The intestinal obstruction secondary to internal hernia is a diagnostic challenge. The absence of specific symptoms and signs during clinical examination often lead to underestimation of the severity and its early surgical treatment. The purpose of this article is to review the clinical presentation of two patients with internal hernia, was well as to describe the clinical, biochemical and radiological findings, with emphasis on L-lactate as an early serum marker of intestinal ischaemia.
Clinical casesCase 1: female, 44 years old, with a history of urolithiasis and 2 caesarean sections. Case 2: female, 86 years old, with a history of open cholecystectomy, incisional and bilateral inguinal hernia repair with mesh placement. Both admitted with abdominal pain and intestinal obstruction data. The only significant laboratory finding was elevation of L-lactate. The abdominal X-rays showed air-fluid levels, and dilated loops of small intestine and colon. Abdominal contrast tomography showed free abdominal fluid, internal hernia and mesenteric torsion. In both cases, exploratory laparotomy was performed with bowel resection of ischaemic segments, with uneventful recovery.
ConclusionsIntestinal ischaemia secondary to internal hernia is difficult to diagnose. In patients with a high suspicion, signs of intestinal obstruction by plain radiography, and the elevation of L-Lactate, could help in the early diagnosis of intestinal ischaemia and its immediate surgical treatment.
La oclusión intestinal secundaria a hernia interna es un reto diagnóstico. La ausencia de síntomas y signos específicos durante la exploración clínica a menudo conducen a una subestimación de la gravedad y a tratamiento quirúrgico temprano.
El propósito de este artículo es revisar la presentación clínica de 2 pacientes con oclusión intestinal secundaria a hernia interna, describir los hallazgos clínicos, bioquímicos y radiológicos, con énfasis en el L-lactato como marcador sérico de isquemia intestinal.
Casos clínicosCaso 1: mujer de 44 años, con historia de litiasis renal y 2 cesáreas. Caso 2: mujer de 86 años, con antecedente de colecistectomía abierta, plastia de pared e inguinal bilateral con colocación de malla. Ambas ingresaron por dolor abdominal y datos de oclusión intestinal. Los reportes de laboratorios demostraron: elevación de L-lactato. Por radiografía, se observaron niveles hidroaéreos, dilatación de asas de intestino delgado y colon. En ambas pacientes la tomografía abdominal con contraste evidenció líquido libre, hernia interna y torsión del mesenterio. En los 2 casos se realizó una laparotomía exploradora con resección intestinal de segmentos isquémicos, con buenos resultados.
ConclusionesLa isquemia intestinal secundaria a hernia interna es difícil de diagnosticar. En pacientes con alta sospecha, signos de obstrucción intestinal y la elevación de L-lactato podrían ser de utilidad para el diagnóstico temprano de isquemia intestinal y tratamiento quirúrgico inmediato.
An internal hernia is made up of a visceral protrusion through a peritoneal or mesenteric opening, within the limits of the peritoneal cavity1. It presents an overall incidence of less than 1% that represents up to 5.8% of all the small bowel obstructions, which, if untreated, reach a global mortality rate higher than 50% in bowel ischaemia and necrosis cases2–4.
Meyer's classification describes internal hernias according to their clinical and radiographic characteristics and anatomical area. The most frequent are: paraduodenals (53%), pericaecals (13%), transmesenterics and Winslow foramen (8%)2–4.
It is difficult to make a preoperative diagnosis of an internal hernia and, frequently, resection of the bowel which was affected during surgery is required1,4. The discrepancy between the clinical signs and the symptoms usually leads to underestimating the seriousness of the condition. The definite diagnosis of bowel ischaemia is more challenging. Its development comes with a mortality rate between 67 and 80% when there is no early surgical treatment5.
Nowadays, L-lactate is measured regularly as an organ ischaemia marker or in any hypoxia situation. Even though lactate has not been recognized as a specific biomarker of bowel ischaemia, the failure in its correction has been associated to a mortality rate of 100%6.
The purpose of this article is to review the clinical presentation of 2 patients with bowel occlusion secondary to internal hernia, to describe the clinical, biochemical and radiological findings, highlighting L-lactate as a serum biomarker of bowel ischaemia.
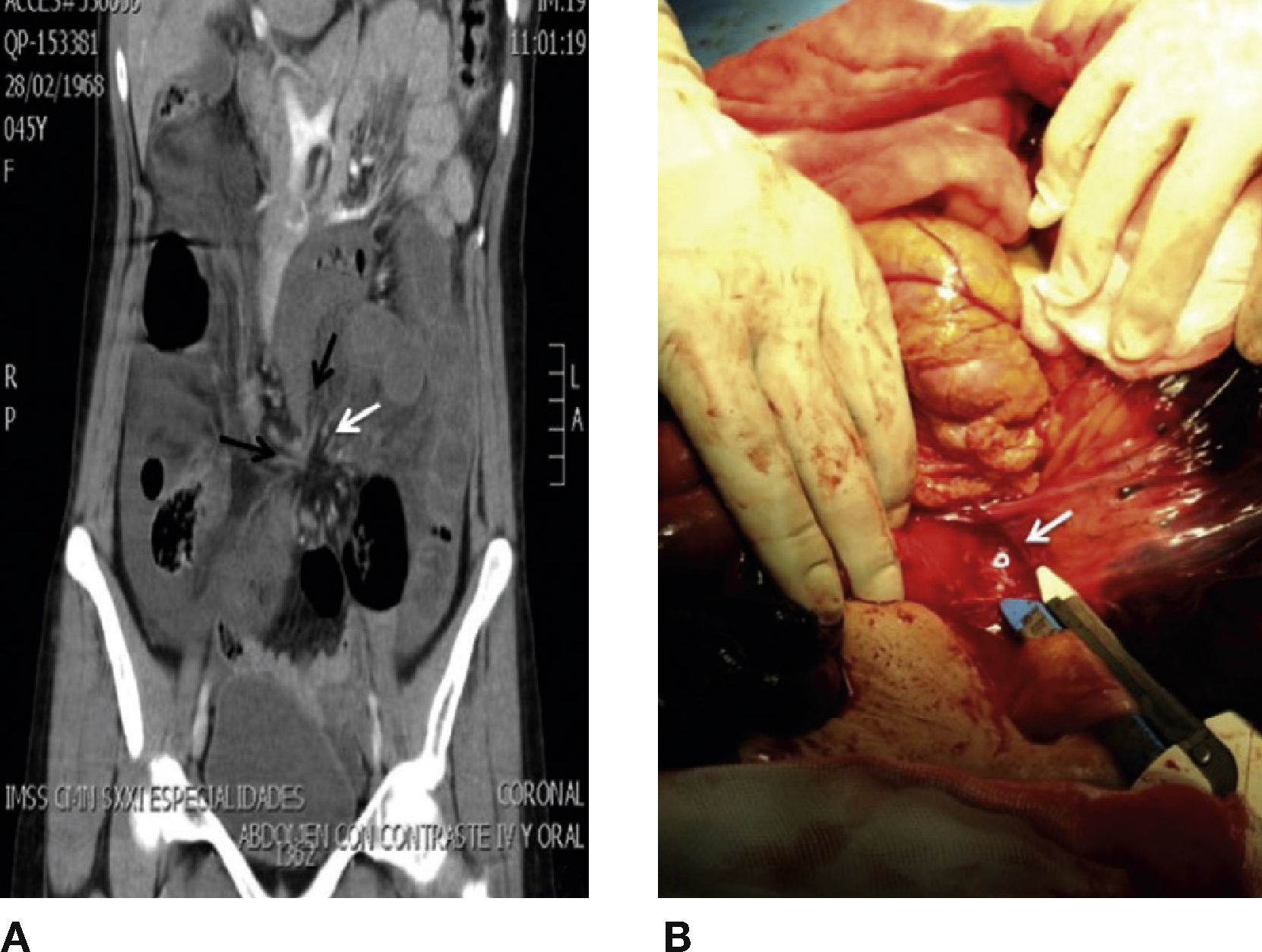
Clinical casesCase 1Woman, 44 years old, with a clinical history of renal lithiasis and 2 caesareans. She arrived at the emergency department due to a sudden colic abdominal pain, of 1-hour evolution, which was located at flank and left iliac fossa. She also suffered from constipation, obstipation and bloating. During abdominal exploration, the following was found: globe-shaped abdomen at the expense of dipose panicle, present peristalsis and slight pain to deep palpation in left flank, without information of abdominal irritation. Blood biometry, blood-chemistry and serum electrolytes did not show alterations; however, the measure of lactate serum was 5.4 mmol/l. Abdominal x-ray evidenced expansion of loops in small bowel and colon, fixed in mesogastrium, with hydro-aerial levels. Abdominal tomography with contrast material evidenced free liquid in both parietocolic gutters, expansion of loops in small bowel, fusiform-shaped stenosis in terminal ileum and distal colon (peak signal), mesentery torsion adjacent to terminal ileum and distal colon (whirlpool signal) (Fig. 1A).
A) Coronal reconstruction of abdominal tomography that evidences fusiform stenosis in terminal ileum and distal colon (peak sign, black arrow), mesentery torsion adjacent to terminal ileum and distal colon (whirlpool sign, black arrow). B) Exploratory laparotomy that shows transmesenteric opening that leads to internal hernia of small bowel and colon.
An exploratory laparotomy was carried out, finding transmesenteric hernia with involvement of 110 cm of terminal ileum and 50 cm of descending colon (Fig. 1B). There was a resection of the ischaemic segments, latero-lateral enteroenterostomy and a terminal colostomy with Hartmann's Procedure.
The patient had an adequate evolution, and she was discharged five days after the operation.
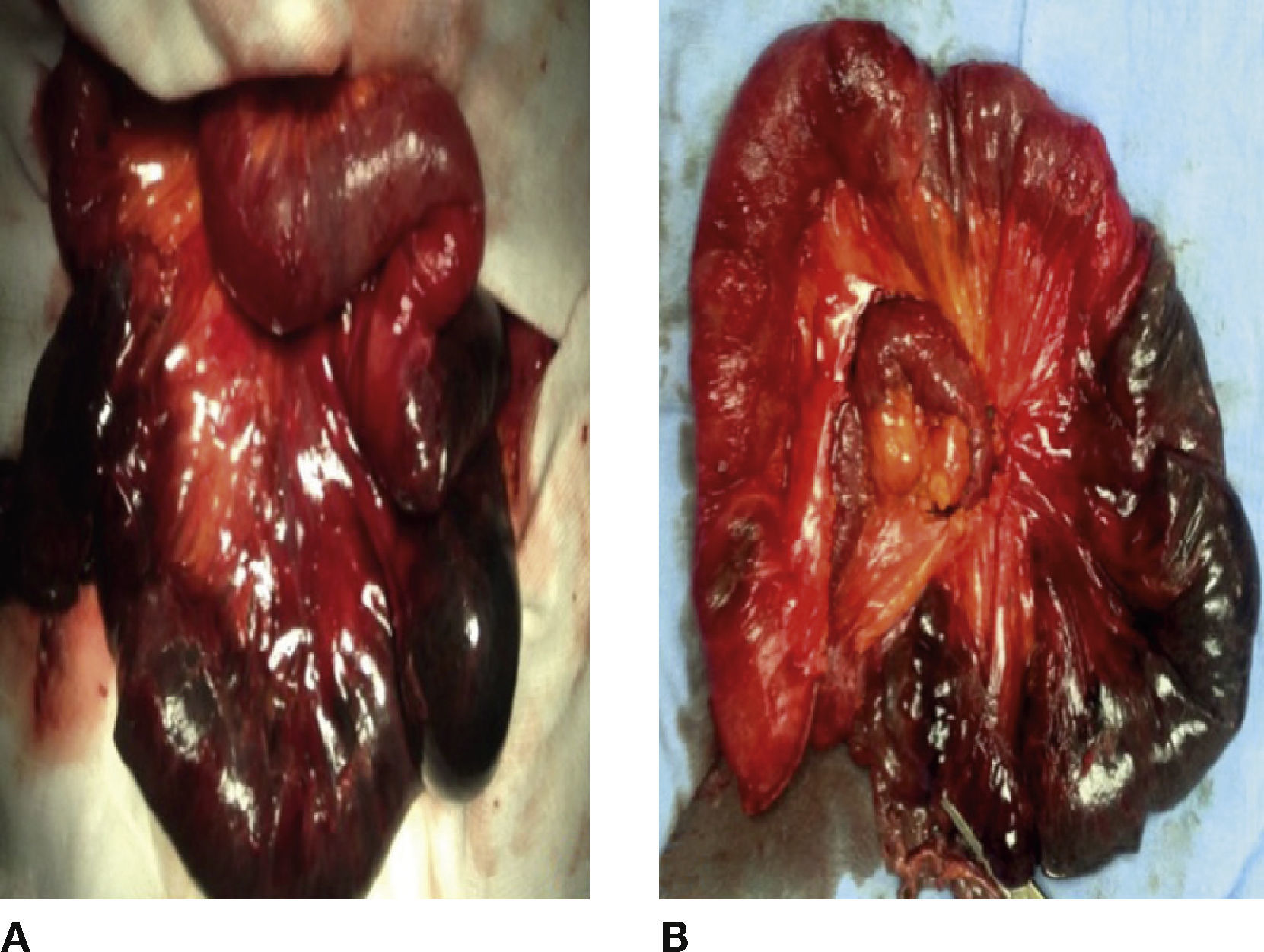
Case 2Woman, 86 years old, with a clinical history of open cholecystectomy, plasty of the wall and bilateral inguinal, with mesh placement. She was admitted with abdominal colic pain, progressive, of 48hours of evolution, poorly localized, with nausea and vomiting with gastro-alimentary content, obstipation and bloating. In the exploration, the following was found: bloating, with hernial defect over the centre line of 10 cm and current peristalsis. During abdominal palpation, the hernial content was reduced, slight pain due to deep palpation in mesogastrium, without information of abdominal irritation. Laboratory reports evidenced: leukocytosis of 10.9 ml/mm3 and serum lactate of 2.2 mmol/l. Simple abdominal x-rays were performed, which evidenced dilation of loops in small bowel, hydro-aerial levels and the presence of gas in the descending colon. Conservative treatment was initiated with hydration and abdominal decompression with nasogastric intubation. After 12hours, she suffered an increase in pain and a rise in serum lactate at 4 mmol/l. The abdominal tomography using contrast material showed perihepatic free liquid in the cul-de-sac and both parietocolic gutters, hernial defect in abdominal anterior wall, with the presence of loops in small bowel and mesentery. The contained small bowel segment was reported dilated with hydro-aerial levels, pneumatosis in wall and mesenteric torsion.
Exploratory laparotomy was conducted and the following was found: abdominal wall defect in centre line of approximately 8 cm, hernial sac with reducible loops in small bowel, internal hernia that included 60 cm of ischaemic jejunum with adhesions and mesenteric root volvuled (Fig. 2 A). All the following were conducted: adherenciolisis, resection of ischaemic segments and latero-lateral enteroenterostomy (Fig. 2B).
The patient had a satisfactory evolution and she was discharged ten days after the surgery.
DiscussionAcute bowel occlusion and its vascular compromise are a surgical urgency with a gravity that is secondary to several factors: the constitution of a third space of bowel lumen (cause of hydroelectrolytic disorders aggravated by an increase in secretions), with absorption reduction inside the occluded bowel, parietal oedema, bleeding towards peritoneal cavity and losses secondary to vomiting. All of the above cause extracellular dehydration, with hypovolaemia and renal failure, which may lead to a rapidly irreversible state of shock. Bacteria proliferation in bowel fluid causes their release and toxicity to flow in the venous circulation, which leads to a septic state5.
Bowel ischaemia has a mortality rate from 67 to 80% when it is not treated promptly5. The type of lesion ranges according to several factors including the following: type of affected vessel, occlusion grade, ischaemia mechanism (occlusive or non-occlusive), its duration and the presence of collateral circulation. Of all the bowel layers, the mucosa is the most vulnerable to the effects of hypoxia. Therefore, initial lesions lie in the mucosa, where there are areas of oedema and sub-mucous haemorrhage, ulceration and, finally, necrosis. Only if ischaemia persists, the condition can be transmural, with chances of perforation, sepsis and peritonitis7.
Once the ischaemia cause is treated, the issue of conservation or resection of initially devitalized loop in the small bowel is raised. Every bowel resection represents a risk of contamination in the peritoneal cavity and anastomotic fistula. Inversely, the conservation of a devitalized bowel loop may represent a risk of postoperative peritonitis due to perforation and, even more rarely, to secondary ischaemic stenosis and postoperative iterative occlusion5.
In contrast to ischaemia's serological markers used in the heart, liver and other organs, which identify ischaemia rapidly and accurately, bowel ischaemia markers have a low level of accuracy and usefulness. There are many possible explanations for this. Firstly, the bowel has a complex structure made up of mucosa, sub-mucosa and an external layer of smooth muscle. The ideal marker of bowel feasibility must be capable of showing that complexity, which allows for the distinction among damages limited to the mucosa or to the total bowel thickness. The second potential obstacle is the first-pass hepatic metabolism. Every bowel ischaemia marker put through the portal-venous route in the system can provide hepatic clearance before reaching systemic circulation. The third explanation is protein overexpression between the liver and the bowel, which makes it difficult to distinguish the specific organ of origin8.
During tissue ischaemia, the metabolism changes from aerobic to anaerobic and cells depend upon anaerobic glycolysis, the final product of which is L-lactate. This metabolic route is common for all tissues in the body; therefore, high levels of L-lactate are not a specific marker of bowel ischaemia. However, authors like Kurimoto et al.8 have found significant increases in the superior mesenteric vein, posterior to arterial compression, which evidence that L-lactate is an ischaemia marker in the colon9.
In bowel ischaemia, there is reduction of luminal oxygen, bacteria that belong to normal gastro-intestinal flora (Escherichia coli, species of Lactobacillus and Klebsiella) increase their anaerobic metabolism and, as a result, there is a production of 2 Lactate Isomers: L-lactate and D-lactate10. In normal conditions, healthy bowel mucosa prevents D-lactate from passing into circulation. During mesenteric ischaemia, the barrier function of the mucous is compromised, which enables bacteria translocation in the bowel wall, increasing mucous capillarity and permeability8, all of which allows D-lactate to pass into portal circulation. D-lactate has been studied as a serum marker of bowel ischaemia; however, the results have not been favourable7,11,12.
Having made L-lactate was significant, because in both cases an important increase was reported, which indicated anaerobical metabolism, secondary to a bad perfusion of bowel tissue. In the second case, the progressive increase enabled the identification of the poor response to conservative management and the persistence of ischaemia from the affected bowel segment.
Despite the fact that L-lactose has not been recognized as a specific marker for bowel ischaemia, the failure to correct it has been associated to mortality rates of 100%. McNelis et al.6 demonstrated that compensation in less than 24hours has a mortality rate of 4%; between 24 and 48hours, mortality rate rises up to 13%, reaching 43% in those whose compensation was longer than this in the Surgical and Intensive Care Unit. The response to a lactate rise involves measures related to the maximization of oxygen flow. The normalization of a serum concentration of arterial lactate would indicate an adequate tissue perfusion at a cellular level, and adequate correction and extraction of oxygen6. In both cases, the arterial lactate correction occurred in less than 24hours after surgical treatment and, despite the need for resection of ischaemical bowel segments, the patients recovered favourably.
ConclusionBowel occlusion secondary to internal hernia is difficult to diagnose before surgery, even though there is a wide variety of diagnosis techniques currently available. The absence of specific signs during the patient's clinical exploration makes the early decision of surgical approach to continue being a challenge. Early diagnosis is essential for the preservation of the compromised bowel segment. In both cases presented, the only altered serum biochemical marker was L-lactate, which represented the presence of an anaerobical metabolism. Progressive increase in the second case indicated the persistence of an inadequate tissue perfusion. The complement of abdominal tomography using contrast material allowed us to identify the affected bowel segments and the perfusion compromise, all of which was determinant in deciding surgical exploration in both cases. Internal hernias rapidly progress into bowel ischaemia. In patients with high levels of suspicion, signs of bowel obstruction by simple x-ray, lactate rise could be useful to determine a prompt diagnosis of bowel ischaemia and immediate surgical treatment.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Tun-Abraham M.E. et al. L-lactato como marcador sérico de isquemia intestinal en pacientes con oclusión intestinal complicada. Cirugía y Cirujanos. 2015; 83: 65-69.