Compared to other surgical areas, laparoscopic liver resection (LLR) has not been widely implemented and currently less than 20% of hepatectomies are performed laparoscopically worldwide. The aim of our study was to evaluate the feasibility, and the ratio of implementation of LLR in our department.
MethodsWe analyzed a prospectively maintained database of 749 liver resections performed during the last 10-year period in a single center.
ResultsA total of 150 (20%) consecutive pure LLR were performed between 2005 and 2015. In 87% of patients the indication was the presence ofprimary or metastatic liver malignancy. We performed 30 major hepatectomies (20%) and (80%) were minor resections, performed in all liver segments. Twelve patients were operated twice and 2 patients underwent a third LLR. The proportion of LLR increased from 12% in 2011 to 62% in the last year. Conversion rate was 9%. Overall morbidity rate was 36% but only one third were classified as severe. The 90-day mortality rate was 1%. Median hospital stay was 4 days and the rate of readmissions was 6%.
ConclusionsThe implementation of LLR has been fast with morbidity and mortality comparable to other published series. In the last 2 years more than half of the hepatectomies are performed laparoscopically in our center.
En comparación con otras áreas quirúrgicas, la resección hepática laparoscópica (RHL) no se ha aplicado de forma generalizada y en la actualidad menos del 20% de las hepatectomías se realiza por vía laparoscópica en todo el mundo. El objetivo de nuestro estudio fue evaluar la aplicabilidad y la proporción de RHL en nuestro departamento.
MétodosLos datos de morbimortalidad y supervivencia se extrajeron de una base de datos prospectiva con 749 resecciones hepáticas realizadas durante un período de 10 años en un solo centro.
ResultadosEntre 2005 y 2015 se realizaron 150 RHL. En el 87% de los pacientes la indicación fue la presencia de tumores hepáticos primarios o metastásicos. Se realizaron 30 hepatectomías mayores (20%) y el 80% fueron resecciones menores, realizadas en todos los segmentos del hígado. Doce pacientes fueron operados 2veces y 2 pacientes tuvieron una tercera RHL. La proporción de RHL aumentó del 12% en 2011 al 62% en el último año. La tasa de conversión fue del 9%. En general, la tasa de morbilidad fue del 36%, pero solo 1/3 se clasificaron como graves. La tasa de mortalidad a los 90 días fue del 1%. La mediana de estancia fue de 4 días y la tasa de reingresos fue del 6%.
ConclusionesLa aplicación de RHL ha sido rápida y progresiva, con resultados de morbimortalidad comparables a las de las series publicadas en la literatura. En los últimos 2 años más de la mitad de las hepatectomías se realiza por vía laparoscópica en nuestro centro.
Laparoscopic liver resection (LLR) is currently experiencing rapid expansion.1,2 In most hospitals, this surgical approach is limited to small or segmental resections, and the application of laparoscopy in recent years has been slow, even in countries where its use has been well developed.3 There is increasing evidence that minimally invasive liver surgery offers significant short-term advantages, with less pain, shorter hospital stays and fewer blood products required, as well as faster recovery and better aesthetic results compared to open liver resection (OLR).4–6 Moreover, a recent study indicates a lower inflammatory response after LLR compared to OLR.7 Numerous current series have defended the safety of laparoscopic surgery compared to open surgery in terms of both perioperative and oncologic morbidity.8–11
Some years ago, Vigano12 established the learning curve for LLR at 60 cases. Recently, however, this concept has been re-evaluated by the same group, which has focused on the importance not only of the surgeon's learning curve but also of the hospital, especially in major hepatectomies (MH).13 However, the application of laparoscopy in the liver has not been as popular as in other anatomical areas. In a recent publication, based on records from the French national healthcare system analyzing more than 40000 hepatectomies, less than 20% of cases were LLR.3
At our medical center, the first LLR was carried out in 2005. In 2010, we started a specific training program for this approach and, progressively, we have significantly increased the proportion of LLR. The objective of our study was to evaluate the applicability and implementation of the laparoscopic approach in the hepatectomies performed in our Hepatobiliary-Pancreatic Unit (HBP) over a period of 10 years.
MethodsThe data for demographics, surgical indication and morbidity/mortality rates were extracted from a prospective database.
All patients with liver lesions were evaluated by a weekly multidisciplinary committee. Surgical indications were established according to current oncologic standards14 and agreed upon by the radiologist, radiation oncologist, and medical oncologist. The indication for a laparoscopic approach was discussed within the surgical team. From the beginning, we have opted for a purely laparoscopic surgical technique; hybrid and hand-assisted techniques were performed in one case each, as we have recently published.15 We have gradually extended the indications of the laparoscopic approach to include the posterior segments and MH.
Also, our MH technique has evolved along with our case experience. For left hepatectomy, from the beginning we opted for an extra-Glissonian approach with en bloc resection of the left pedicle16 and extrahepatic control of the left hepatic vein, followed by hepatic transection, but in the last 2 cases we introduced the dorsal approach of the middle hepatic vein and its exposure from the root to the periphery, as described by Okuda.17 As for the right hepatectomy, in the first cases the liver was initially mobilized, with dissection of the pedicle and selective ligation of its elements, followed by transection. After the 5th case, the extra-Glissonian approach of the right pedicle was introduced, in accordance with the technique described by Cho, since it was our method of choice in OLR.18–20 Furthermore, from the 8th case on, we adopted the caudal approach, so that the right liver was mobilized at the end of the transection. From the 12th case on, we incorporated the proximal dissection of the hepatic middle vein, as described by Honda et al.21
Our technique with intraoperative ultrasound, liver transection, hilar clamping and extraction of the surgical specimen has already been described.15 Blood loss was estimated by measuring the fluid suctioned from the surgical field and the weight of the surgical gauze.
We did not routinely leave in abdominal drains after LLR or OLR.22
Specific morbidities, such as liver failure, biliary fistula or postoperative bleeding, were recorded systematically according to the definitions of the International Study Group of Liver Surgery.23–25 During daily hospital rounds or postoperative ambulatory consultations, other general complications were also recorded, such as superficial or deep surgical site infections and respiratory infections or complications. Organ-space infection was determined by any intraabdominal collection requiring endoscopic, percutaneous or surgical drainage or associated with the systemic inflammatory response syndrome requiring treatment with antibiotics.26 Postoperative complications were stratified according to the Dindo-Clavien classification.27 Grade IIIa complications or higher were considered severe morbidities.
All the oncology patients were followed by a member of the HPB team one week after discharge and every 3–6 months with tumor markers and thoracoabdominal CT.
Our hospital's ethics committee approved the present study.
Statistical AnalysisQualitative variables are expressed in number and percentage. Quantitative variables are expressed as median and range. All the results were analyzed with the SPSS version 20.0 statistical software package (IBM SPSS Statistics, Chicago, IL, USA).
ResultsBetween May 2005 and November 2015, we conducted 749 liver resections at our hospital. From among these, we analyzed a total of 150 LLR performed in 136 patients.
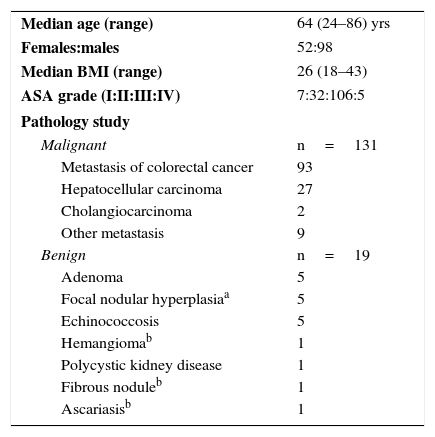
The demographic data of the patients and the final results of the pathology study are enumerated in Table 1: 87% corresponded with malignant disease and only 13% with benign disease.
Patient and Pathological Characteristics.
| Median age (range) | 64 (24–86) yrs |
| Females:males | 52:98 |
| Median BMI (range) | 26 (18–43) |
| ASA grade (I:II:III:IV) | 7:32:106:5 |
| Pathology study | |
| Malignant | n=131 |
| Metastasis of colorectal cancer | 93 |
| Hepatocellular carcinoma | 27 |
| Cholangiocarcinoma | 2 |
| Other metastasis | 9 |
| Benign | n=19 |
| Adenoma | 5 |
| Focal nodular hyperplasiaa | 5 |
| Echinococcosis | 5 |
| Hemangiomab | 1 |
| Polycystic kidney disease | 1 |
| Fibrous noduleb | 1 |
| Ascariasisb | 1 |
ASA: classification of the American Society of Anesthesiologists; BMI: body mass index.
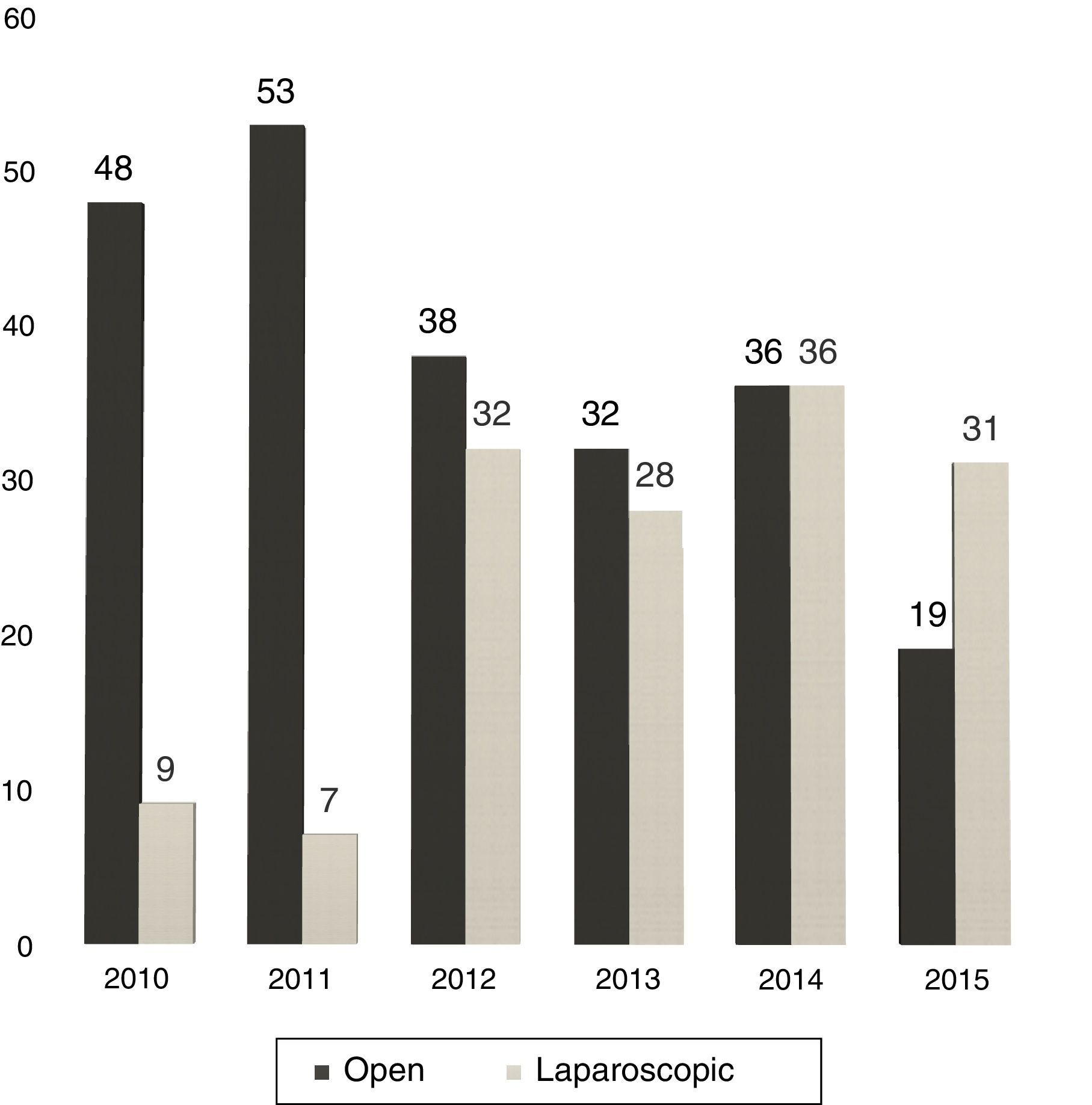
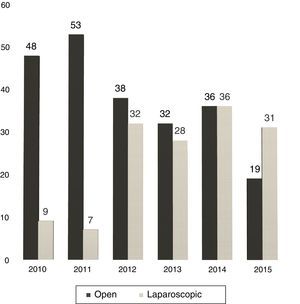
Between 2005 and 2010, the use of LLR was almost anecdotal but, in the latter years, without varying the surgical indication, we have experienced a progressive increase in the percentage of LLR: from 16% in 2010 to 62% currently (Fig. 1).
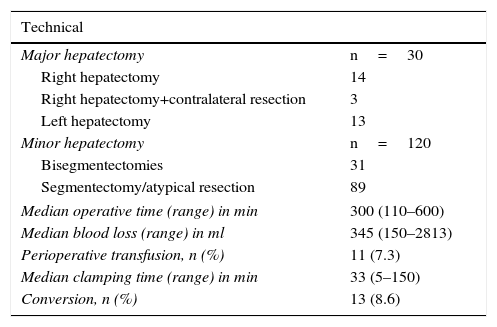
Thirty MH (20%) were conducted, and 120 (80%) were minor resections. Details of the resection type are summarized in Table 2. The minor hepatectomies were performed in the liver segments, including posterior segments (meaning VII, VIII and IVa), in 20 of 89 cases. Twelve patients were operated on twice, and 2 patients were reoperated with a third LLR, all due to recurrence of metastatic colorectal cancer (mCRC). Three patients underwent LLR after previous OLR. In one particular case, a limited mCRC resection was performed after extended right hepatectomy with the substitution of the inferior vena cava and reconstruction of the left hepatic vein under in situ hypothermic perfusion, carried out one year earlier.
Surgical Data.
| Technical | |
|---|---|
| Major hepatectomy | n=30 |
| Right hepatectomy | 14 |
| Right hepatectomy+contralateral resection | 3 |
| Left hepatectomy | 13 |
| Minor hepatectomy | n=120 |
| Bisegmentectomies | 31 |
| Segmentectomy/atypical resection | 89 |
| Median operative time (range) in min | 300 (110–600) |
| Median blood loss (range) in ml | 345 (150–2813) |
| Perioperative transfusion, n (%) | 11 (7.3) |
| Median clamping time (range) in min | 33 (5–150) |
| Conversion, n (%) | 13 (8.6) |
min: minutes; ml: milliliters.
In 5 patients, LLR was done simultaneously with the resection of the primary tumor: total gastrectomy in 2 cases and right colectomy in 3 cases. In another, we conducted partial resection of segment VIII and a right thoracoscopic atypical lung resection in a single surgery. In 11 patients, complementary radiofrequency ablation of small deep lesions was used, both percutaneously before the pneumoperitoneum as well as intraoperatively.
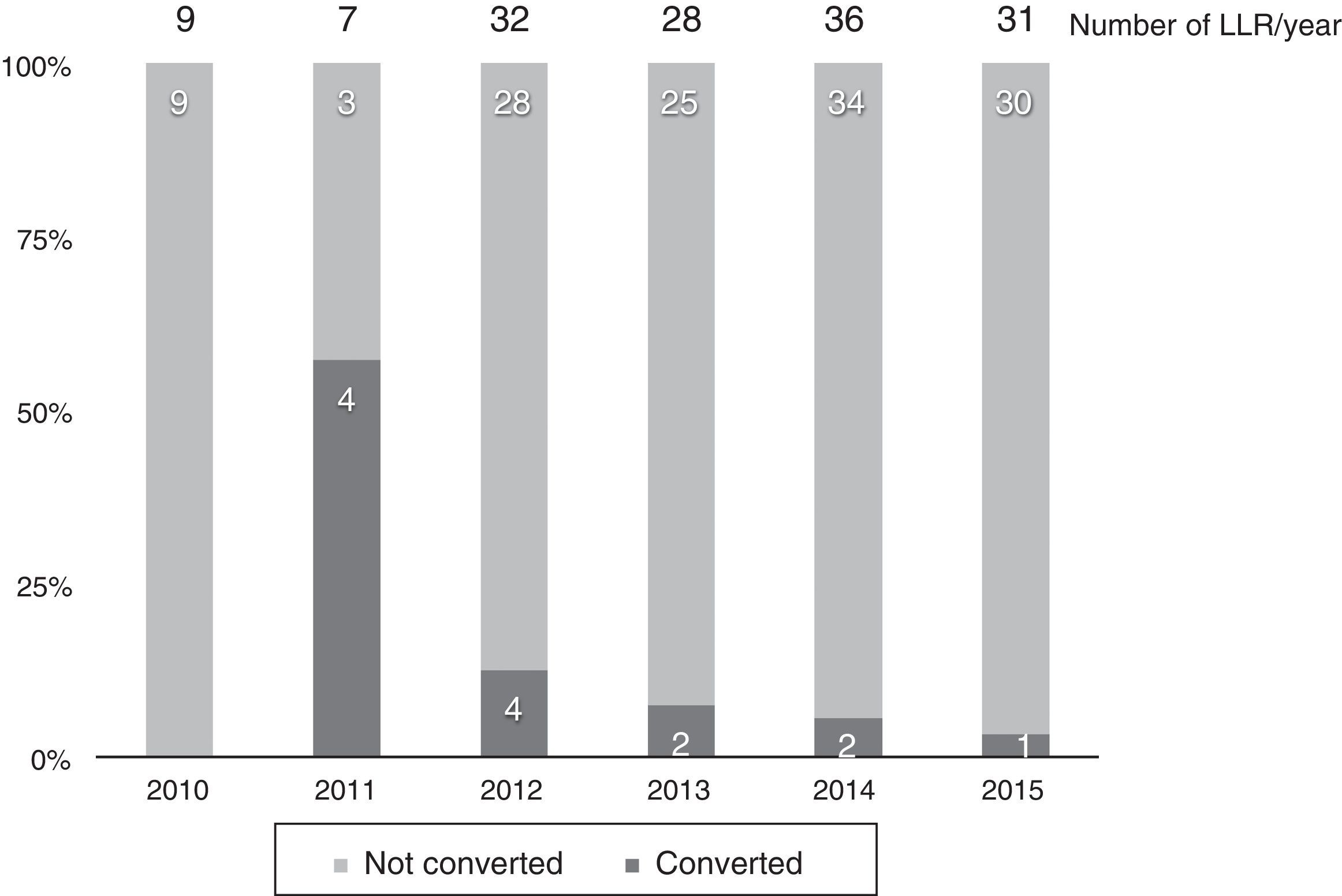
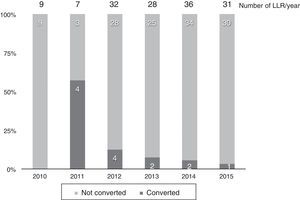
Thirteen cases (8.6%) were converted to laparotomy during LLR because of the inability to continue (6 cases), excessive bleeding (6 cases) or the presence of dense adhesions in one case. The conversion rate progressively decreased, and in the last year it was 3% (Fig. 2).
Mean surgical time was 5h and most patients (77%) needed at least one clamping period. Our intraoperative transfusion rate was low (7.3%), with an overall transfusion rate of 12% (Table 2).
Our parenchyma-saving policy involves a tendency toward narrow surgical margins, which achieves, in malignant cases, an average margin of 0.4 (0–5) cm. Ten patients (6.7%) were classified as R1 after the final pathology report.
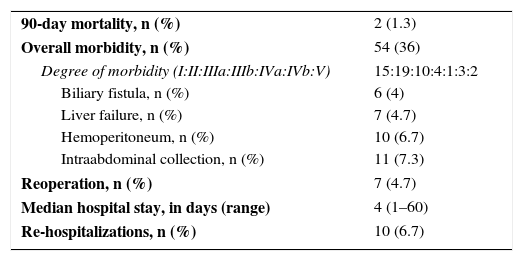
In general, the postoperative morbidity rate was 36%, but only 13% had severe complications (≥IIIa) according to the Dindo-Clavien classification (Table 3). Seven patients required reoperation: 2 cases due to the complications of the simultaneous total gastrectomy (leak and occlusion), 2 cases due to postoperative bleeding, one due to an unnoticed intraoperative duodenal lesion and another 2 cases for the withdrawal of packing.
Postoperative Data.
| 90-day mortality, n (%) | 2 (1.3) |
| Overall morbidity, n (%) | 54 (36) |
| Degree of morbidity (I:II:IIIa:IIIb:IVa:IVb:V) | 15:19:10:4:1:3:2 |
| Biliary fistula, n (%) | 6 (4) |
| Liver failure, n (%) | 7 (4.7) |
| Hemoperitoneum, n (%) | 10 (6.7) |
| Intraabdominal collection, n (%) | 11 (7.3) |
| Reoperation, n (%) | 7 (4.7) |
| Median hospital stay, in days (range) | 4 (1–60) |
| Re-hospitalizations, n (%) | 10 (6.7) |
Two patients (1%) died after LLR. One was an 82-year-old man with a metachronous metastasis requiring right hepatectomy after portal embolization and prolonged chemotherapy. He presented progressive liver failure with ascites, varicose bleeding and encephalopathy and died after 24 days due to sepsis related with infection of the ascitic fluid. The second case was a 74-year-old woman with a large metastasis and partial response after chemotherapy, who underwent left hepatectomy and needed percutaneous drainage to treat a postoperative biliary collection. She presented late-onset bleeding of the biloma requiring laparotomy for hemostatic control. After reoperation, the patient presented bilateral necrotizing pneumonia and died on the 123rd day post-op.
The median hospital stay was 4 days, and the re-admittance rate was very low (7%). Ten patients presented prolonged postoperative hospitalization related with the development of collections (9 cases) and pneumonia (one patient with chronic obstructive pulmonary disease).
DiscussionThe laparoscopic approach has become the approach of choice in several surgical procedures, such as cholecystectomy, fundoplication, splenectomy and adrenalectomy.28–31 Nonetheless, its implementation in liver resection is low. In a retrospective study of more than 40000 hepatectomies, only one-fifth of cases were done by laparoscopy. In fact, the hospitals with the greatest number of annual cases presented the lowest percentage of LLR.3 At the Second International Consensus Conference on Laparoscopic Liver Resections held in Morioka in 2014, a survey was presented with data from surgeons around the world. It was reported that, although 86% of the surgeons declared an increase in LLR over the previous 5 years at their hospitals, only 13.5% perform more than 40% of hepatectomies laparoscopically.32 At this conference, it was concluded that minor LLR are currently standard practice (IDEAL 3) and that major LLR are still innovative procedures being explored (IDEAL 2).33,34
At our hospital, the proportion of LLR has grown from 12% in 2011 to 62% in 2015 (31 out of 50 hepatectomies).
At the first consensus conference held in Louisville in 2008, it was declared that the most favorable indication for LLR is a solitary lesion measuring less than 5cm located in favorable segments (from II to VI). In addition, resections of segments I, VII and VIII and MH are “reserved for surgeons with experience and expertise in more limited laparoscopic resections”.35 However, in our experience, we achieved these milestones at the beginning of the learning curve. Our first LLR of the posterior segments was performed after 15 LLR, the first right hepatectomy after 29, and our first left hepatectomy after 41 LLR.
One of the concerns of the Louisville declaration was the possibility that laparoscopy would increase the indication of resection in benign lesions. Our series analyzed this possibility, and only 13% of the resections were due to benign lesions, some of which had been preoperatively diagnosed as malignant.
Four out of every 5 of our LLR are minor, which is coherent with the parenchymal preservation policy36 that is currently preferred by our team in the treatment of mCRC. In our series, mCRC represented 60% of the indication for LLR and is perfectly accessible laparoscopically.37,38 However, in some patients it is necessary to perform standard right and left hepatectomies. In this scenario, a randomized trial demonstrated the safety of an extra-Glissonian approach18 and we feel very safe with this technique, even for selective clamping in minor hepatectomies.39,40 We also find this approach to be extremely useful after right portal embolization, where it is common to find important inflammatory changes in the interior of the Glisson sheath that hinder the dissection of the vascular structures. In the 8th right hepatectomy, we evolved toward the so-called “caudal approach”41: the liver was mobilized after completing the transection of the parenchyma and the exposure of the inferior vena cava. In the latter 2 cases, proximal exposure was used of the middle hepatic vein at the beginning of the transection, as described by Honda,21 in the right anterior sectionectomy and the transection of the parenchyma from the caudal plane to the surface. The justification was to improve the surgical margin in cases with lesions proximal to large vessels (meaning the middle hepatic vein) and reduce the incidence of complex vascular lesions, fundamentally of the main hepatic vein tributaries. This approach would also have an oncological effect by avoiding the mobilization of the liver, as Fan supports in OLR.42 Intraoperative ultrasound is essential to clearly define the vascular anatomy.
This “vessel-oriented” approach is useful in order to be very conservative in the amount of parenchyma resected. In cases of proximity of the lesion to hepatic veins or Glisson pedicles, we try to guarantee their conservation, assuming that an R1 resection is possible. In fact, R1 resections are still a topic of debate among important workgroups. In the era of modern chemotherapy, there is controversy as to whether there are differences in recurrence-free and global survival rates between the R0 and R1 groups when done “out of necessity”,43–46 and currently there are groups that argue that margins should always be free.47,48 Unfortunately, since 2/3 of our patients have undergone surgery in the last 2 years, the follow-up periods are limited and do not provide for a conclusive analysis of long-term oncologic parameters (overall survival and relapse-free survival) in our series.
The main objective of the laparoscopic approach is to reduce the aggressiveness of the surgical intervention, which would be reflected in the reduction of morbidity and, consequently, shorter hospitalization. Our morbidity rate could be considered high when compared with other series.49–52 However, our rigorous method, which has prospectively recorded all medical and surgical complications, could explain this fact. Moreover, our median stay is comparable to, if not less than, other series. In fact, even more important than external validation is the evaluation of our own results from previous experience in open liver resections. We recently published the comparison of our first 50 consecutive LLR with a well-matched group of 100 OLR performed in our unit during the same time period. We have been able to observe a decrease in the rate of complications, especially infectious ones, and a significant reduction in hospital stay.15 At present, our morbidity and mortality rates remain at the same level after having tripled our experience and increased the complexity of cases treated laparoscopically.
The implementation of the laparoscopic approach for liver resections at our hospital has been achieved at a high rate, especially in the last 5 years. Constant refinement of the laparoscopic surgical technique has allowed us to progressively increase the complexity of the procedures, while maintaining low morbidity and short hospital stays. In the last 2 years, we have been able to perform more than half of hepatectomies laparoscopically, and the current limitations to the indication of laparoscopy would be hilar cholangiocarcinoma, the need for vascular reconstruction and 4 or more planned limited simultaneous resections.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr. Fernando Rotellar Sastre, hepatobiliary surgeon at the Clínica Universitaria de Navarra, for his critical review of this study.
Please cite this article as: López-Ben S, Ranea A, Albiol MT, Falgueras L, Castro E, Casellas M, et al. Evolución de la cirugía laparoscópica en una unidad hepatobiliar de alto volumen: 150 hepatectomías laparoscópicas consecutivas. Cir Esp. 2017;95:261–267.