It remains unclear whether liver resection is justified in patients with non-colorectal non-neuroendocrine liver metastases (NCNNLM). A single-center study was conducted to analyse overall survival (OS), disease-free survival (DFS), and potential prognostic factors in patients with different types of NCNNLM.
MethodA retrospective analysis of all patients who underwent liver resection of NCNNLM from January 2006 to July 2019 was performed.
ResultsA total of 62 patients were analyzed. 82.3% presented metachronous metastases and 74.2% were unilobar. The most frequent primary tumor site (PTS) were breast (24.2%), urinary tract (19.4%), melanoma (12.9%), and pancreas (9.7%). The most frequent primary tumor pathologies were breast carcinoma (24.2%), non-breast adenocarcinoma (21%), melanoma (12.9%) and sarcoma (12.9%). The most frequent surgical procedure performed was minor hepatectomy (72.6%). R0 resection was achieved in 79.5% of cases. The major complications’ rate was 9.7% with a 90-day mortality rate of 1.6%. The 1, 3 and 5-year OS/DFS rate were 65%/28%, 45%/36% and 46%/28%, respectively. We identified the response to neoadjuvant therapy and PTS as possible prognostic factors for OS (P =0.06) and DFS (P =0.06) respectively.
ConclusionBased on the results of our series, NCNNLM resection produces beneficial outcomes in terms of OS and DFS. PTS and the response to neoadjuvant therapy could be the main prognostic factors after resection.
No está totalmente aclarado si la resección hepática está justificada en pacientes con metástasis hepáticas no colorrectales no neuroendocrinas (MHNCNN). Hemos realizado un estudio en un solo centro para analizar la supervivencia global (SG), la supervivencia libre de enfermedad (SLE) y posibles factores pronósticos en pacientes con diferentes tipos de MHNCNN.
MétodoSe realizó un análisis retrospectivo de todos los pacientes que se sometieron a resección hepática de MHNCNN desde enero de 2006 hasta julio de 2019.
ResultadosSe analizaron un total de 62 pacientes. El 82,3% presentó metástasis metacrónicas y el 74,2% fueron unilobares. El sitio original del tumor primario (STP) más frecuente fue mama (24,2%), tracto urinario (19,4%), melanoma (12,9%) y páncreas (9,7%). Las histopatologías tumorales primarias más frecuentes fueron el carcinoma de mama (24,2%), el adenocarcinoma no mamario (21%), el melanoma (12,9%) y el sarcoma (12,9%). El procedimiento quirúrgico realizado con mayor frecuencia fue la hepatectomía menor (72,6%). La resección R0 se logró en el 79,5% de los casos. La tasa de complicaciones mayores fue del 9,7% con una tasa de mortalidad a los 90 días del 1,6%. La tasa de SG / SLE a 1, 3 y 5 años fue de 65% / 28%, 45% / 36% y 46% / 28%, respectivamente. Identificamos la respuesta a la terapia neoadyuvante y el STP como posibles factores pronósticos de SG (p = 0,06) y SLE (p = 0,06) respectivamente.
ConclusiónSegún los resultados de nuestra serie, la resección de MHNCNN produce resultados beneficiosos en términos de SG y SLE. El STP y la respuesta a la terapia neoadyuvante podrían ser los principales factores pronósticos tras la resección.
The liver is one of the most common sites of metastasis from solid tumors1. Liver surgery is widely accepted for the treatment of resectable colorectal liver metastases (CRLM)—with over 50% overall survival at 5 years2—, and neuroendocrine liver metastases (NELM)—with 74% overall survival at 5 years3. However, indication of surgical treatment for non-colorectal non-neuroendocrine liver metastases (NCNNLM) remains unclear due to the heterogeneity of the primary tumor types and the limited number of published studies1,4. As to de PTS, the breast is the most frequent location, followed by urinary tract, melanoma, and pancreas4–6. Survival rates are lower for Digestive Origin (DO) liver metastases4,7. Some retrospective studies have suggested that liver resection may be as safe and effective as liver resection of CRLM, although this determination should be made based on survival rates and specific risk factors for each of the various types of primary tumors1,4,7.
To further explore this matter, we carried out a single-center study to analyze the overall survival (OS) and disease-free survival (DFS) rates, as well as predictive risk factors for recurrence and mortality in patients with NCNNLM who underwent liver resection or ablation.
MethodData collectionWe performed a retrospective, single-center study of patients who underwent liver resection for NCNNLM, from January 2006 to July 2019. Data were obtained from a prospectively maintained database of all patients who had undergone surgery for liver metastases of any type at our department, as part of a high-volume center for hepatobiliary and pancreatic surgery. The study was submitted and approved by our Hospital Research Ethics Committee (date 30th October 2019, study code 18002909).
Inclusion criteria: Patients diagnosed with NCNNLM who underwent resection, either alone or combined with ablation of liver metastases, whose primary tumor was completely resected or resectable at the time they were diagnosed with liver metastasis. In addition, patients with extrahepatic disease who met the prior inclusion criteria were still considered as long as their extrahepatic disease was resectable and responsive to neoadjuvant therapy, if any, following the RECIST 1.1 guidelines. Exclusion criteria: Patients with direct invasion of the liver due to extension of the primary tumor, and those with a primary or benign liver injury, as evidenced by pathological review. Patients with peritoneal carcinomatosis were also excluded, even if they had undergone a resection.
Diagnostic management included establishing the patient’s medical history, and performing clinical examinations and imaging tests, including computed tomography (CT) scans or magnetic resonance imaging (MRI), to define the number and location of hepatic metastases, and to determine the presence of extrahepatic disease. Where extrahepatic disease was suspected, a positron emission tomography (PET) was performed.
Data for this analysis were obtained by reviewing patients’ medical histories using a specific NCNNLM questionnaire, designed to identify patients and tumor characteristics, type of treatment, surgical intervention details, and short and long-term results. The variables considered were the age and sex of the patients, the type and site of the primary tumor, and the presence and site of extrahepatic metastases at the time of primary tumor diagnosis. Other variables considered included time of diagnosis of the liver metastasis (“synchronous metastasis” was defined as liver lesion diagnosed at the time of resection of the primary tumor); length of time from treatment of the primary tumor to diagnosis of metastasis, when metachronous; distribution (unilobar or bilobar); number and size of metastases; neoadjuvant medical therapy (chemotherapy, immunotherapy, or hormone therapy administered before surgery) prior to liver resection; and response to neoadjuvant medical therapy per RECIST 1.1 guidelines. Perioperative clinical results, surgical approach, intraoperative complications, and postoperative outcomes were recorded. Details of the postoperative course were collected. Some of the key short-term data recorded included length of hospital stay, complications’ ranking (according to the Clavien-Dindo Classification8, “severe complication” is defined as greater than or equal to IIIa), re-operation, re-admission, and operative mortality (<90 days after surgery). The long-term data recorded were adjuvant medical therapy, DFS, and OS. For survival rate calculations, we considered patients who were alive 90 days after the surgery.
Surgical approachThe decision for NCNNLM treatment, in cases of synchronous or methacronic liver metastases, was taken by a multidisciplinary team meeting, which include radiologists, oncologists, and surgeons. Surgery was performed when the overall surgical strategy could achieve complete tumor resection, and the disease was controlled by medical treatment. In addition, percutaneous biopsy prior to surgical resection was performed on a case-by-case basis.
Surgical management was planned based on the number, size, and distribution of metastases, as well as remnant liver volume (remnant liver volume to body weight ratio greater than 0.5%, estimated by CT and/or MRI)9. The surgical procedures performed were major hepatectomy (resection of 3 or more anatomical liver segments), minor hepatectomy (resection of less than 3 liver segments and wedge resection)10, and radiofrequency or microwave ablation procedures. Ablation procedures were preferentially reserved for central located lesions ≤30 mm and high risk of liver recurrence (disease-free interval <12 months, bilateral tumor, >1 tumor) to preserve liver parenchyma for future resections if needed. Surgical margins were also recorded after resection of metastases (R0: negative microscopic margin, R1: positive microscopic margin, R2: positive macroscopic margin)11.
Follow-upPatient follow-up management included testing for tumor markers specific to each type of tumor and conducting chest-abdominal CTs or abdominal MRIs every three months for the first two years; then twice a year on the following year; and ultimately once a year for five years. Local recurrence was defined as the reappearance of the tumor within the surgical fields, while systemic recurrence was defined as the recurrence of the disease outside the surgical field.
Statistical analysisContinuous variables are represented by the mean (standard deviation) if they follow a normal distribution, or median (interquartile range) if they do not follow a normal distribution.
Categorical variables are represented by the frequencies of their categories. For the univariate analysis of prognostic factors, survival rates were estimated using the Kaplan–Meier survival curve and compared using the Log-rank test. Factors that showed statistical significance in univariate analysis of P ≤0.2 were entered into the Cox proportional hazard model for multivariate analysis. For this purpose, independent variables where P ≤ .05 were considered significant.
All statistical calculations were performed with IBM SPSS Statistics 21.0 software.
ResultsA total of 62 patients underwent liver resection for NCNNLM during the study period. From 2006 to 2009, 14 patients were included: 3 adenocarcinomas, 2 other mixed carcinomas, 4 melanomas, 4 breast carcinomas, 1 germ-cell tumor. From 2010 to 2012, 14 patients were included: 3 adenocarcinomas, 2 other mixed carcinomas, 3 sarcomas, 5 breast carcinomas, 2 clear cell renal cell carcinoma. From 2013 to 2015, 15 patients were included: 2 adenocarcinomas, 3 melanomas, 1 sarcoma, 6 breast carcinomas, 2 germ-cell tumors, 1 clear cell renal cell carcinoma. From 2016 to 2019, 19 patients were included: 5 adenocarcinomas, 5 other mixed carcinomas, 1 melanoma, 4 sarcomas, 1 germ-cell tumor, 3 clear cell renal cell carcinomas.
Table 1 shows patient and tumor characteristics, details of liver resections, and histopathological outcomes. The median age of patients was 58.7 years (interquartile range [IQR]: 48.1–64.7years). The male/female ratio was 1:1.6. The PTS were the breast (n = 15, 24.2%), urinary tract (n = 12, 19.4%), melanoma (n = 8, 12.9%), pancreas (n = 6, 9.7 %), gynecologic tract (n = 5, 8.1%), stomach (n = 4, 6.5%), retroperitoneum (n = 4, 6.5%), lungs (n = 2, 3.2%), bowels (n = 2, 3.2%), testicles (n = 2, 3.2%), ORL (n = 1, 1.6%) and thyroid (n = 1, 1.6%). Twenty-nine (29) patients (46.8%) were found to have abdominal primary sites, 12 of which (19.4%) had a DO. Synchronous metastases were present in 17.7% of the total cohort, whereas metachronous metastases in 82.3%. As for distribution of liver lesions, they were unilobar in 46 patients (74.2%). Imaging test, CT or MRI, were performed in all patients. PET was performed where extrahepatic disease was suspected (62.9%), except for those patients diagnosed between 2005 and 2007. The median time from treatment of the primary tumor to diagnosis of metachronous metastasis was 28.6 months (IQR: 11.4–60.8 months).
Patient demographics.
| Variables | Median (IQR) or n (%) |
|---|---|
| Total (n) | 62 (100) |
| Age, year | 58.7 (48.1–64.7) |
| Male sex | 24 (38.7) |
| Synchronous: metachronous | 11 (17.7): 51 (82.3) |
| Abdominal primary tumor | 29 (46.8) |
| Digestive primary tumor | 12 (19.4%) |
| Time from treatment of the primary tumor to diagnosis of the metachronus metastasis, month | 28.6 (11.4–60.8) |
| Unilobar: bilobar | 46 (74.2): 16 (25.8) |
| Extrahepatic metastases | 5 (8.1) |
| Neo-adjuvant medical treatment prior to liver resection | 30 (48.4) |
| Response to adjuvant medical treatment prior to liver resection | 25 (83.3) |
| Liver surgery: | |
| Major liver resection | 13 (21) |
| Minor liver resection | 45 (72.6) |
| Only ablation procedure | 4 (6.5) |
| Surgery approach, laparoscopic: open approach | 4 (6.5): 58 (93.5) |
| Operative time, minutes | 185 (150–230) |
| Intraoperative blood transfusion | 8 (12.9) |
| Postoperative complications, major or equal to IIIa grade, Clavien-Dindo classification | 6 (9.7%) |
| Tumor pathology: | |
| Breast carcinoma | 15 (24.2) |
| Adenocarcinoma other than breast | 13 (21) |
| Melanoma | 8 (12.9) |
| Sarcoma | 8 (12.9) |
| Clear cell renal cell carcinoma | 6 (9.7) |
| Germ-cell tumor | 4 (6.5) |
| Other mixed carcinomas | 8 (12.9) |
| Major liver metastasis size, mm | 24 (16–42) |
| Liver metastasis number | 1 (1–3) |
| R0 resection | 35 (79.5) |
| Adjuvant medical treatment after liver resection | 31 (50) |
IQR, interquartile range; abdominal primary tumor, primary tumor that is located within the abdominal cavity; digestive primary tumor, primary tumor whose origin is located in the digestive tract.
Neoadjuvant medical therapy prior to liver resection was administered to 30 patients (48.4%), 83.3% of whom were responsive (14 partial response, 3 complete response, 8 stable disease). Median number of liver metastases was 1 (IQR: 1–3 lesions). Median size of the largest hepatic metastasis was 24 mm (IQR: 16–42 mm). Extrahepatic metastatic disease was present in 5 patients (8.1%) —3 patients with lung disease, and 2 patients with bone disease.
Major hepatectomy was performed in 13 patients (21%), minor hepatectomy in 45 (72.6%), and stand-alone ablation in 4 (6.5%). Negative resection margins (R0) were achieved in 79.5% of hepatectomies. R1 margin was present in 20.5% of the patients. The R0 rate according to histological subtype was 90.9% for adenocarcinomas other than breast, 100% for other mixed carcinomas, 80% for melanomas, 55.6% for breast carcinoma and 80% for clear cell renal cell carcinoma.
Twenty-six (26) patients (41.9%) presented postoperative complications. According to the Clavien-Dindo Classification, 8.1% of these complications were Grade I, and 24.2% were Grade II. Six (6) patients (9.7%) experienced severe complications. Main complications were intra-abdominal infection in 8 patients (12.9%), and bile leak in 3 patients (4.8%). One (1) patient (1.6%) died during the immediate postoperative period due to multiple organ failure. The median postoperative hospital stay was 5 days (IQR: 4–8 days).
In relation to breast carcinoma, the study includes from 2006 to 2009: 1 HER2-positive, and 3 unknown subtypes; from 2010 to 2012: 2 Luminal A, 2 Luminal B, 1 Triple-negative, and 1 unknown subtypes; from 2013–2015: 3 Luminal A, and 3 Luminal B subtypes. Only 8 patients (50%) with Luminal A and Luminal B subtypes, who were included since 2010, received neoadjuvant medical treatment; 6 of them (50%) were treated with hormonal therapy or chemotherapy and they showed partial/stable radiological response.
Regarding sarcomas, the study includes from 2010 to 2012: 3 leiomyosarcomas; from 2013 to 2015: 1 adenosarcoma; from 2016 to 2019: 2 GIST, 1 Ewing sarcoma and 1 leiomyosarcoma. In order to GISTs, both tumors were >10 mitoses per 50HPF (Field High Power) and >5 cm size; these patients received Imatinib as a neoadjuvant treatment with a partial radiological response. Non-GIST tumors did not receive neoadjuvant treatment, only Ewin sarcoma received Gemcitabine-Docetaxel with a stable radiological response.
As for melanomas, the study includes from 2006 to 2009, 1 acral lentiginous melanoma and 4 choroidal melanomas; from 2013 to 2015: 2 choroidal melanomas and 1 lentigo maligna melanoma; from 2016 to 2019: 1 nodular melanoma. The cutaneous melanomas presented a Breslow Depth between 1.7–2 mm. 4 tumors (50%) received neoadjuvant treatment, 3 cutaneous melanoma and 1 choroidal melanoma, only cutaneous melanomas presented partial/stable radiological response, the treatments received were: chemotherapeutic agents in acral lentiginous, monoclonal antibody in lentigo maligna melanoma and a serine-threonine kinase (B-RAF) inhibitor in nodular melanoma.
The median follow-up period spanned over 29 months (IQR: 12.2–64.4 months). The 1, 3, and 5-year OS rates were 65%, 46%, and 36%, respectively, with a median OS of 44.7 months (95%CI 16.9–72.5). OS depending on site and pathology are represented in Table 2. During follow-up, recurrence of the disease was identified in 39 patients (62.9%). This recurrence was exclusively intrahepatic in 16 patients (41%), extrahepatic in 13 patients (33.3%), and both intra and extrahepatic in 10 patients (25.6%). After the first surgery, the 1, 3, and 5-year DFS was 45%, 28%, and 28%, respectively, with a median of 14.75 months (95%CI 0.5–29). The treatment for liver recurrence was primarily medical (chemotherapy or monoclonal antibody treatment depending on the primary tumor pathology) (53.8%) although surgical procedures (resection and/or ablation) were performed in 23.1% of the cases. Those patients with exclusively liver recurrence were treated with surgery resection (12.5%), medical adjuvant treatment (43.8%), ablation (6.3%) or a combined surgery resection and ablation procedure (12.5%). Those patients with intra and extrahepatic recurrence received medical adjuvant treatment (70%) or ablation procedure (10%).
Univariate and multivariate Cox regression analyse for overall survival.
| Variables | Survival | Univariate analysis | Mulivariate analysis | |||
|---|---|---|---|---|---|---|
| 5y-OS | HR | 95% CI for HR | P value | P value | ||
| Gender | Male | 37% | .661 | |||
| Female | 36% | |||||
| Age | <65 years old | 34% | 0.491 | 0.187–1.288 | .140 | .983 |
| ≥ 65 years old | 46% | |||||
| Timing of metastases | Synchronous | 64% | .472 | |||
| Metachronous | 32% | |||||
| Extrahepatic metastases | No | 39% | .980 | |||
| Yes | 50% | |||||
| Extent of liver resection | Minor hepatectomy | 35% | 2.520 | 0.332–19.128 | .112 | .251 |
| Major hepatectomy | 10% | 3.486 | 0.408–29.745 | |||
| Ablation (reference) | 100% | |||||
| Intraoperative blood transfusion | No | 33% | .834 | |||
| Yes | 21% | |||||
| Operative time | <180 min | 57% | .379 | |||
| ≥180 min | 18% | |||||
| Primary tumor pathology: | Adenocarcinoma other than breast | 18% | .862 | |||
| Other mixed carcinomas | 22% | |||||
| Melanoma | 21% | |||||
| Sarcoma | 47% | |||||
| Breast carcinoma | 37% | |||||
| Germ-cell tumor | 75% | |||||
| Clear cell renal cell carcinoma | 20% | |||||
| Primary tumor site: | Stomach | 71% | .683 | |||
| Gynecologic | 16% | |||||
| Urinary tract | 26% | |||||
| Melanoma | 21% | |||||
| Retroperitoneal | 50% | |||||
| Breast | 37% | |||||
| Bowels | 50% (3y) | |||||
| Lung | 0% (1y) | |||||
| Pancreas | 60% (2y) | |||||
| Testicle | 100% | |||||
| Thyroid | 100% (1y) | |||||
| Distribution of metastases | Unilobar | 24% | .647 | |||
| Bilobar | 45% | |||||
| Liver metastasis size | ≤30 mm | 25% | .388 | |||
| >30 mm | 33% | |||||
| Liver metastasis number | ≤3 | 24% | .908 | |||
| >3 | 40% | |||||
| Margin resection | R0 | 17% | .419 | |||
| R1 | 42% | |||||
| Neo-adjuvant medical treatment | No | 31% | .424 | |||
| Yes | 38% | |||||
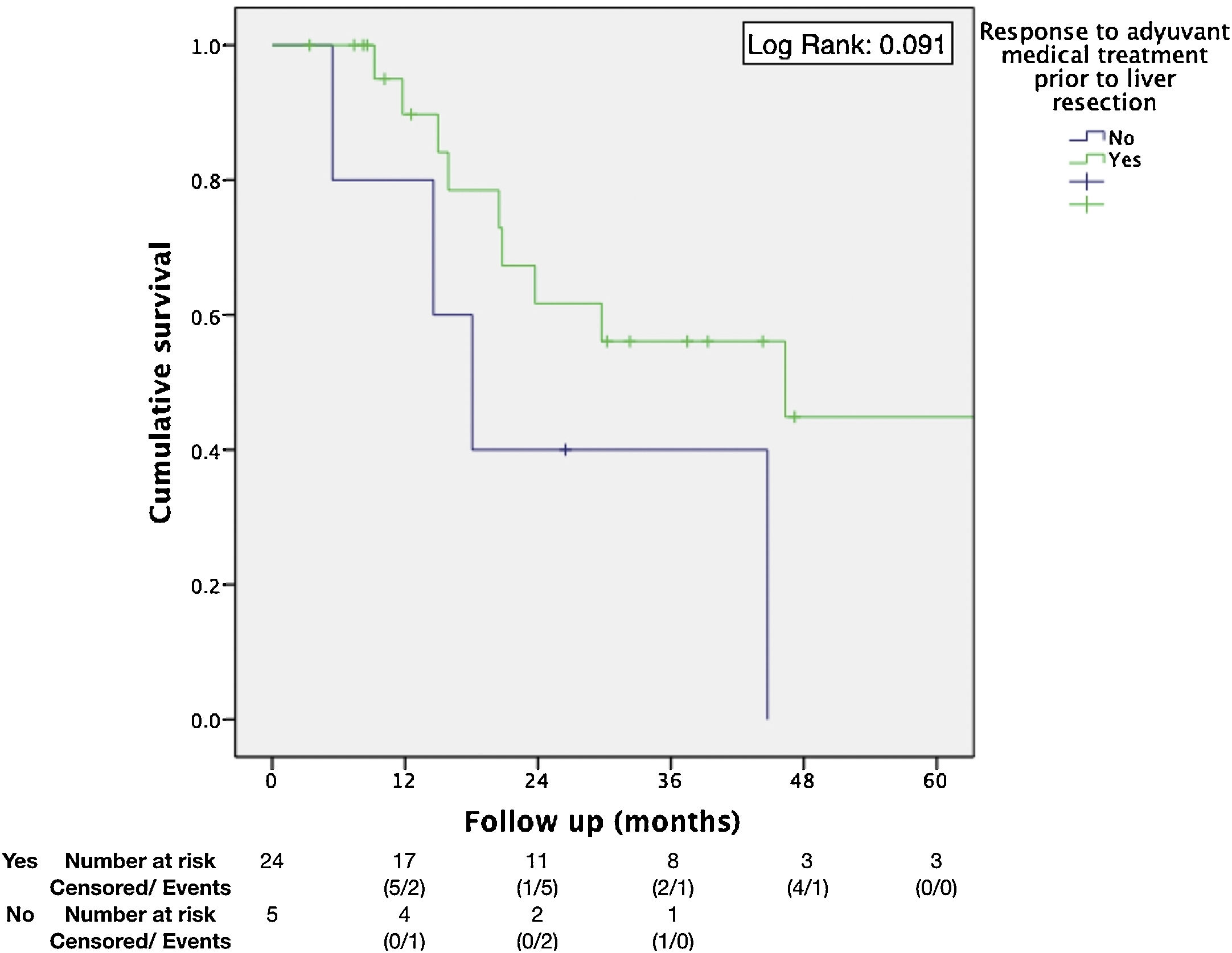
| Response to neo-adjuvant medical treatment | No | 40% (2y) | 0.368 | 0.110–1.231 | .091 | .064 |
| Yes | 47% | |||||
| Surgery approach | Open | 27% | .799 | |||
| Laparoscopy | 60% (3y) | |||||
| Biliar leak | No | 33% | 3.296 | 0.961–11.299 | .044 | .128 |
| Yes | 33% (2y) | |||||
| Intraabdominal infection | No | 43% | .466 | |||
| Yes | 15% | |||||
| Postoperative complications (Clavien-dindo classification) | <3a grade | 34% | .769 | |||
| ≥3a grade | 52% (4y) | |||||
| Adjuvant medical treatment after liver resection | No | 50% | .250 | |||
| Yes | 20% | |||||
| Primary tumor of abdominal origen | No | 36% | .865 | |||
| Yes | 24% | |||||
| Primary tumor of digestive origen | No | 28% | .336 | |||
| Yes | 35% | |||||
| Time from treatment of the primary tumor to diagnosis of the metachronous metastasis | <30 months | 11% | 0.480 | 0.191–1.207 | .111 | .349 |
| ≥30 months | 45% | |||||
HR, hazard ratio; CI, confidence interval; y, year; OS, overall survival; abdominal primary tumor, primary tumor that is located within the abdominal cavity; digestive primary tumor, primary tumor whose origin is located in the digestive tract.
Regarding adenocarcinomas other than breast, extrahepatic recurrence was identified in 4 patients (44.4%), intrahepatic recurrence in 2 patients (22.2%) and recurrence in both locations in 3 patients (33.3%). Among the adenocarcinomas, those located in the stomach, extrahepatic recurrence was treated with radiotherapy and hepatic recurrence received ablation. Gynecological adenocarcinoma was treated with hormone therapy. At the urinary tract level, recurrence received antiangiogenic treatment. At the bowels, pulmonary and pancreatic levels the recurrence was treated with chemotherapy.
In relation to other mixed carcinomas, extrahepatic recurrence was identified in 1 patient (16.7%), intrahepatic recurrence was observed in 4 patients (66.7%) and 1 patient (16.7%) presented recurrence in both locations. Within this subtype, those located at the gynecological level were treated with chemo-radiotherapy, those located at the urinary tract and the lung were treated with monoclonal antibodies, and at the thyroid, recurrence was treated with antiangiogenic treatment.
Regarding to melanoma, extrahepatic recurrence was presented in 2 patients (33.3%), intrahepatic recurrence was identified in 3 patients (50%) and recurrence in both locations was presented in 1 patient (16.7%). Hepatic recurrence was treated with resection and ablation procedures and chemotherapy, extrahepatic recurrence was treated with surgical resection and monoclonal antibodies.
Regarding sarcomas, extrahepatic recurrence was presented in 1 patient (25%), intrahepatic recurrence 2 patients (50%) and both recurrence 1 patient (25%). Three of these patients were treated. The one with retroperitoneal location presented hepatic recurrence and it was treated with surgical resection. Gynecologic and pancreatic locations were treated with chemotherapy.
Breast carcinoma presented 2 patients (22.2%) with extrahepatic recurrence, 4 patients (44.4%) with intrahepatic recurrence, and 3 patients (33.3%) with intra and extrahepatic recurrence. Intrahepatic recurrence was treated in 1 case with resection and surgical ablation, in the rest with chemotherapy; extrahepatic recurrence or both was treated with chemotherapy.
In relation to germ-cell tumors, the only patient who presented recurrence did so in both locations and did not receive treatment; the location of the primary tumor was retroperitoneal.
Clear cell renal cell carcinoma presented extrahepatic recurrence in 3 patients (75%) and intrahepatic recurrence in 1 patient (25%). The patient who presented hepatic recurrence was treated with surgical resection, those who presented extrahepatic recurrence were treated with radiosurgery, surgery and monoclonal antibodies.
When considering the PTS, the most frequent origin was the breast (n = 15), followed by the urinary tract (n = 12; 7 patients with renal cancer, 2 with bladder cancer, 1 with ureter cancer, and 1 with prostate cancer). Melanoma metastases were in third place, with 8 patients (5 with choroidal melanoma and 3 with cutaneous melanoma), and in fourth place were pancreatic tumors (1 patient with leiomyosarcoma, 1 with pseudopapillary carcinoma, and 4 with ductal adenocarcinoma).
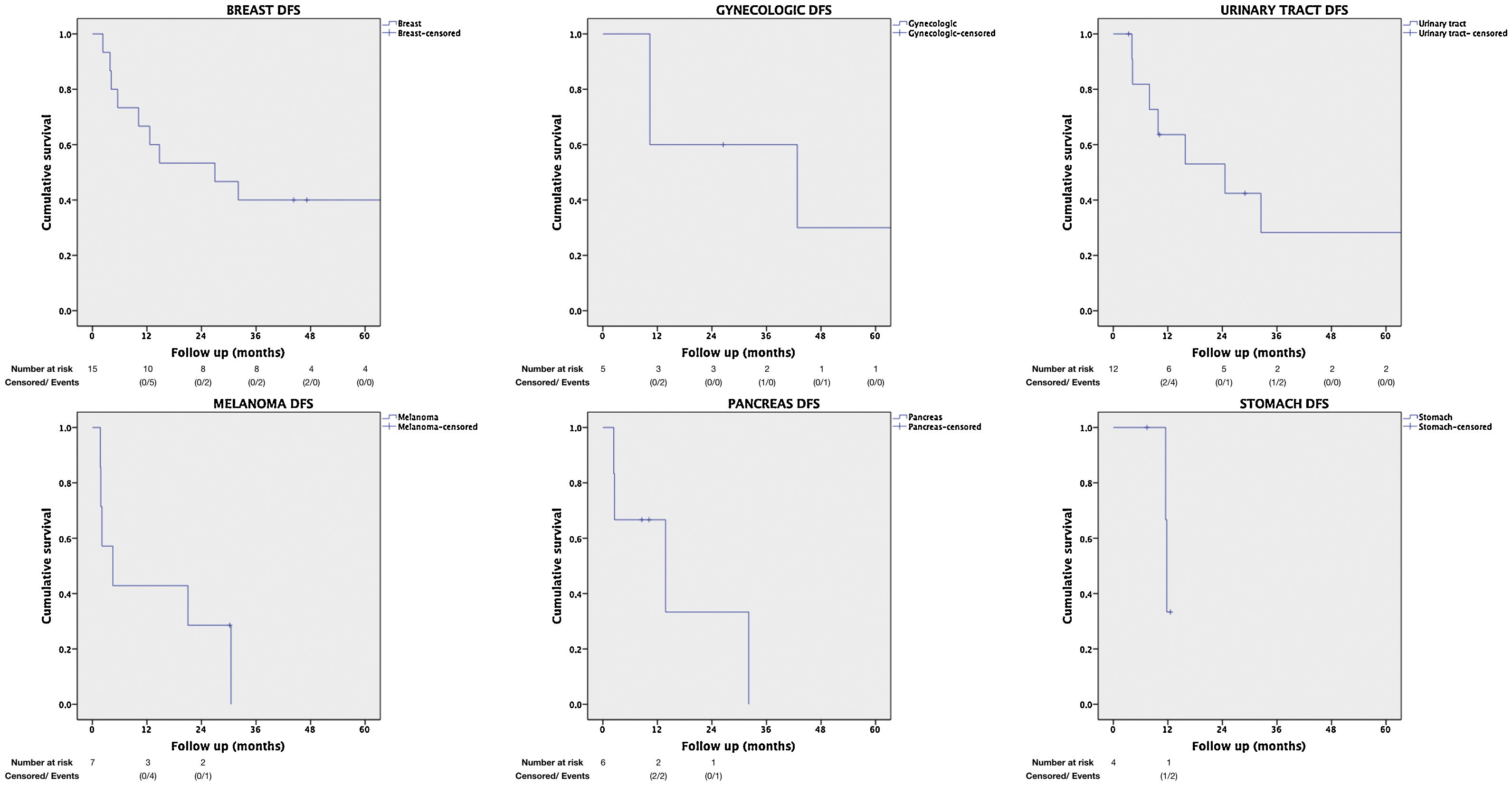
Table 2 presents the remaining less frequent sites, as well as OS and DFS rates per PTS.
The distribution based on primary tumor histology was as follows: breast carcinoma (24.2%), non-breast adenocarcinoma (21%), melanoma (12.9%), sarcoma (12.9%), clear cell renal cell carcinoma (9.7%), germ-cell tumor (6.5%), and other carcinomas (12.9%). When survival rates were analyzed based on the primary tumor histology, a higher 5-year OS was observed in patients with germ-cell tumor (75%), sarcoma (47%), breast carcinoma (37%), and clear cell renal cell carcinoma (30%). Patients whose primary tumor histology was non-breast adenocarcinoma or melanoma had a lower 5-year OS, 23% and 21%, respectively. No statistically significant difference was observed between groups (Log-rank 0.86).
Patients were also classified according to whether they had a primary tumor of DO. Twelve (12) patients (19.4%) presented a primary tumor of DO vs. 50 patients (80.6%) a primary tumor of different origin. The 5-year OS rate was 35% (median of 36.07 months) for patients with a primary tumor of DO vs. 36% (median of 46.32) for patients with a primary tumor of different origin (Log-rank test P = .41).
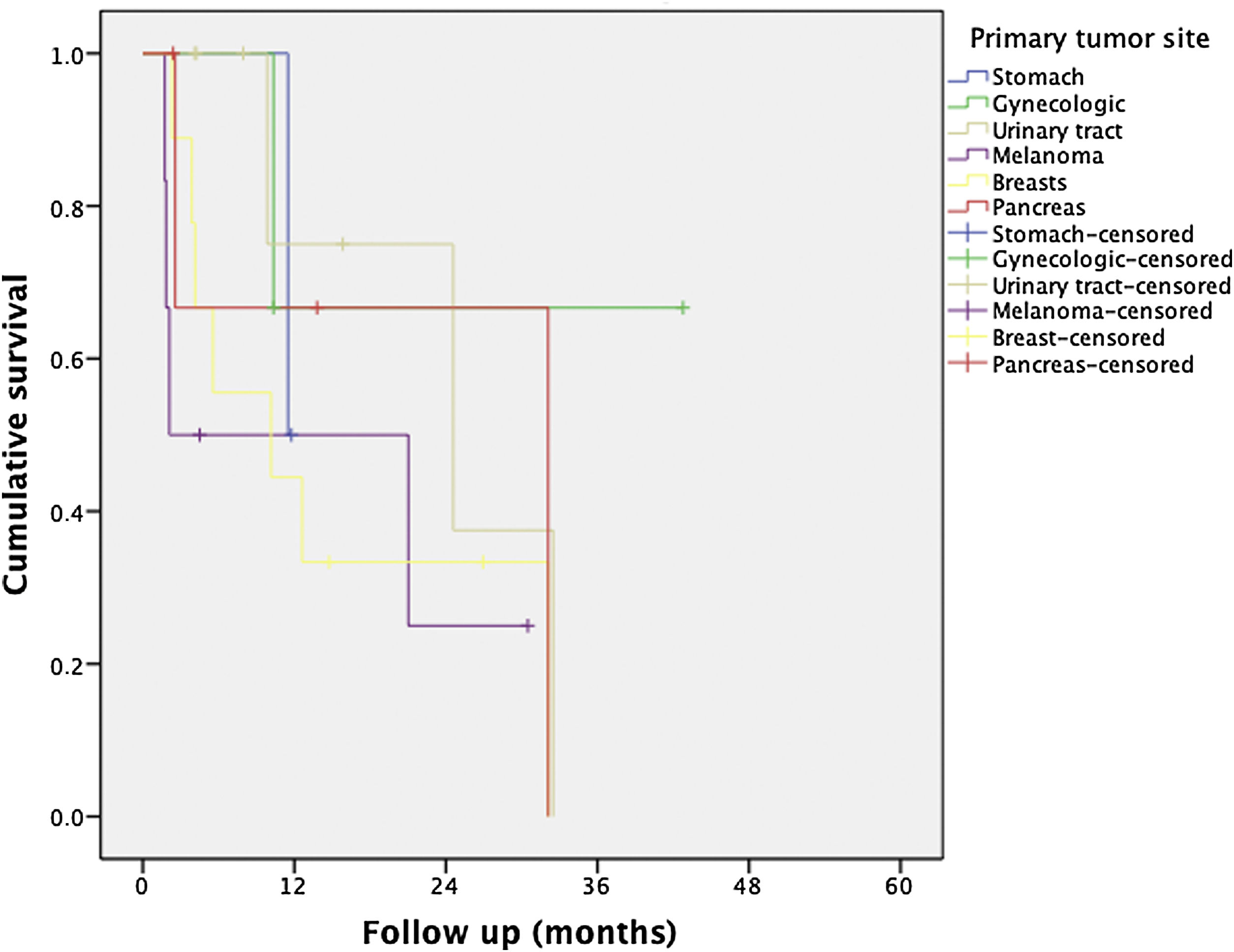
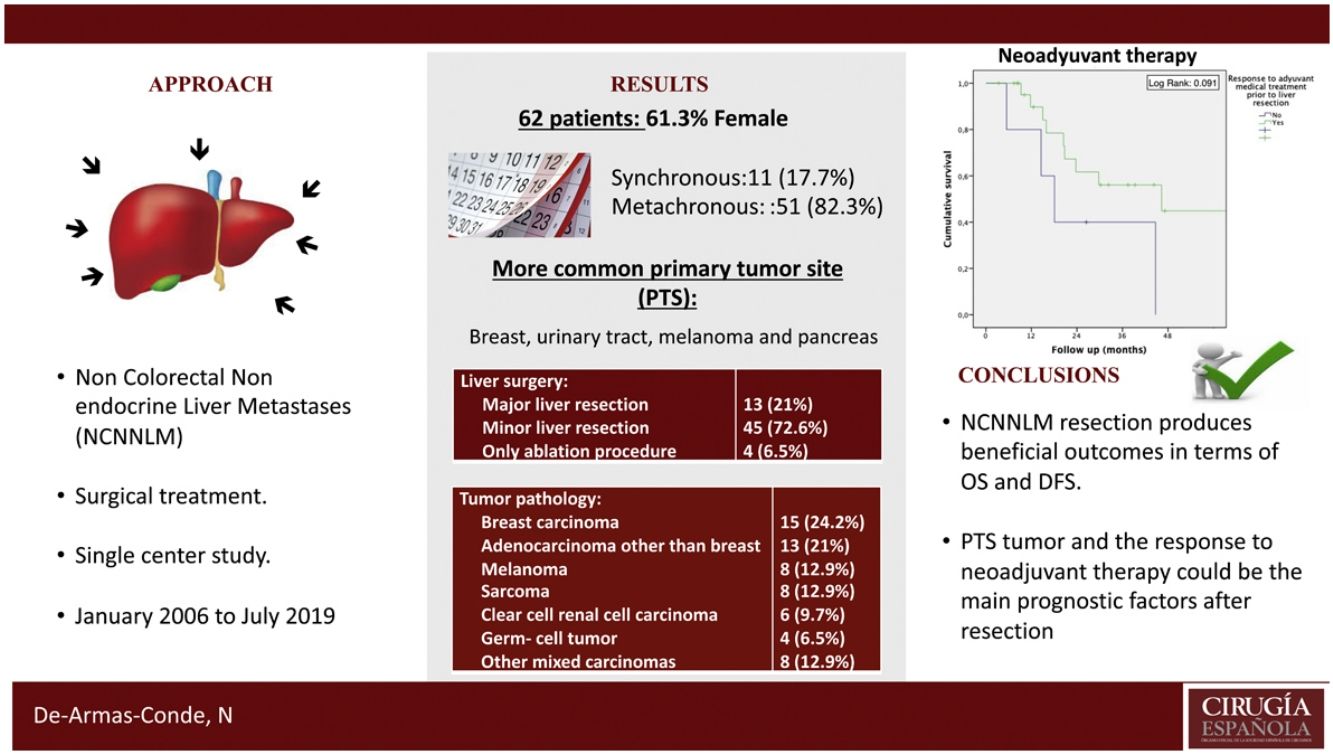
Regarding the identification of prognostic factors, the univariate analysis identified five factors potentially associated with poor OS: age under 65, extended hepatectomy, 30 months or less from time of treatment of the primary tumor to the diagnosis of metachronous metastases, presence of postoperative bile leak, and non-response to neoadjuvant medical therapy. However, using the Cox proportional hazard model for multivariate analysis, none of these prognostic factors were independently associated with lower survival rates (Table 3); only non-response to neoadjuvant medical therapy administered prior to liver resection came close to reaching statistical significance (P = .064) (Fig. 1). The univariate analysis also identified seven factors associated with poor DFS: age under 65, metachronous metastases, operation time equal to or greater than 180 min, PTS, spread of unilobar liver metastases, bile leak, and intra-abdominal infection. However, in the multivariate analysis by Cox proportional hazards model which included all of these factors as well, none of them was independently associated with poorer disease-free survival; only the PTS came close to reaching statistical significance (P = .062) (Fig. 2).
Univariate and multivariate Cox regression analyses for disease-free survival.
| Variables | Survival | Univariate analysis | Mulivariate analysis | |||
|---|---|---|---|---|---|---|
| 5y-DFS (%) | HR | 95% CI for HR | P value | Pvalue | ||
| Gender | Male | 26% | .748 | |||
| Female | 29% | |||||
| Age | <65 years old | 22% | 0.439 | 0.183–1.054 | .057 | .260 |
| ≥65 years old | 48% | |||||
| Timing of metastases | Synchronous | 58% | 0.422 | 0.149–1.193 | .093 | .427 |
| Metachronous | 21% | |||||
| Extrahepatic metastases | No | 35% | .929 | |||
| Yes | 40% | |||||
| Extent of liver resection | Minor hepatectomy | 11% (3y) | .639 | |||
| Major hepatectomy | 29% | |||||
| Ablation | 25% | |||||
| Intraoperative blood transfusion | No | 33% | .403 | |||
| Yes | 14% | |||||
| Operative time | <180 min | 46% | 1.711 | 0.812–3.603 | .151 | .106 |
| ≥180 min | 22% | |||||
| Primary tumor pathology: | Adenocarcinoma other than breast | 17% | .265 | |||
| Other mixed carcinomas | 17% | |||||
| Melanoma | 10% (2y) | |||||
| Sarcoma | 25% (3y) | |||||
| Breast carcinoma | 40% | |||||
| Germ-cell tumor | 75% | |||||
| Clear cell renal cell carcinoma | 15% (2y) | |||||
| Primary tumor site: | Stomach | 43% (1y) | 1 | 0.153–6.527 | <.001 | .062 |
| Gynecologic | 30% | 1 | 0.209–4.786 | |||
| Urinary tract | 29% | 1 | 0.238–4.200 | |||
| Melanoma | 10% (2y) | 1 | 0.185–5.404 | |||
| Retroperitoneal | 33% (1y) | 1 | 0.113–8.876 | |||
| Breasts | 40% | 1 | 0.253–3.945 | |||
| Bowels | 50% (3y) | 1 | 0.134–7.460 | |||
| ORL | 1 | 0.000– 4697975.16 | ||||
| 0.020–49.337 | ||||||
| Lung | 1 | 0.181–5.534 | ||||
| Pancreas | 30% (1y) | 1 | 0.006–162.5919 | |||
| Thyroid | 1 | |||||
| Testicle (reference) | 100% | |||||
| Distribution of metastases | Unilobar | 20% | 1.981 | 0.871–4.503 | .095 | .161 |
| Multilobar | 50% | |||||
| Liver metastasis size | ≤30 mm | 28% | .825 | |||
| >30 mm | 28% | |||||
| Liver metastasis number | ≤3 | 26% | .736 | |||
| >3 | 31% | |||||
| Margin resection | R0 | 18% | .422 | |||
| R1 | 33% | |||||
| Neo-adjuvant medical treatment prior to liver resection | No | 25% | .909 | |||
| Yes | 35% | |||||
| Response to adjuvant medical treatment prior to liver resection | No | 13% (2y) | .278 | |||
| Yes | 39% | |||||
| Surgery approach | Open | 28% | .5 | |||
| Laparoscopic | 25% (3y) | |||||
| Biliar leak | No | 32% | 2.474 | 0.751–8.154 | .123 | .978 |
| Yes | 33% (1y) | |||||
| Intraabdominal infection | No | 37% | 1.860 | 0.838–4.132 | .121 | .198 |
| Yes | 38% (1y) | |||||
| Postoperative complications (Clavien-dindo classification) | <3a grade | 31% | .401 | |||
| ≥3a grade | 40% (1y) | |||||
| Adjuvant medical treatment after liver resection | No | 40% | .798 | |||
| Yes | 25% | |||||
| Primary tumor of abdominal origen | No | 32% | .874 | |||
| Yes | 22% | |||||
| Primary tumor of digestive origen | No | 29% | .875 | |||
| Yes | 19% (3y) | |||||
| Time from treatment of the primary tumor to diagnosis of the metachronous metastasis | <30 months | 17% | .514 | |||
| ≥30 months | 28% | |||||
HR, hazard ratio; CI, confidence interval; y, year; DFS, disease-free survival; abdominal primary tumor, primary tumor that is located within the abdominal cavity; digestive primary tumor, primary tumor whose origin is located in the digestive tract.
Liver resection for CRLM and NELM is currently the standard treatment to improve these patients’ OS. Studies have proven that patients with CRLM who have undergone a successful liver resection have a 5-year OS rate of 36–63%12,13. The systematic review by Bellver Oliver et al. estimated that the quality standards for surgery of CRLM were 84% and 34% at 1-year and 5-year OS respectively14 (Fig. 3).
However, there is an ongoing debate on the role of surgical resection for NCNNLM. In most cases, metastases reach the liver through systemic circulation, thus a systemic tumor spread should be assumed15. The diversity and rarity of NCNNLM have impeded consensus on the most appropriate treatment for these patients. Nevertheless, resection for this type of metastasis has increasingly been presented as a safe and viable treatment option4,16. Liver resection for NCNN metastases can be justified primarily because the 5-year DFS ranges between 21–26%, and the 5-year OS ranges between 36%–41%1,4,17. These results are similar to the survival rates of CRLM, although considerations have to be made for individual differences which may affect the prognosis of these patients, mainly the origin of the primary tumor and the response to neoadjuvant therapy.
In analyzing the demographic characteristics, liver metastases characteristics, and the surgical procedure, we found that patients are more likely to develop metastasis when they are between the ages of 50 and 60, while the metastases are frequently metachronous, unilobar, single, and without extrahepatic disease. The most frequent surgical procedure performed was minor hepatectomy with a negative resection margin. These characteristics are comparable to those published by Sim et al.—80.8% unilobar, 67.9% metachronous, median number 1, and only 25.6% with extrahepatic disease15. In terms of postoperative complications, our study shows major complications in 9.7% of patients, and a mortality rate of 1.6%, which is similar to or even lower than other cohorts, with 16%–25% of severe complications and a rate mortality of 1.5%1,5.
As to the PTS, the breast is the most frequent location (24.2%), followed by urinary tract (19.4%), melanoma (12.9%), and pancreas (9.7%). Our distribution is similar to that of other European studies4–6,18, but dissimilar to the that of the Asian studies, in which the most frequent site is the stomach1,7. Regarding the classification by PTS, we included melanoma as a location in order to unify our patients, since most of them are choroidal melanoma and only two are cutaneous melanoma; which is in line with the publication by Slotta et al. where melanoma is also considered as a location6. According to the histology of the primary tumor, breast carcinoma and non-breast adenocarcinoma are the most common malignancies, followed by melanoma, sarcoma, and clear cell renal cell carcinoma, similar to results found by other studies that consider histopathological distribution4,19. For the 62 patients we analyzed, the 5-year OS is 36%, which is in line with the publications by Adam et al. and O'Rourke et al., with their respective 5-year OS rates at 36% to 38.5%4,17, another recent study improves these results with a 52.9% 5-year OS20. In addition, the 5-year OS is even similar to the OS after resection of CRLM, making the resection of NCNNLM an acceptable procedure. Our results in terms of the 5-year DFS, which is 28%, are also similar to the data published by Slotta et al. and O’Rourke et al., who report 5-year DFS rates of 25% and 26.5%, respectively6,17, or to recent publication like Bohlok et al. who reports a 30% 5-year DFS18.
Although these OS results make resection a reasonable treatment option, when correlating survival with the primary origin of the tumor, differences appear. Our distribution in terms of survival is similar to that published in other studies in overall terms4,7,19, but it differs in cases of DO. In these other studies, survival rates are lower for DO; however, in our study, the stomach is the location with the second-best prognosis. This difference is probably due to the fact that in our cohort half the stomach tumors were adenocarcinomas and the other half were sarcomas, whereas the other studies indicated all tumors were adenocarcinomas.
Several articles have mentioned that the origin of the primary tumor is an independent prognostic factor4,17 with a lower OS for those of gastrointestinal or pancreatic origin5,6. This is a good reason to consider liver resection on a case-by-case basis. In our study, we did not find differences in terms of OS; however, the origin of the primary tumor may be a possible prognostic factor for DFS (P = .062), with better DFS rates for primary testicular, breast, gynecological and urinary tract tumors when compared to other sites, such as pancreas, stomach, or melanomas, which is in line with the publication by Labgaa et al. where primary tumor type was associated with recurrence on univariate analysis19.
Another independent factor associated with OS is the response to neoadjuvant medical therapy4,5,7. In our study, neoadjuvant medical therapy was administered to 48.4% of patients, a rate similar to other publications1,5, with a response in 83.3% of them and with a better OS (median of 46.32 months vs. 18.07 months; P = .064). Other prognostic factors have been mentioned in different studies4,17 such as age, time from the treatment of the primary tumor to the diagnosis of metachronous metastasis, extrahepatic disease, resection margins, extended hepatectomy, and diameter of the tumor. However, these factors have not been confirmed in our study.
Our cohort has certain limitations. The main limitation of our study is the small sample size. The small number of cases of some tumour types means that statistical significance is not reached in several of the analyses performed. Secondly, it is a retrospective analysis, which is a disadvantage in and of itself. Thirdly the heterogeneity of the primary tumors, their diverse inherent biology, and the range of treatment options hinder the ability to interpret data and draw conclusions. Since NCNNLMs are rare and cases are few; therefore, prospective studies or randomized controlled trials are difficult to conduct.
ConclusionsDespite the limitations, according to our results, we could consider that NCNNLM resection is a valid procedure in selected cases in the context of multidisciplinary care, even in those of primary tumors traditionally associated with a poor prognosis, with beneficial outcomes in terms of OS and DFS. Primary tumor site and response to neoadjuvant therapy could be the main prognostic factors after resection of NCNNLM. DO does not worsen prognosis versus non-digestive origin after NCNNLM resection.
More multicenter and prospective studies considering all the most recent molecular and genetics variables are required to establish a consensus on treatment.
Ethics approvalThe study was approved by the Ethical Committee of our institution.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
None.
Please cite this article as: De-Armas-Conde N, Ramon-Rodriguez J, Prada-Villaverde A, Jaén-Torrejimeno I, López-Guerra D, Blanco-Fernández G. Influencia del origen e histopatología tumoral tras la resección de metástasis hepáticas de origen no colorrectal no neuroendocrino. Experiencia unicéntrica. Cir Esp. 2022.