said improvement was sustained throughout follow-up, and complete remission was obtained in 68.7% of cases with type 2 diabetes mellitus; the findings were comparable to several studies conducted (the majority with a non Latin population).8,9,11
Prior to bariatric surgery, in 1955, Friedmann et al.14 reported 3 cases of diabetic patients who underwent subtotal gastrectomy for peptic ulcers, which showed a reduction or elimination of the therapeutic requirements for insulin to control their disease. Years later, with knowledge of the procedures used in patients with morbid obesity, Poires et al.15 demonstrated that bariatric surgery has a long-term effect in the resolution of type 2 diabetes mellitus, with improvement in the clinical manifestations and laboratory parameters in these patients, in over 90% of them; they even observed that before leaving hospital these patients already had better glycaemic control than before being operated on. These results were not well accepted by the medical community due to the retrospective and non specific nature of the study. However, after many posterior studies, it was not until 2009 that a systematic and meta-analysis review was made with 621 studies which demonstrated the impact of bariatric procedures for type 2 diabetes mellitus management, associated with obesity.16 In this study, overall “resolution” rates of 78% were found, but this concept remained unclear and used the definition previously established by the ADA: normal fasting blood sugar levels or glycosylated haemoglobin levels under 6%.17
Following the modification of remission criteria in 2009,18 reported percentages were lowered, as demonstrated by Pournaras et al.17 in a study conducted in 160 patients who underwent gastric bypasses, where the old and new definition was compared and showed a reduction in remission rates of 57.5 to 40.6% 23 months after follow-up. The adaptation of said criteria by the ADA was the beginning of formal acknowledgement of bariatric surgery as an effective option in the treatment of type 2 diabetes mellitus associated with obesity, and management algorithms of several international associations were even incorporated. 14,19
Short-term surgical outcome (particularly with gastric bypass) is better than that of a conventional approach.6,10 However, there is a considerable variability in remission and improvement percentages. The difference between studies (including meta-analysis, systemic reviews and randomised clinical trials) is a result of the remission and improvement criteria used by the authors. Several recent studies established short-term remission rates ranging from 43.2% to 75%.20–22Although almost all current definitions agree with the suspension of medical treatment and glycaemia levels <100mg/dl during the first year, the discrepancy comes from the cut-off point of the glycosylated haemoglobin levels. Latest recommendations show a glycosylated haemoglobin level of <5.7% as treatment goal.20,23 However, few studies are based on this figure since in general the parameters of 6%–6.5% are used.21 It is important to mention that glycosylated haemoglobin higher than 5.7% is associated with a high risk of type 2 diabetes mellitus, cardiovascular disease and mico/macrovascular disease.24 We therefore consider it reasonable to use this parameter as a cut-off point to define remission. In our study, the remission rate at 12 months was almost 69%, and was comparable with previous reports from studies in other population samples.25
There are many reasons and theories why bariatric surgery has such a profound and lasting impact, beyond simple weight loss as the primary aetiology of the improvement or control of the hyperglycaemic status. The already established causes include regularisation of several metabolic sequences, beta cell function and insulin sensitivity, together with intestinal absorption of glucose which is beneficial for glycaemic control and is potentially synergic.26 Several significant changes in dietary habits also promote the selection of healthy foods,27 associated with postoperative changes in taste and smell,28 and with neural responses in the brain's satiety centres. Neuro-hormonal mechanisms also play a major role, where they are linked to a peptide which is similar to type 1 glucagon (GLP-1) and peptide YY (PYY), which increase their production after sleeve gastrectomy and/or gastric bypass, and particularly after the latter. At the same time, the concentrations of hunger and satiety hormones change, which has an influence on calorie intake.26
Regarding long-term outcome, a study recently reported the evolution of 78 patients who had undergone a gastric bypass, with a follow-up of 11 years. Complete remission was observed in 52.6% of cases and partial remission in 6.4% of cases. In the said study, overall recurrence of the disease was 15.4%. Furthermore, the microvascular complication rate was 11.5% and macrovascular was 5%. For those patients where there was no early remission of type 2 diabetes mellitus, an 8.8 times bigger risk of microvascular complications occurred, compared with the patients in remission. The authors related the evolution time of the diabetes mellitus and high levels of glycosylated haemoglobin and insulin in the preoperative period to a lower remission rate and increase in the risk of presenting with vascular complications.29
Further randomised and controlled studies need to be conducted aimed at better results, with regard to certain selection criteria, as there has been a recurrence or poor response in relation to the following parameters: advanced age, high BMI, high glycosylated haemoglobin levels, number of drugs and/or insulin requirements in the preoperative period,30 as well as low C peptide levels.31
One of the greatest concerns with regard to the implementation of these interventions are surgical complications, particularly short term ones (<30 days), even with possible fatal consequences in several cases. This interpretation has developed in accordance with experience and with the appearance of laparoscopic surgery. The morbidity associated with bariatric surgery has fallen to overall rates of 17% (7%–23%) and 21% for gastric bypass (12%–33%).32 These results were observed in specialised centres, with a high flow of patients with these diseases, similarly to our own hospital where the early complication percentage was 13.6%.
To conclude, bariatric surgery is a safe and effective option for the treatment of patients with type 2 diabetes mellitus or carbohydrate intolerance, with obesity. The limitations of our study are retrospective, with a low sample size and short follow-up period. Despite this, the significance of analysis stems from the fact it is the first of its type conducted with a Mexican population which has a detailed and complete follow-up, and above all, has a low cut-off rate for glycosylated haemoglobin, to define remission.
ConclusionBariatric surgery effectively and safely improves the metabolic state of patients who have been diagnosed with type 2 diabetes mellitus and/or carbohydrate intolerance in obese patients during the first year, which leads to high complete remission rates. Bariatric surgery also has a positive effect on other aspects such as the improvement in systemic blood pressure, lipid and anthropometric parameters, during the first year of follow-up.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Ramírez-Avilés E, Espinosa-González O, Amado-Galván M, Maydón-González H, Sepúlveda-Guerrero E, Zerrweck-López E. Evolución de los pacientes con diabetes mellitus tipo 2 e intolerancia a los carbohidratos posterior a cirugía bariátrica en la población mexicana. Cir Cir. 2017;85:135–142.