Laparoscopic pancreaticoduodenectomy (PD) is not widely accepted, and its use is controversial. Only correct patient selection and appropriate training of groups experienced in pancreatic surgery and laparoscopy will be able to establish its role and its hypothetical advantages.
MethodsOut of 138 pancreatic surgeries performed in a two-year period (2017–2019), 23 were laparoscopic PD. We evaluate its efficacy and safety compared to 31 open PD.
ResultsThere were no cases of B/C pancreatic or biliary fistula, nor any cases of delayed gastric emptying in the laparoscopic group, but hemorrhage required one reoperation. The conversion rate was 21% (five cases): one due to bleeding, and the remainder for non-progression. The converted patients showed no differences compared to those completed by laparoscopy. There were no differences between laparoscopic and open PD in surgical time, postoperative complications, reintervention rate, readmissions or mortality. R0 resection in tumor cases was 85% for laparoscopy and 69% in open surgery without statistical significance. The postoperative hospital stay was shorter in the laparoscopic PD group (eight vs. 15 days).
ConclusionsIn a selected group, laparoscopic PD can be safely and effectively performed if carried out by groups who are experts in pancreatic surgery and advanced laparoscopy. The technique has the same postoperative results as open surgery and is oncologically adequate, with less hospital stay. Proper patient selection, a step-by-step program and a lax and early conversion prevents serious operating accidents.
La duodenopancreatectomía (DPC) laparoscópica no es ampliamente aceptada y su uso es controvertido. Únicamente una correcta selección de los pacientes y un aprendizaje adecuado por grupos con experiencia en cirugía pancreática y laparoscopia podrán establecer cuál es su papel y sus hipotéticas ventajas.
MétodosDe 138 cirugías pancreáticas realizadas en un periodo de dos años (2017–2019) se realizaron 23 DPC laparoscópicas, incluyendo patología benigna y maligna. Se valora la eficacia y seguridad y se compara con 31 DPC abiertas en el mismo periodo.
ResultadosNo hubo casos de fístula pancreática B/C, biliar, ni retraso en vaciamiento gástrico en el grupo laparoscópico, pero apareció una hemorragia que obligó a una reintervención. El índice de conversión fue del 21% (cinco casos), uno por hemorragia y el resto por no progresión. Los convertidos no mostraron diferencias frente a los que se completó por laparoscopia. No existieron diferencias entre la DPC laparoscópica y abierta en tiempo quirúrgico, complicaciones postoperatorias, índice de reintervenciones, reingresos ni mortalidad. La resección R0 en los casos tumores fue del 85% por laparoscopia y del 69% en cirugía abierta sin significación estadística. La estancia postoperatoria fue inferior en el grupo DPC laparoscópica, ocho vs. 15 días.
ConclusionesEn un grupo seleccionado, la DPC laparoscópica puede realizarse de forma segura y eficaz si se realiza por grupos expertos en cirugía pancreática y en laparoscopia avanzada. Obtiene los mismos resultados que la cirugía abierta en el postoperatorio y es oncológicamente adecuada con menor estancia hospitalaria. Una selección adecuada de los pacientes, un programa establecido por pasos con una conversión laxa y precoz evita accidentes operatorios graves.
Minimally invasive surgery, both laparoscopic and robotic, has spread to most fields of surgery in recent decades1–3. In general, it has demonstrated its effectiveness by improving patient progress and postoperative recovery, while maintaining the oncological results achieved in open surgery.
Predictably, pancreatic surgery has followed the same pathway. Due to its idiosyncrasy, high technical difficulty and possible hemorrhagic accidents, the application of minimally invasive surgery has been disparate. There have been two speeds of incorporation depending on the location of the lesion. While the use of these procedures has been extensive and rapid for the resection of the body and tail of the pancreas, laparoscopic PD was generally abandoned and, nowadays, is only performed in small groups4–6 after the first laparoscopic PD performed by Gagner and Pomp7 in 1994. For this reason, there is still doubt about the role of laparoscopic PD, and the results published to date are controversial8,9.
Cohort studies suggest that, performed by experienced surgeons and in high-volume hospitals, these procedures are safe and maintain the benefits of minimally invasive surgery10,11. A meta-analysis has reported less blood loss and less delay in gastric emptying along with a decrease in the mean hospital stay. However, surgical times increase significantly11, and a recent multicenter, prospective and randomized clinical trial has reported higher rates of complications and mortality related to the laparoscopic technique12. Currently, the question remains about whether these techniques should be used in a generalized manner, as discussed at the Miami consensus meeting13.
These discrepancies have placed laparoscopic PD under scrutiny, requiring research and development with new studies to establish its role in terms of indications, training, safety and efficacy.
This study compares the short-term results of laparoscopic PD in a selected group of patients versus open PD, performed in the same period of time by the same surgical group, with the aim to assess its feasibility and safety in the immediate postoperative period.
MethodsDesignWe have conducted a prospective, non-randomized study at two tertiary hospitals in the metropolitan area of Barcelona (Hospital Universitari Germans Trias i Pujol and Hospital Universitari Mútua de Terrassa), with a joint reference population of 1 200 000 inhabitants, comparing the results of laparoscopic PD and open surgery during the same period.
Inclusion and exclusion criteriaThe study included all patients who had undergone elective PD, regardless of benign or malignant etiology. The exclusion criteria were: patients who received neoadjuvant treatment (due to the added difficulty this represents and the possibility of requiring complex vascular resections); and, patients who underwent robotic resection (which was considered a different surgical technique).
Study populationFrom October 2017 to December 2019, 138 pancreatic surgeries were performed, 98 of which were PD. We analyzed 54 cases that met the inclusion criteria: 23 who were treated with laparoscopic surgery, and 31 with open surgery. In all cases, a fast-track protocol was followed with early reintroduction of enteral feeding (within the first 24 h).
Surgical techniqueLaparoscopic pancreaticoduodenectomyThe patient is placed in the supine position, with legs spread, on a vacuum mattress. Pneumoperitoneum is created with a Veress needle, and five 12-mm trocars are placed in a semicircle (supraumbilical, right and left paraumbilical, and right and left flanks) along with a sixth 5-mm subxiphoid trocar.
The greater and lesser omentum are divided to proceed with the division of the gastric antrum, using an endostapler. The common hepatic artery is identified and followed up to the gastroduodenal artery and the pyloric artery, which are divided between Hem-o-lok®. The hepatic hilum is dissected, and lymphadenectomy of the area is performed. The superior mesenteric vein is identified at the lower pancreatic margin and dissected to the portal vein. Subsequently, an extensive Kocher maneuver is performed, and the first jejunal loop is divided using an endostapler to uncross the duodenum. Pancreatic transection is performed using the monopolar scalpel. The mesopancreas is divided using bipolar forceps until the uncinate process, and the pancreatic head of the superior/portal mesenteric vein are fully mobilized. Lastly, cholecystectomy is performed, and the main bile duct is divided, placing the surgical specimen in an extraction bag.
Reconstruction is performed using a single loop. Firstly, the end-to-side pancreaticojejunostomy is carried out with two continuous 3/0 barbed sutures (Stratafix®) and a 5/0 PDS duct-to-mucosa suture with interrupted stitches over a 6−8Fr tutor. In cases in which the Wirsung duct is not identified, a pancreaticogastrostomy is performed. At about 20 cm, in the same jejunal loop, the end-to-side hepaticojejunostomy is performed with continuous 3/0 barbed suture (Stratafix®) except if it was narrow, in which case it was performed with interrupted 4/0 PDS stitches. Finally, the antecolic gastroenteric anastomosis is performed with a 60 mm endostapler, closing the defect with continuous 2/0 barbed suture (Stratafix®).
Two drains are placed, the right one after the hepaticojejunostomy and the left one after the gastroenteroanastomosis with the end adjacent to the pancreaticojejunostomy.
A Pfannenstiel incision is made for the extraction of the piece that is closed by planes with continuous 2/0 PDS.
Open pancreaticoduodenectomyThe open technique is very similar to the laparoscopic one, although this varied over time thanks to the advances we made with the laparoscopic technique. Access is made through subcostal laparotomy, and a sequence of steps is followed as in laparoscopic surgery. The single-loop reconstruction and type of pancreatic anastomosis were maintained. Hepaticojejunostomy, which was initially performed with interrupted 4/0 PDS stitches, was now performed following the same scheme as in laparoscopic surgery. Similarly, the gastrojejunal anastomosis, usually performed with 3/0 Novosyn® interrupted stitches, was now done with an endostapler and Stratafix®. Finally, the subcostal laparotomy is closed in planes with continuous 2/0 PDS sutures
Main variablesThe main variables studied were: surgical time, hospital stay, conversion rate, morbidity measured using the Dindo-Clavien scale14 and 90-day associated mortality.
Conversion was defined by any accessory incision in the laparoscopy group that was not for one of the trocars or for the extraction of the surgical specimen.
Scheduled assisted laparoscopy was defined as a resection performed laparoscopically with anastomoses performed through a right subcostal incision (15 cm).
Secondary variablesWe have also considered secondary variables, which included: pancreatic fistula, biliary fistula, postoperative hemorrhage, delayed gastric emptying, state of the resection margin, number of lymph nodes obtained, and 30-day readmission rate.
Pancreatic fistulae, postoperative bleeding, and delayed gastric emptying were classified following the definitions established by the International Study Group of Pancreatic Surgery (ISGPS)15–17. Biliary fistulae were classified following the definitions of the International Study Group of Liver Surgery18.
Regarding resection margins, R0 was defined as a distance from the tumor margin >1 mm, R1 if the distance with the tumor margin was <1 mm, and R2 if there was a macroscopic tumor in the resection margin19.
Statistical analysisThe statistical analysis involved an intention-to-treat analysis using SPSS® software (version 24). The normal distribution was studied using the Kolmogorov–Smirnov test and the different variables using the Mann–Whitney U and chi-squared tests. Quantitative variables are expressed as means and range, and qualitative variables as number and percentage. Differences with a P value <.05 were considered statistically significant.
ResultsIn the established period, 98 PD were performed, 54 of which met the inclusion and exclusion criteria described. These 54 procedures were distributed as 31 open PD and 23 laparoscopic PD, and only 3 procedures in this group were assisted by laparoscopy.
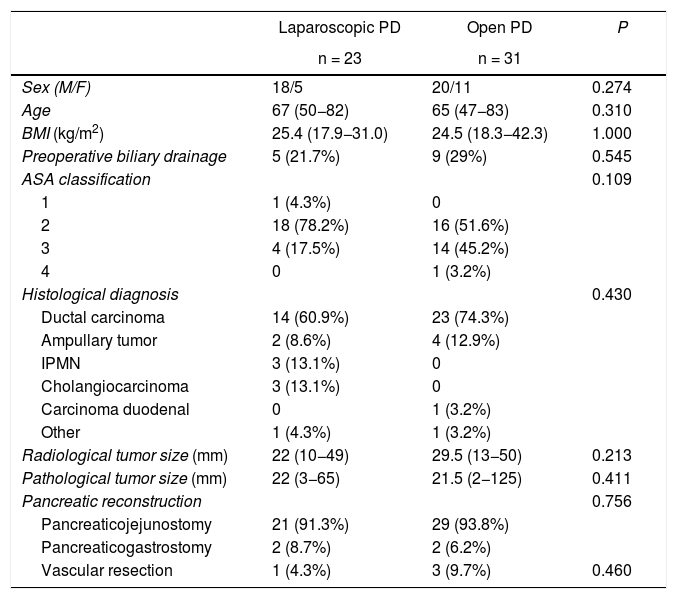
Characteristics of the patientsDespite having made a selection of the most suitable patients for laparoscopic surgery, no significant differences were found between the patient characteristics of the two groups. There were no differences in sex, age, body mass index, American Society of Anesthesiologists (ASA) classification, preoperative biliary drainage, tumor size, histological diagnosis, type of pancreatic reconstruction or need for vascular resection (Table 1). It should be noted that the need for vascular resection was assessed intraoperatively; in the case of laparoscopy, partial lateral resection was performed with an endostapler, while in open surgery the resections were complete, with end-to-end anastomosis.
Baseline characteristics and demographic variables of the sample.
| Laparoscopic PD | Open PD | P | |
|---|---|---|---|
| n = 23 | n = 31 | ||
| Sex (M/F) | 18/5 | 20/11 | 0.274 |
| Age | 67 (50−82) | 65 (47−83) | 0.310 |
| BMI (kg/m2) | 25.4 (17.9−31.0) | 24.5 (18.3−42.3) | 1.000 |
| Preoperative biliary drainage | 5 (21.7%) | 9 (29%) | 0.545 |
| ASA classification | 0.109 | ||
| 1 | 1 (4.3%) | 0 | |
| 2 | 18 (78.2%) | 16 (51.6%) | |
| 3 | 4 (17.5%) | 14 (45.2%) | |
| 4 | 0 | 1 (3.2%) | |
| Histological diagnosis | 0.430 | ||
| Ductal carcinoma | 14 (60.9%) | 23 (74.3%) | |
| Ampullary tumor | 2 (8.6%) | 4 (12.9%) | |
| IPMN | 3 (13.1%) | 0 | |
| Cholangiocarcinoma | 3 (13.1%) | 0 | |
| Carcinoma duodenal | 0 | 1 (3.2%) | |
| Other | 1 (4.3%) | 1 (3.2%) | |
| Radiological tumor size (mm) | 22 (10−49) | 29.5 (13−50) | 0.213 |
| Pathological tumor size (mm) | 22 (3−65) | 21.5 (2−125) | 0.411 |
| Pancreatic reconstruction | 0.756 | ||
| Pancreaticojejunostomy | 21 (91.3%) | 29 (93.8%) | |
| Pancreaticogastrostomy | 2 (8.7%) | 2 (6.2%) | |
| Vascular resection | 1 (4.3%) | 3 (9.7%) | 0.460 |
ASA: American Society of Anesthesiologists; PD: pancreaticoduodenectomy; M/F: males/females; BMI: body mass index; IPMN: intraductal papillary mucinous neoplasm.
Between parentheses, interquartile range and percentages.
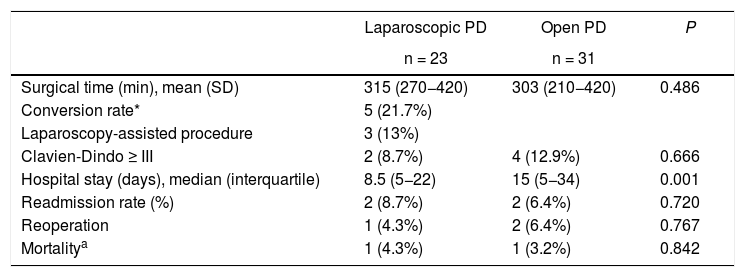
Significant differences in hospital stay were identified. In the laparoscopic surgery group, the mean stay was 8.5 days (5–22) compared to 15 days (5–34) in the open surgery group (P = .001).
No significant differences were obtained in surgical times (315 [270–420] and 303 [210–420] minutes, respectively [P = .486]), major complications (2 [8.7%] and 4 [12.9%] cases, respectively [P = .666]), or mortality (one case in each group, 4.3% and 3.2%, respectively [P = .842]) (Table 2).
Results of the main variables.
| Laparoscopic PD | Open PD | P | |
|---|---|---|---|
| n = 23 | n = 31 | ||
| Surgical time (min), mean (SD) | 315 (270−420) | 303 (210−420) | 0.486 |
| Conversion rate* | 5 (21.7%) | ||
| Laparoscopy-assisted procedure | 3 (13%) | ||
| Clavien-Dindo ≥ III | 2 (8.7%) | 4 (12.9%) | 0.666 |
| Hospital stay (days), median (interquartile) | 8.5 (5−22) | 15 (5−34) | 0.001 |
| Readmission rate (%) | 2 (8.7%) | 2 (6.4%) | 0.720 |
| Reoperation | 1 (4.3%) | 2 (6.4%) | 0.767 |
| Mortalitya | 1 (4.3%) | 1 (3.2%) | 0.842 |
SD: standard deviation; PD: pancreaticoduodenectomy.
In parentheses, interquartile range and percentages.
Five of the 23 laparoscopic surgeries were converted, representing a conversion rate of 21.7%. Only one was due to a complication (a critical portal vein hemorrhage), while the other four cases were converted due to lack of progression, which was defined as the inability to advance in the surgical technique for more than 20 min. Another three patients (13%) were presented preoperatively as assisted procedures, with no complications until assistance was initiated.
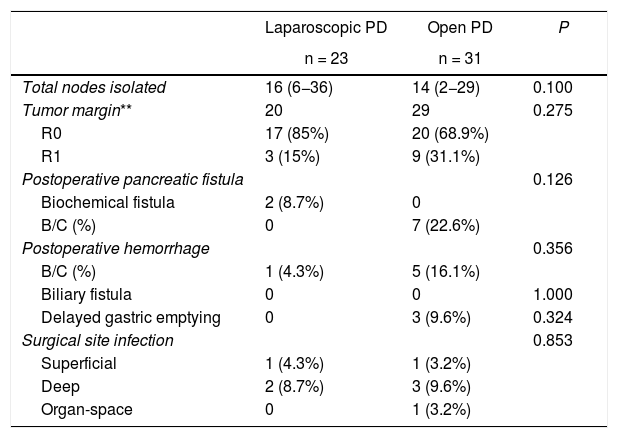
Secondary variablesThere were no statistically significant differences in the grade B/C pancreatic fistula rate, finding no cases in the laparoscopy group and 7 (22.6%) in the open group (P = .126). In the laparoscopy group, two cases (8.7%) presented biochemical fistula.
There were no differences observed in biliary fistula (no patient in either group), postoperative bleeding (one case [4.3%] in laparoscopic PD, and 5 [16.1%] in open PD [P = .356]), delayed gastric emptying (0 and 3 [9.6%], respectively [P = .324]), nor in surgical infection (P = .853). Two cases were readmitted in both groups (8.7% and 6.4%, respectively [P = .720]). In the open surgery group, one readmission was due to hemorrhage requiring urgent digital subtraction angiography, and the other was due to a grade C pancreatic fistula with associated hemorrhage that required reoperation. In the laparoscopy group, both readmissions were due to deep infection of the surgical site, presenting purulent exudate from the surgical wound and requiring drainage. Regarding reoperations, there was one case in the laparoscopy group due to bleeding and two cases in the open group (4.3% vs 6.4%, P = .767), one due to hemorrhage and the other due to grade C fistula associated with bleeding (Table 2).
In the group of neoplastic patients (n = 49), no significant differences have been identified in the R0 rate (17 out of 20 cases [85%] in laparoscopic PD and 20 out of 29 cases [68.9%] in open PD [P = .075]) or in the number of nodes obtained (16 [6–36] and 14 [2–29], respectively [P = .100]) (Table 3).
Results of the secondary variables.
| Laparoscopic PD | Open PD | P | |
|---|---|---|---|
| n = 23 | n = 31 | ||
| Total nodes isolated | 16 (6−36) | 14 (2−29) | 0.100 |
| Tumor margin** | 20 | 29 | 0.275 |
| R0 | 17 (85%) | 20 (68.9%) | |
| R1 | 3 (15%) | 9 (31.1%) | |
| Postoperative pancreatic fistula | 0.126 | ||
| Biochemical fistula | 2 (8.7%) | 0 | |
| B/C (%) | 0 | 7 (22.6%) | |
| Postoperative hemorrhage | 0.356 | ||
| B/C (%) | 1 (4.3%) | 5 (16.1%) | |
| Biliary fistula | 0 | 0 | 1.000 |
| Delayed gastric emptying | 0 | 3 (9.6%) | 0.324 |
| Surgical site infection | 0.853 | ||
| Superficial | 1 (4.3%) | 1 (3.2%) | |
| Deep | 2 (8.7%) | 3 (9.6%) | |
| Organ-space | 0 | 1 (3.2%) | |
PD: pancreaticoduodenectomy.
In parentheses, interquartile range and percentages.
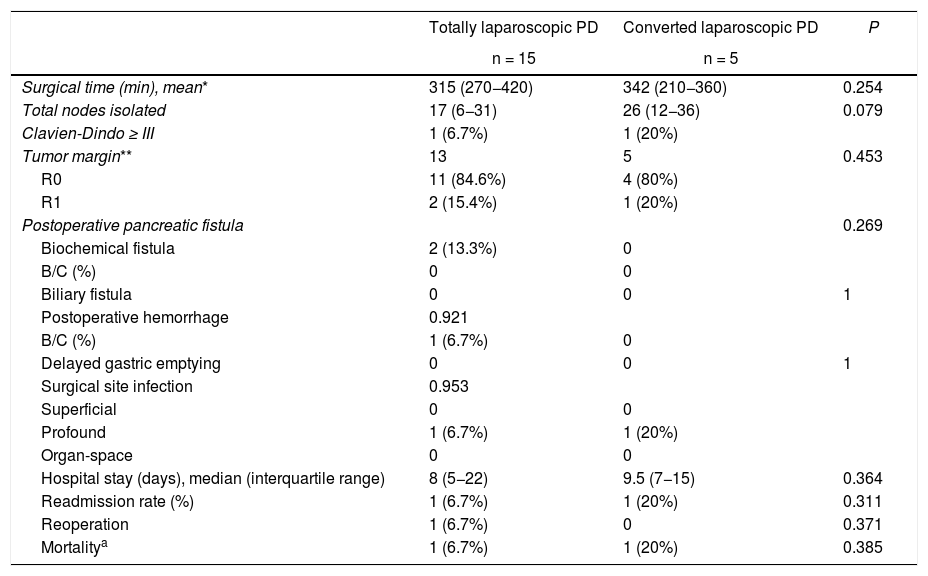
When we analyzed separately the patients in whom PD was completed by laparoscopy from those who were converted, no differences were observed in the different variables analyzed, showing a very similar hospital stay (8 [5–22] and 8.5 [7–19] days, respectively) (Table 4).
Comparative analysis between laparoscopic approach and conversion.
| Totally laparoscopic PD | Converted laparoscopic PD | P | |
|---|---|---|---|
| n = 15 | n = 5 | ||
| Surgical time (min), mean* | 315 (270−420) | 342 (210−360) | 0.254 |
| Total nodes isolated | 17 (6−31) | 26 (12−36) | 0.079 |
| Clavien-Dindo ≥ III | 1 (6.7%) | 1 (20%) | |
| Tumor margin** | 13 | 5 | 0.453 |
| R0 | 11 (84.6%) | 4 (80%) | |
| R1 | 2 (15.4%) | 1 (20%) | |
| Postoperative pancreatic fistula | 0.269 | ||
| Biochemical fistula | 2 (13.3%) | 0 | |
| B/C (%) | 0 | 0 | |
| Biliary fistula | 0 | 0 | 1 |
| Postoperative hemorrhage | 0.921 | ||
| B/C (%) | 1 (6.7%) | 0 | |
| Delayed gastric emptying | 0 | 0 | 1 |
| Surgical site infection | 0.953 | ||
| Superficial | 0 | 0 | |
| Profound | 1 (6.7%) | 1 (20%) | |
| Organ-space | 0 | 0 | |
| Hospital stay (days), median (interquartile range) | 8 (5−22) | 9.5 (7−15) | 0.364 |
| Readmission rate (%) | 1 (6.7%) | 1 (20%) | 0.311 |
| Reoperation | 1 (6.7%) | 0 | 0.371 |
| Mortalitya | 1 (6.7%) | 1 (20%) | 0.385 |
Conv.: conversion; PD: pancreaticoduodenectomy.
In parentheses, interquartile range and percentages.
Although the development of minimally invasive surgery in general surgery has been irregular, we could say that the incorporation of pancreatic surgery is recent, slow and not extensive.
The use of laparoscopic distal pancreatectomy has become more widespread and accepted in recent years. However, the use of laparoscopic PD is more limited because the aggression of the resection is significant regardless of the approach and due to the proximity of large vessels with the potential for difficult-to-control hemorrhage.
Many surgical groups do not include laparoscopic PD in their daily practice. It is difficult to learn, surgical times are long, and there are purportedly serious intraoperative problems that call into question its application. Under these conditions, it is difficult to determine the future of this procedure12.
Previous studies have described controversial results in terms of safety and efficacy20,21 but, in most, positive results depend on the volume of PD performed at the hospital, both open and laparoscopic, placing the cut-off at a number of procedures greater than 20 per year13,22. In our case, the advantage of the collaboration of two referral hospitals in pancreatic surgery with the same criteria, the same protocols, and the same shared surgical group has enabled us to obtain an extensive series of more than 50 cases of PD in just over 24 months.
This series demonstrates that laparoscopic PD can be performed with similar safety to open surgery. It guarantees the criteria for adequate oncological surgery while maintaining the benefits of minimally invasive surgery with a shorter postoperative stay.
We believe that these results are due, in part, to the fact that the surgical group has extensive experience in open pancreatic surgery and advanced laparoscopic surgery, as well as the development of the technique in phases. Before starting the laparoscopic PD program, as a learning curve, most of the steps had been partially and progressively performed in several patients. Furthermore, once the program had started, the first patients who were anticipated to be complex were scheduled for laparoscopy-assisted PD, where the reconstruction was performed as open surgery.
On the other hand, an early and flexible conversion policy (if adequate progress is not made before a complication appears) seems fundamental to us. In our study, only one case was converted due to a hemorrhagic complication after a mesenteric vein injury that was resolved without problems due to the experience in open surgery, while in the other cases the reason was the lack of progression with non-urgent conversion.
Following this strategy and with clear standardization, the surgical times did not show significant differences and remained around 5 h. We feel this time is appropriate as it is well below the 10 h reported in some series, which we consider too long for both the patient and for the surgical team23.
The reconstruction phase affects surgical time considerably. The use of barbed sutures and extensive experience in laparoscopic suturing greatly shortens the time. In robotic series, the ease in the anastomosis and suture procedure is cited as an advantage, although the future role of robotic surgery remains to be seen8,24,25.
The surgical technique in the two groups is well established and similar. In open surgery, continuous barbed suture was used in the pancreaticojejunostomy, which we had designed for laparoscopic surgery and was ultimately used in both groups.
We are aware that this is a non-randomized prospective study in which the comparison is not strict and the patients who underwent the laparoscopic procedure were selected, possibly making the surgery easier. However, when demographic data, history, and tumor size were compared, no significant differences were found. Even so, we believe that it is essential to select only those cases that are considered favorable for laparoscopic surgery. We have ruled out borderline-resectable cases that received neoadjuvant treatment as we considered them, a priori, more complex.
Comparative studies between laparoscopic PD and open surgery show a conversion rate in the laparoscopic group between 0% and 23%8,24,26. Our series is situated in the upper margin, which we believe is due to our policy of early conversion in the event of non-progression, while the surgical times are somewhat lower than the lower margin, partly due to the same reasoning.
We have not found differences in the general complications of the groups using the Dindo–Clavien classification, nor have we found any when we analyzed only those specific to this type of surgery, such as pancreatic fistula, biliary fistula, delayed gastric emptying and bleeding. When we assessed the absolute numbers, there was a lower tendency in the laparoscopic group, a fact that should be confirmed if the series is expanded and this trend is maintained.
As in the present study, other comparative series do not show differences in blood loss. A similar fact occurs when specific pancreatic complications are analyzed8,22. The reoperation rate varies from 3% to 24% for the laparoscopic group and between 2% and 11% for open PD, while the rates in our series were at the lower end21,24. In all publications, mortality is slightly higher in the laparoscopy group21, including one case (4.3%) in our series, showing no differences with the laparotomy group and considered acceptable for this procedure.
To date, 3 randomized studies have been conducted: PLOT, PADULAP and LEOPARD-2. The first 2 showed a benefit in favor of minimally invasive surgery. However, concern arose after the LEOPARD-2 trial, in which there was a significant difference in 90-day mortality (10% in the laparoscopic PD group vs 2% in open PD), a fact that led to premature closure of the study12,25,27.
In contrast, we should highlight 2 single-center studies by groups with extensive experience in laparoscopic PD. The first is the Dutch group, which used laparoscopic, robotic and hybrid techniques, reporting a longer operative time, less blood loss and shorter hospital stay in minimally invasive surgery8. The second is a study by the Mayo Clinic in Rochester with 113 laparoscopic cases vs 225 open surgeries, where the operative times were very similar between both groups (around 360 min), the conversion rate was 4%, and the mortality rate was 1%24. These results are similar with our series in terms of surgical time, with lower conversion and mortality rates, and they do not concur with the LEOPARD-2 study12.
In cancer patients, laparoscopic resection has achieved a very high R0 resection rate. It is used in association with extensive excision of the lymph nodes, with an average of 16 nodes, which is similar to the 14 obtained in open surgery and can be considered optimal. These results confirm that the proposed en bloc resection by adequate vascular planes is possible and feasible. It should be taken into account that the patients were selected and considered feasible a priori, but even so, in one case we need to perform venous resection to ensure R0 resection.
We do not have data about the evolution of patients who are started by laparoscopy and must be converted; there are discrepancies regarding a possible harmful effect of this conversion, which may go beyond indicating difficulties28. We know that the need for a quick conversion with unwanted steps can make a difficult situation worse, and this situation should be avoided. In our series, the evolution of converted patients was similar to those in whom the procedure was completed laparoscopically. This is another fact that reaffirms our protocol of conversion when faced with lack of progression during surgery. It is essential for us not to modify and worsen the natural history of an aggressive surgery like PD.
The current role of surgery in patients with a neoplasm in the head of the pancreas is to perform a standardized R0 resection with minimal morbidity and mortality, maintaining physical and nutritional status, and achieving rapid recovery so that patients can then undergo effective adjuvant treatment29. With this approach, open PD is well established, and its development has reached its limit. What remains to be demonstrated is whether the laparoscopic approach with the same oncological criteria has the advantages of minimally invasive surgery and will be beneficial in the future.
Our study has the limitation of being a prospective study of selected patients who were not randomized, with a reduced n. For this reason, new studies in coming years will be required to clarify in what situations laparoscopic PD can be performed safely and whether it is feasible; what the results are and whether they are comparable with open surgery; whether it is effective, who should perform it, and whether it is reproducible. This makes for an interesting challenge, but, with a sufficient number of patients and the collaboration of different units and surgeons, these questions will be able to be answered.
ConclusionsIn a selected group of patients, laparoscopic PD is safe and effective when performed by experts in pancreatic surgery and advanced laparoscopy techniques. A stepwise program with adequate patient selection and lax and early conversion avoids surgical accidents.
Compared with open surgery, laparoscopic PD obtains the same results in the initial postoperative period and is oncologically adequate in tumor cases with a shorter hospital stay.
It has been suggested that greater experience and number of cases may make the trend towards a lower rate of postoperative complications significant. However, multi-center and prospective studies are required to confirm this.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Espin Alvarez F, García Domingo MI, Cremades Pérez M, Herrero Fonollosa E, Navinés López J, Camps Lasa J, et al. Luces y sombras de la duodenopancreatectomía cefálica laparoscópica. Cir Esp. 2021;99:593–601.