Hemosuccus pancreaticus is caused by a pseudoaneurysm in the lumen of the pancreatic duct and is connected with a pancreatic pseudocyst.1 It is not an exceptional event as it is reported in 5%–10% of patients with chronic pancreatitis and associated hemorrhage.2
The formation of a pseudoaneurysm is the result of the erosion and weakening of the pancreatic and peripancreatic artery walls due to inflammation caused by the release of pancreatic enzymes.3 The most frequently affected blood vessels are the splenic and hepatic arteries, although any branch of the celiac trunk can be compromised. Another factor that has been related with the genesis and rupture of aneurysms and pseudoaneurysms is Salmonella typhi infection.3
Angiography is the method of choice for identifying the exact location of the hemorrhage, with a reported sensitivity of 96%.2 Bleeding can be stopped with either of 2 procedures: surgery, involving ligation of the bleeding vessel or total or partial resection of the affected area; and, selective transcatheter embolization, which can be done during diagnostic angiography. In recent years, the use of this latter technique has increased as it provides precise localization of the lesion and better preservation of the adjacent parenchyma; it is associated with lower morbidity and mortality and has a success rate between 67 and 100%. Surgery is the treatment option when radiological embolization fails.1–6
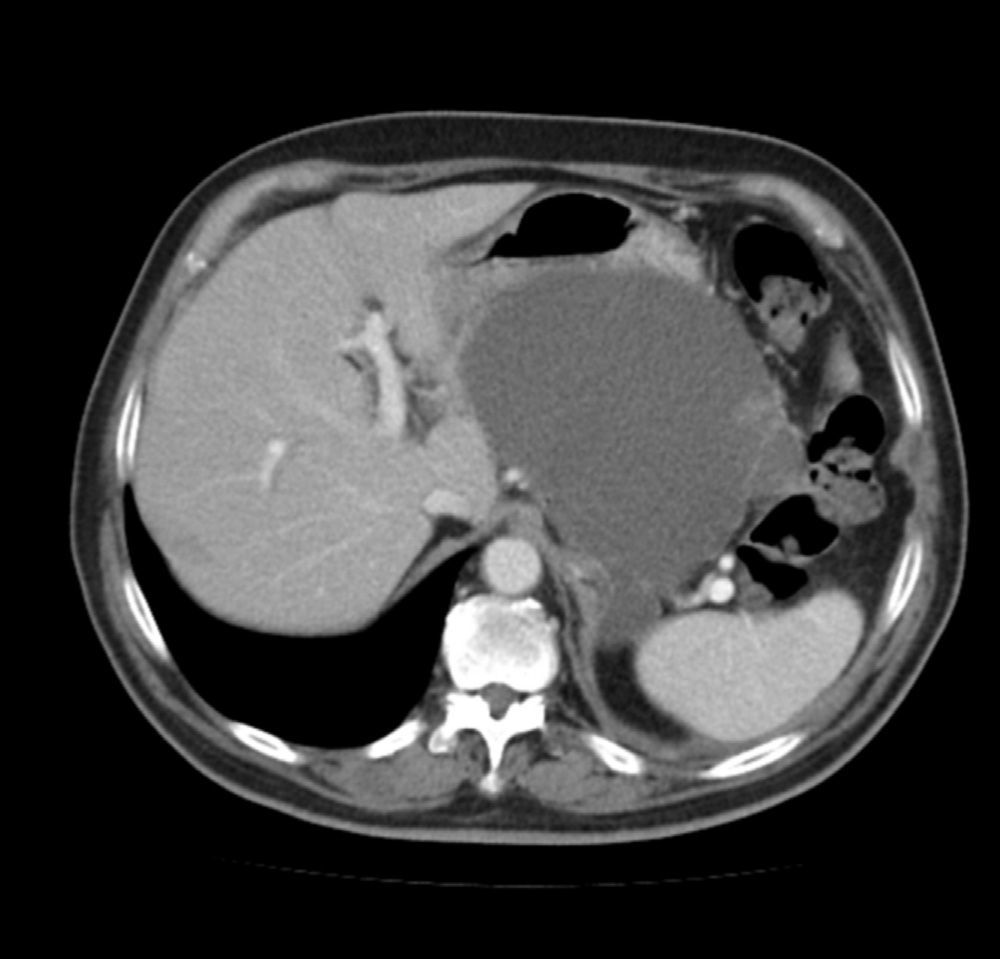
We present a case in which it was necessary to control a hemorrhage originating from a pancreatic pseudocyst secondary to hemosuccus pancreaticus. The patient is a 72-year-old male with a history of severe acute pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) 15 months earlier. He had developed a pancreatic pseudocyst measuring 12cm in diameter, which compressed the stomach and caused vomiting (Fig. 1). Endoscopic drainage of the pseudocyst was attempted but not possible, so we decided to operate and performed cystogastrostomy using a transgastric approach. The anterior gastric wall was opened and, through the lumen of the stomach, the cavity of the pseudocyst was accessed through the posterior gastric wall. The contents of the pseudocyst were drained and a cystogastric anastomosis was created manually with a continuous polyglactin 3/0 suture.
On the 6th day post-op, the patient presented with symptoms of massive hematemesis in association with arterial hypotension, which improved partially after the transfusion of 4 units of packed red blood cells. Upper gastrointestinal endoscopy could not identify a clear bleeding point and, given the patient's persistent hemodynamic instability and the inability to do an urgent angiography at our hospital, we decided to review the previous surgery. The gastric lumen was accessed through the anterior wall of the stomach, but no bleeding point was identified in the mucosa. Numerous clots were extracted from the cavity of the pseudocyst, where the origin of the continuous bleeding was confirmed, compatible with hemosuccus pancreaticus. The cystogastrostomy was extended to improve the access to the cavity of the pseudocyst, although our attempts to detect the bleeding point were unsuccessful. In the end, we decided to pack the cavity, using 25 oxidized cellulose hemostats (Surgicel®, Ethicon, Johnson & Johnson, Somerville, NJ, USA).
The patient had an uneventful recovery and was discharged on the 12th day after the second procedure. Nine months later, the patient is asymptomatic and his gastric compression symptoms have disappeared.
Even though there are currently several hemostatic substances and devices available, hemorrhages that originate in deep cavities or locations with difficult surgical access are difficult to control. In some cases, simple compression on the bleeding area is sufficient to control the hemorrhage, but in others it is necessary to maintain the compression for hours in order to achieve proper hemostasis.7 In these cases, it is necessary to pack the cavity, which is usually done with gauze or dressings that are left in the cavity for at least 24–48h. The main indication of packing is when a hemorrhage is not able to be controlled after having attempting proper hemostasis for a reasonable amount of time, and the patient is becoming hemodynamically unstable or presents coagulopathy. After 24–48h, the coagulopathy improves with adequate treatment, and during the post-surgery follow-up it is often observed that the bleeding has completely ceased with compression.7,8
The main disadvantage to packing with gauze or surgical dressings is the need for a second intervention to remove these “foreign bodies”, even though in many cases the hemorrhage has already stopped. The possibility to pack a cavity with absorbable material could avoid a second intervention in many cases, especially when the patient is hemodynamically stable and there is no clinical or analytical evidence of persistent bleeding.
Surgicel® is a hemostatic agent made up of an oxidized cellulose polymer that is completely reabsorbed in 7–14 days. In the literature, there are few case reports of intraabdominal packing with oxidized cellulose polymers. Muguti et al.9 describe a case of massive hemorrhage from a duodenal diverticulum that was controlled using local packing with Surgicel®. In retroperitoneal surgery, there is a series of 7 patients who presented with bleeding through the retroperitoneal veins, which was controlled with this method.10
Please cite this article as: Ruiz-Tovar J, Oller I, Barreras JA, Santos J, Calpena R. Taponamiento quirúrgico con polímero de celulosa oxidada en paciente con hemosuccus pancreaticus. Cir Esp. 2015;93:47–48.