A systematic review of the literature was performed with the aim to determine differences in the rate of respiratory complications after esophagectomy for esophageal cancer using minimally invasive access vs traditional thoracic access.
MethodsA literature search was performed using Medline and Cochrane Library, identifying studies that compared the 2 types of thoracic access, regardless of the type of abdominal access (laparotomy/laparoscopy). The studies selected described respiratory complications in absolute numbers and different categories. Studies that considered minithoracotomy as a minimally invasive technique were excluded. Inclusion criteria were studies describing the different types of respiratory complications (9 in total), and analysing the most common complications: respiratory infection, respiratory failure and pleural effusion.
ResultsNine studies were selected (one prospective randomised trial and 8 case control studies) including 1190 patients, 1167 of whom were operated on for esophageal cancer: 482 patients by thoracotomy and 708 by thoracoscopy. Three studies included definitions of respiratory complications, and one stratified them. The more frequent complications that allowed a meta-analysis were respiratory infections, pleural effusion, and respiratory failure. No significant differences were found between the 2 types of access in the global analysis.
DiscussionThe type of thoracic access (thoracotomy or thoracoscopy) does not seem to influence the development of respiratory complications after esophagectomy for cancer. However, the design of the studies analysed, the absence of clear definitions and stratification of the complications make this conclusion questionable. A consensus on the definition of complications and further prospective randomised clinical trials are necessary.
Revisión sistemática de la literatura con el objetivo de determinar diferencias entre el abordaje torá cico mínimamente invasivo y por toracotomía tradicional para la esofagectomía por cáncer de esófago, en términos de complicaciones respiratorias.
MétodosLa búsqueda se ha realizado a través de las bases de datos Medline y Cochrane Library, identificando los estudios que comparaban las 2 variantes técnicas mencionadas, independientemente del tipo de abordaje a nivel abdominal (laparotomía/laparoscopia). Se seleccionaron aquellos estudios que describían las complicaciones respiratorias desglosadas por categorías y en datos absolutos. Se excluyeron los estudios en que se consideraba la minitoracotomía en el grupo de abordaje torá cico mínimamente invasivo. Los criterios de selección fueron: consideramos los estudios en los que se describieron las complicaciones respiratorias desglosadas (9 en total) y analizamos las complicaciones más frecuentes (infecciones respiratorias, insuficiencia respiratoria y derrame pleural).
ResultadosSeleccionamos 9 estudios (un ensayo clínico prospectivo y aleatorizado, y 8 estudios de casos y controles) totalizando 1.190 pacientes, de los cuales 1.167 fueron intervenidos por cá ncer de esó fago, 482 pacientes por toracotomía y 708 por toracoscopia. En 3 estudios se encontraron definiciones de las infecciones respiratorias y la estratificación por gravedad de las complicaciones descritas se encontró en un estudio. Las complicaciones má s frecuentes y que permitieron realizar un metaaná lisis fueron: las infecciones respira- torias, el derrame pleural y la insuficiencia respiratoria. No se identificaron diferencias estadísticas significativas entre los 2 abordajes en el aná lisis global en cuanto a la tasa de complicaciones respiratorias mencionadas.
DiscusiónEl tipo de abordaje torá cico (toracotomía o toracoscopia) no parece influir de forma significativa en el desarrollo de complicaciones respiratorias postesofagectomía por cáncer. Sin embargo, el diseño de los estudios analizados, los criterios de definición heterogé neos y la ausencia de una estratificación adecuada de las complicaciones hacen cuestionable esta constatación. Se necesitan más ensayos clínicos prospectivos y aleato-rizados y un consenso en cuanto a la forma de definir las complicaciones respiratorias postoperatorias postesofagectomía.
Esophagectomies to treat malignancy are one of the most complex interventions in digestive surgery. This procedure is associated with a high complication rate, and respiratory complications rank first with regard to frequency and severity. These complications are usually reported in 40%–50% of cases,1 but the actual incidence varies significantly depending on the entities included in this category and the criteria used to define them. The minimally invasive approach, which is a possible key factor in reducing these complications, has gradually been incorporated within the armamentarium of oesophageal surgery. However, this incorporation has occurred more slowly compared with other areas of surgery due to the technical complexities of the procedures involved.
The assessment of the effects of the type of surgical approach on clinical outcomes is based on experience with patient series in which different minimally invasive surgery methods (i.e., thoraco-laparoscopy or hybrid procedures) are compared with either transthoracic or open transhiatal esophagectomy. Based on these studies, systematic reviews (SRs) have been published that highlight the benefits of the endoscopic approach (e.g., reducing blood loss and hospital stays) without showing the differences related to oncological resection criteria. One SR2 described a trend towards fewer respiratory complications when using minimally invasive techniques, whereas three other SRs3–5 did not find differences when compared with open procedures. However, none of the studies included in these analyses were designed to evaluate the effect of surgical techniques on respiratory complications. A recent retrospective study6 addressed this issue and found a trend towards the decreased incidence of respiratory complications in a minimally invasive surgery group.
ObjectivesThe objective of this study was to evaluate if there are differences in the incidence of respiratory complications between the minimally invasive thoracic approach and traditional thoracotomies for esophagectomies used to treat patients with oesophageal cancer.
Materials and MethodsSearch StrategyA SR of the literature was performed according to the recommendations published in the “Preferred Reporting Items for SRs and Meta-Analyses” (PRISMA) consensus document.7
An electronic search was conducted in Medline and the Cochrane Library; we were unable to use the Embase database due to a lack of institutional or individual access. This search was performed using the following MeSH terms: esophagectomy; thoracoscopy; oesophageal neoplasms/surgery; oesophageal neoplasms/therapy; esophagectomy/adverse effects; surgical procedures, minimally invasive; respiratory tract infections; respiratory insufficiency; and pleural effusion. The search was performed without time or language restrictions; however, it was limited to studies among humans. The final revision was conducted on June 26, 2012, and the results are shown in Fig. 1.
Selection Criteria- 1.
Clinical studies comparing groups of patients who underwent esophagectomies by thoracotomy versus thoracoscopic esophagectomies, regardless of the abdominal approach (laparotomy or laparoscopy). For studies in which the patients and results were described within subgroups based on the abdominal approach type, the data were aggregated by the thoracic approach.
- 2.
Studies that specifically reported respiratory complications were grouped into categories. Studies reporting respiratory complications using absolute values or the percentages of the overall complications without specifying the categories were excluded.
- 3.
Studies that included minithoracotomies in the group of minimally invasive thoracic approaches were excluded.
All the studies that met the aforementioned criteria were included in this study without design constraints.
Data CollectionTwo masked investigators (S.M. and C.B.) reviewed the full text of the papers retrieved using the aforementioned search strategy. All references included in these articles were manually reviewed to identify other possible references. Disagreements regarding the reported variables were decided by consensus.
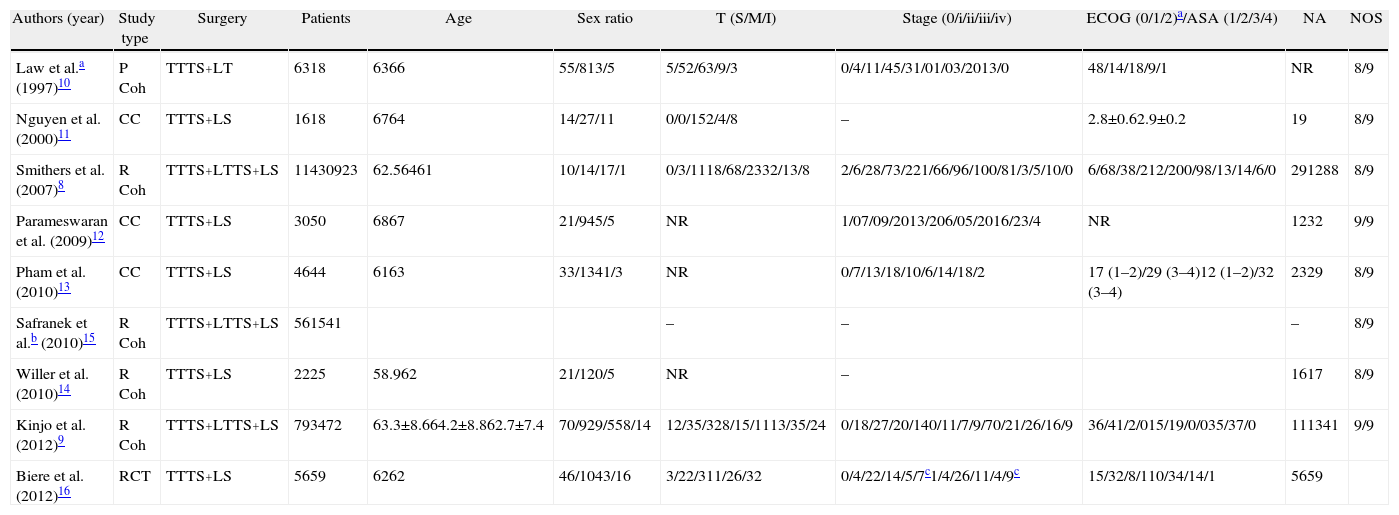
The data collected included the author's name, journal, year of publication, sample demographics, categories of respiratory complications, and associated mortality rates (Tables 1 and 2).
Characteristics of Included Studies.
| Authors (year) | Study type | Surgery | Patients | Age | Sex ratio | T (S/M/I) | Stage (0/i/ii/iii/iv) | ECOG (0/1/2)a/ASA (1/2/3/4) | NA | NOS |
| Law et al.a (1997)10 | P Coh | TTTS+LT | 6318 | 6366 | 55/813/5 | 5/52/63/9/3 | 0/4/11/45/31/01/03/2013/0 | 48/14/18/9/1 | NR | 8/9 |
| Nguyen et al. (2000)11 | CC | TTTS+LS | 1618 | 6764 | 14/27/11 | 0/0/152/4/8 | – | 2.8±0.62.9±0.2 | 19 | 8/9 |
| Smithers et al. (2007)8 | R Coh | TTTS+LTTS+LS | 11430923 | 62.56461 | 10/14/17/1 | 0/3/1118/68/2332/13/8 | 2/6/28/73/221/66/96/100/81/3/5/10/0 | 6/68/38/212/200/98/13/14/6/0 | 291288 | 8/9 |
| Parameswaran et al. (2009)12 | CC | TTTS+LS | 3050 | 6867 | 21/945/5 | NR | 1/07/09/2013/206/05/2016/23/4 | NR | 1232 | 9/9 |
| Pham et al. (2010)13 | CC | TTTS+LS | 4644 | 6163 | 33/1341/3 | NR | 0/7/13/18/10/6/14/18/2 | 17 (1–2)/29 (3–4)12 (1–2)/32 (3–4) | 2329 | 8/9 |
| Safranek et al.b (2010)15 | R Coh | TTTS+LTTS+LS | 561541 | – | – | – | 8/9 | |||
| Willer et al. (2010)14 | R Coh | TTTS+LS | 2225 | 58.962 | 21/120/5 | NR | – | 1617 | 8/9 | |
| Kinjo et al. (2012)9 | R Coh | TTTS+LTTS+LS | 793472 | 63.3±8.664.2±8.862.7±7.4 | 70/929/558/14 | 12/35/328/15/1113/35/24 | 0/18/27/20/140/11/7/9/70/21/26/16/9 | 36/41/2/015/19/0/035/37/0 | 111341 | 9/9 |
| Biere et al. (2012)16 | RCT | TTTS+LS | 5659 | 6262 | 46/1043/16 | 3/22/311/26/32 | 0/4/22/14/5/7c1/4/26/11/4/9c | 15/32/8/110/34/14/1 | 5659 |
ASA: American Society of Anaesthesiologists (this scale estimates the risk from anaesthesia for different patient states); CC: case–control study; P Coh: prospective cohort study; R Coh: retrospective cohort study; RCT: randomised clinical trial; ECOG: Eastern Cooperative Oncology Group (this scale measures the quality of life of patients with cancer); LT: laparotomy; LS: laparoscopy; NA: neoadjuvant; NOS: Newcastle-Ottawa Scale (this tool assesses the methodological quality of nonrandomised, case–control, and cohort studies); NR: not reported; SR: sex ratio; TS: thoracoscopy; TT: thoracotomy.
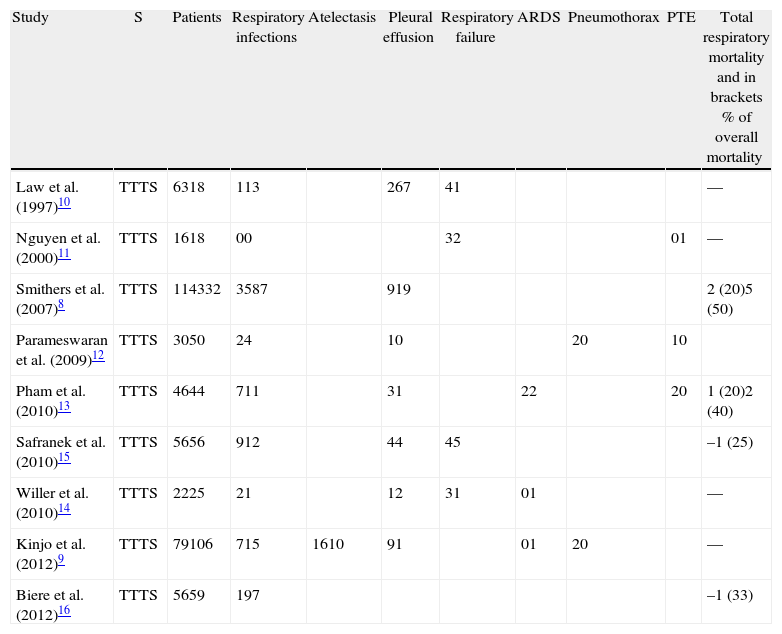
Respiratory Complications.
| Study | S | Patients | Respiratory infections | Atelectasis | Pleural effusion | Respiratory failure | ARDS | Pneumothorax | PTE | Total respiratory mortality and in brackets % of overall mortality |
| Law et al. (1997)10 | TTTS | 6318 | 113 | 267 | 41 | –– | ||||
| Nguyen et al. (2000)11 | TTTS | 1618 | 00 | 32 | 01 | –– | ||||
| Smithers et al. (2007)8 | TTTS | 114332 | 3587 | 919 | 2 (20)5 (50) | |||||
| Parameswaran et al. (2009)12 | TTTS | 3050 | 24 | 10 | 20 | 10 | ||||
| Pham et al. (2010)13 | TTTS | 4644 | 711 | 31 | 22 | 20 | 1 (20)2 (40) | |||
| Safranek et al. (2010)15 | TTTS | 5656 | 912 | 44 | 45 | –1 (25) | ||||
| Willer et al. (2010)14 | TTTS | 2225 | 21 | 12 | 31 | 01 | –– | |||
| Kinjo et al. (2012)9 | TTTS | 79106 | 715 | 1610 | 91 | 01 | 20 | –– | ||
| Biere et al. (2012)16 | TTTS | 5659 | 197 | –1 (33) |
S: surgery; ARDS: acute respiratory distress syndrome; PTE: pulmonary thromboembolism; TT: thoracotomy; TS: thoracoscopy.
The following respiratory complications were included in the final analysis: respiratory infections, respiratory failure, and pleural effusion. The first two are well known as major respiratory complications. Only three complications are addressed in this meta-analysis due to the number of reported cases. Cochrane Review Manager (RevMan) version 5.1 was used for data analyses.
The Cochrane Collaboration tool for assessing the risk of bias was used to assess the methodological quality of the only multicentre, randomised, prospective clinical trial (Biere et al.16). This evaluation determined that the aforementioned study has a low risk of bias with regard to all aspects (i.e., random sequence generation, allocation concealment, blinding, attrition bias, selective reporting of results, and other biases). The Newcastle-Ottawa scale (Table 1) was developed to evaluate non-randomised, case-control, and cohort studies, and it was used to assess the other studies. According to this scale, the studies included in our analysis have an adequate methodological quality. However, this scale was unable to assess a crucial aspect (i.e., the definition of the analysed complications) mentioned in two studies.8,9
Relative risk (RR) was calculated for all studies. The Mantel–Haenszel method was applied to obtain overall RR. RR values were described using 95% confidence intervals (CIs). Probability values of P<.05 were considered significant. To investigate the differences between subgroups, a significance test (described by Borenstein et al.17 and included in RevMan version 5.1) was implemented. The I2 test was also used; this test describes the percentage of variability in the evaluation of the effects between subgroups due to real differences between these groups rather than random effects.
The random effects model was applied because patients were operated on at various centres and at different stages of the learning curve, and technical variations existed.
ResultsNine studies were identified using the aforementioned selection criteria8–16 with a total of 1190 patients; 482 with open chest stage and 708 with thoracoscopic stage. Of these patients, 1167 underwent surgery for oesophageal cancer (adenocarcinoma, squamous cell carcinoma, or other) and 27 underwent surgery for Barrett's oesophagus with high-grade dysplasia, peptic stricture, or another unspecified benign condition. The demographic characteristics of these studies, the tumour stage, the Eastern Cooperative Oncology Group/American Society of Anaesthesiologists (ECOG/ASA) assessment, and the use of neoadjuvant therapies are described in Table 1.
Depending on the design used, one multicentre randomised clinical trial,16 three case-control studies,11–13 four retrospective cohort studies,8,9,14,15 and one prospective cohort study10 were found. The primary objective of two studies was to analyse postoperative respiratory complications.9,16
The terms used to describe the surgical techniques were open Ivor-Lewis esophagectomy, minimally invasive esophagectomy (MIE; thoraco-laparoscopy), and hybrid esophagectomy (thoracoscopy + laparotomy or thoracostomy + laparoscopy).
One study10 compared open Ivor-Lewis esophagectomy with the hybrid approach (thoracoscopy + laparotomy), four studies compared Ivor Lewis esophagectomy with MIE,11–14 and three studies8,9,15 compared Ivor Lewis, MIE, and hybrid esophagectomy.
The spectrum of respiratory complications described by the authors included bronchopneumonia, atelectasis, respiratory failure, acute respiratory distress syndrome, pleural effusion, pneumothorax, and thromboembolism. Respiratory complications were defined in three studies.
- -
Smithers et al.8 reported significant respiratory infection (defined as clinically suspected respiratory infection), which is usually associated with fever (with or without radiological/microbiological confirmation) and requires treatment using intravenous antibiotics for re-admission to the intensive care unit with or without mechanical ventilation.
- -
Kinjo et al.9 reported postoperative complications including respiratory complications (which were defined as those greater than Grade 2 according to the National Cancer Institute Terminology Criteria for Adverse Events; NCI-CTCAE, version 3.0). These authors included cases of pleural effusion and pneumothorax only when they appeared after removing pleural drainage. Atelectasis cases were included only after radiological/bronchoscopy confirmation.
- -
Biere et al.16 reported postoperative pulmonary infection (which was defined as a symptomatology suggestive of pneumonia or bronchopneumonia) confirmed by chest radiograph or chest computed tomography (assessed by different radiologists) and a positive sputum culture during the first 2 weeks after surgery until hospital discharge.
Atelectasis is often associated with thoracoabdominal surgery, but only Kinjo et al.9 mentioned this condition as a complication. These authors comprehensively described all postoperative adverse effects that were most likely due to its subclinical character. Acute respiratory distress syndrome represents the most severe form of acute lung injury with diffuse alveolar involvement, characterised by PaO2/FiO2<200 and the presence of bilateral pulmonary infiltrates in the absence of acute cardiogenic pulmonary oedema. Its aetiology is varied and includes both pulmonary and extrapulmonary diseases; thus, its mention as an isolated respiratory complication without specifying the trigger event does not allow its inclusion in the current analysis. Pulmonary thromboembolism is a major vascular complication rather than a respiratory complication; however, three studies included this condition in the latter category.11–13 Pneumothorax was described in two studies, but these four cases were too few for a meta-analysis of this complication.
A list of respiratory complications is provided in Table 2.
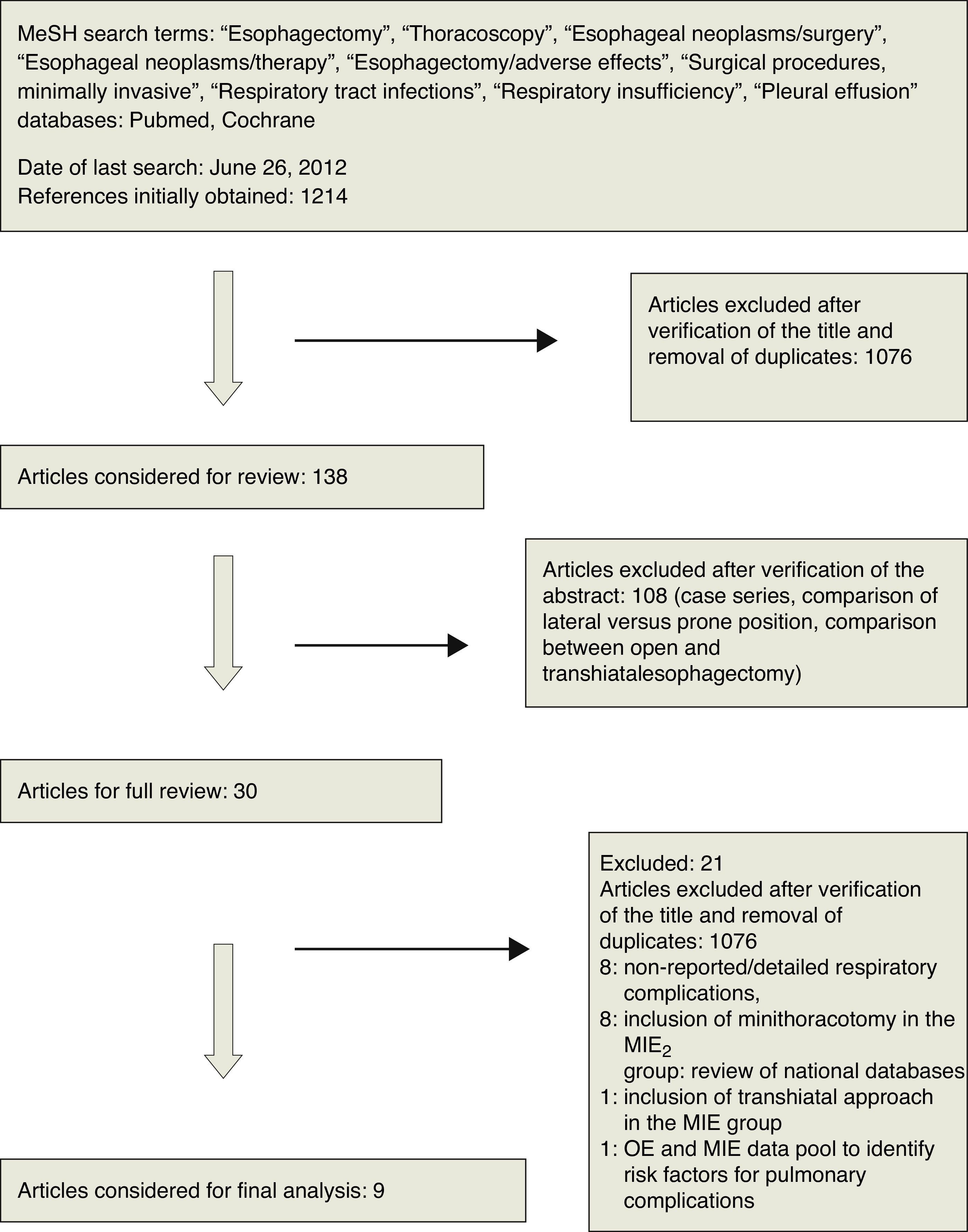
Respiratory InfectionsRespiratory infectious complications were described in eight of nine studies, defined as pneumonia,9,12–14 bronchopneumonia,10 or respiratory infections.8,15,16 Smithers et al.8 subclassified these infections into moderate and severe categories depending on whether the treatment was conducted in a conventional hospital ward or an intensive care unit.
The overall analysis did not reveal a significant difference between esophagectomies with open thoracic versus thoracoscopic approaches with regard to the incidence of respiratory infections (P=.8; RR=0.95, 95% CIs=0.66–1.38; Fig. 2). However, an additional analysis showed a significant difference among subgroups (Chi2=6.38; P=.01; I2=84.3%), which was generated by design differences. The randomised study subgroup was composed of only one study (which sought to assess the effect of surgical approach on respiratory complications, demonstrating the superiority of the minimally invasive technique; P=.009).
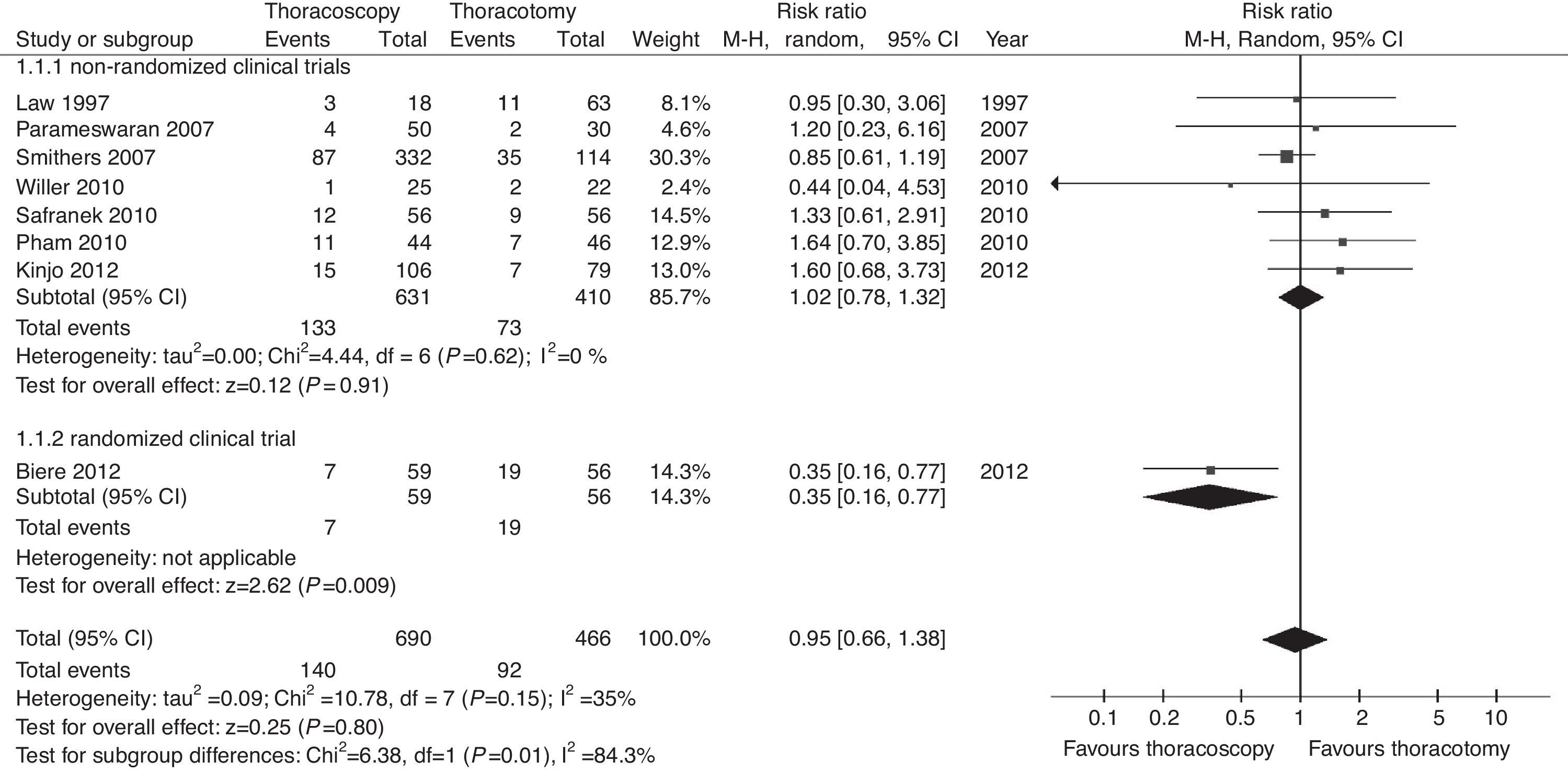
Respiratory FailureFour studies reported cases of post-esophagectomy respiratory failure10,11,14,15 but did not mention the cause of the condition.
No significant differences between groups were found with regard to the incidence of respiratory failure (P=.68; RR=0.84, 95% CIs=0.38–1.90). The results for this outcome were homogeneous (I2=0%; Fig. 3).
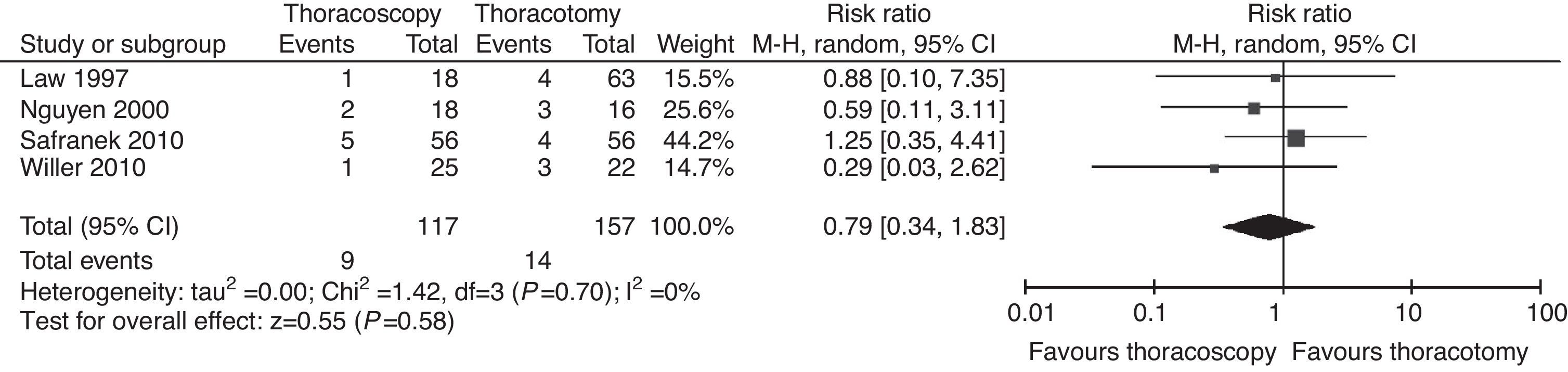
Pleural EffusionSeven studies described pleural effusion among the causes of postoperative morbidity.8–10,12–15 The difference in the incidence of pleural effusion was not significant (P=.2; RR=0.71; 95% CIs=0.41–1.20; (Fig. 4). Unlike the aforementioned complications, moderate heterogeneity was found between studies (I2=18%) except that Kinjo et al.9 only described pleural effusions when they appeared after chest tube removal. All such effusions were National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) Grade 2, which required therapeutic intervention (diuretics or thoracentesis). None of the other six studies mentioned the clinical significance of pleural effusion; however, its inclusion among the complications suggests that these effusions are symptomatic, equivalent to those described by Kinjo et al.,9 which suggests heterogeneity within the random effect.
DiscussionPerforming an esophagectomy for cancer remains an aggressive intervention associated with a 3%–10% mortality rate18 and a >50% morbidity rate.19 Respiratory complications represent the most common type of post-esophagectomy complications. Their causal factors are related to patient condition (age, a history of alcoholism, smoking, altered dental hygiene, preoperative pulmonary reserve, cachexia, and performance status) and the intervention (respiratory muscle involvement, the disruption of bronchial innervation and lymphatic drainage with impaired bacterial clearance, recurrent laryngeal nerve injury with vocal cord paralysis, and prolonged selective ventilation).1
Several retrospective studies have assessed these characteristics to identify the most important factors. Avendano et al.18 reported respiratory complications in 87% of their sample and concluded that the previous alteration of respiratory function (FEV1<65%) was the primary factor associated with mechanical ventilation and prolonged hospital stay. Three large retrospective series analysed major respiratory complications (defined as bronchopneumonia and postoperative respiratory failure with reintubation). Ferguson et al.1 applied a multivariate analysis and found a 27% complication rate among their sample and the following three associated factors: age, FEV1, and performance status. Nakamura et al.20 applied a logistic regression method and reported respiratory complications in 19% of their sample; they identified three significant factors: the absence of preoperative respiratory physiotherapy, the absence of preoperative prophylactic corticosteroids, and intraoperative blood loss >630 cc. Law et al.21 reported respiratory complications in 15.9% of their sample, which were responsible for 55% of hospital deaths. The predictors included age over 70 years, proximal tumours, and prolonged interventions. Therefore, there is a wide range of causes that could be potentially modified.
The need to reduce respiratory complications has led to the incorporation of numerous changes in pre- and perioperative care including improving oral hygiene, smoking cessation, epidural analgesia, bronchoscopic aspiration of secretions, and encouraged postoperative physiotherapy. In Asia, the use of prophylactic corticosteroids and sivelestat (a neutrophil elastase inhibitor) has been associated with improved cardiorespiratory parameters and decreased cardiorespiratory morbidity over the first postoperative week, especially in connection with the decreased need for prolonged mechanical ventilation.22–24
Within this framework, the effect of surgical technique modifications on postoperative respiratory complications must be assessed.
Three MIE variants are known: laparoscopic transhiatal resection and the thoracoscopic-laparoscopic approach with either intra-thoracic or cervical anastomosis. Cuschieri et al.25 described the first thoracoscopic esophagectomy; DePaula26 described transhiatal laparoscopic esophagectomy; and Luketich et al.27 were the first to publish their extensive experience with the minimally invasive approach. The wide spread use of MIE techniques has been slow due to their complexity, but progress has occurred via hybrid approaches that combine open chest time with the minimally invasive approach. The only data related to the incorporation of MIE in clinical practice come from the UK; these data show exponential growth from 0.6% in 1996–1997 to 16% in 2008, according to the National Health Service (NHS) Registry.28 These authors argued that the only benefit of their technique over open esophagectomy is the significantly lower mortality rate per year; however, the data were not stratified by tumour stage or with regard to the minimally invasive technique.
Four SRs have assessed the results of nonrandomised studies that compared open esophagectomy with MIE (full or hybrid).
Although these reviews agree with regard to the feasibility and safety of the minimally invasive approach, and judge it as equivalent to the open chest approach from an oncological standpoint, and superior in terms of postoperative mortality,2,3,5 the conclusions regarding respiratory complications are not similar. Biere et al.3 and Sgourakis et al.5 did not find significant differences in the rate of respiratory complications between the two approaches, whereas Verhage4 and Nagpal et al.2 concluded that MIE is superior to the open chest approach. However, these three SRs included retrospective studies, and respiratory complications were evaluated globally and not by subgroups. Furthermore, Nagpal et al.2 reviewed five studies, and three included transhiatal and thoracoscopic interventions interchangeably in the MIE group; moreover, one study compared laparoscopic transhiatal esophagectomy with the open chest approach. These observations cast doubt on the authors’ conclusions.
The variability of the procedures also increases the difficulty of the analysis. One SR of 46 MIE series found respiratory complications ranging between 0% and 76% depending on the definitions used and the range of mentioned complications.19 The authors calculated an average respiratory complication incidence of 22%, which is similar to that of open chest surgery; furthermore, they suggest that the term “minimally invasive” should be replaced with “minimal access”. However, the three larger and more homogeneous MIE series report a respiratory infection rate ranging between 1.54% and 7.7%, which suggests a significant improvement over open chest esophagectomy.27,29,30
Given that one of the significant contributions of MIE is reduced parietal thoracic aggression,6 we conducted our review to evaluate the relationship between respiratory morbidity and thoracic approach type. We limited our review to studies that disaggregated respiratory complications, and we included only the most common complications (i.e., respiratory infections, respiratory failure, and pleural effusion) in the analysis. Using the described methodology, we selected nine studies for the final analysis: one randomised clinical trial, three case-control studies, and five cohort studies (four retrospective and one prospective). In these reviews, the authors used additional databases (Embase and Google Scholar), and three of the four SRs used more primary studies than the current review (12 in Nagpal et al.,2 11 in Verhage et al.,4 and 10 in Biere et al.3). Sgourakis et al.5 analysed eight studies. After removing duplicates, 19 primary studies remained from the 40 studies included in these four SRs. Only three of these studies8,10,11 met the criteria for inclusion in our review.
Importantly, both types of studies included and the objectives of the previous reviews were completely different. Global comparative analyses were performed with regard to open chest and MIE techniques (including the transhiatal approach) to evaluate intraoperative parameters (e.g., duration and blood loss), administrative parameters (e.g., hospital stay), postoperative complications, and oncological parameters (e.g., lymph nodes removed). Respiratory complications were assessed globally without addressing the criteria used to define them and without accounting for the differences between transhiatal and transthoracic interventions on postoperative respiratory function. Thus, we believe that our review, which focused on the specific and categorical analysis of respiratory complications by type of thoracic approach (a key factor in our opinion), is superior to the aforementioned reviews with regard to the objective defined in the introduction. We also included five studies published over the last two years, which were subsequently added to the aforementioned reviews.
Our review did not demonstrate a difference between transthoracic and thoracoscopic esophagectomy with regard to the aforementioned complications. A subgroup analysis of respiratory infections revealed a clear advantage of a minimally invasive approach (in the only prospective randomised study included) compared with the similarity between the approaches in non-randomised studies.
Although comparable in terms of mean patient age and sex distribution, the studies are not similar in terms of tumour location and performance status (Table 1).
The limitations of this analysis are numerous. The literature search was performed using two databases for the reasons mentioned above. However, we found all the studies used in the previous SRs and 13 additional studies published between 2009 and 2012 (of which we selected five) according to our review selection criteria. Thus, despite not being able to use other databases, it is unlikely that we missed any important study.
Only one study in this review was a randomised clinical trial. All but three included studies8,9,16 reported respiratory complications without defining this term. None of the studies indicated the presence or absence of previous respiratory diseases or the use of neoadjuvant therapy among patients who developed these complications. Only two studies8,9 reported data related to preoperative respiratory function. Several authors noted the effect of transient/permanent vocal cord paralysis secondary to recurrent laryngeal nerve injury with regard to the development of respiratory infections. Among them, Hulscher et al.31 demonstrated its correlation with a higher rate of re-intubation, longer mechanical ventilation, and intensive care unit stay, despite the fact that pneumonia infections did not increase. This complication was observed in varying proportions across all studies of this meta-analysis; however, only Biere et al.16 found that this incidence was significantly more common in the open chest esophagectomy group.
The dichotomous distribution of thoracic approach type, without additional stratification by abdominal incision type (laparotomy/laparoscopy), might be a confounding factor; however, the advantages of laparoscopy with regard to postoperative respiratory dynamics are well documented.
On the contrary, we must account in the differences of adequate working space during thoracoscopy (selective vs non-selective intubation with or without CO2) and patient position during thoracoscopic time. Four SRs specified the type of ventilation used for thoracoscopic time (selective).8–10,16 Non-selective intubation might help reduce the rate of atelectasis and subsequent respiratory infections. Lateral decubitus was used in five studies,10–14 prone decubitus was used in two studies,8 and both variants were used in one study.9 A recent review on the use of prone decubitus32 identified five studies that described the results obtained with this approach: three retrospective studies (one with historical controls) compared lateral with prone decubitus and two non-comparative observational studies. Aside from the ergonomic improvements related to their use, the authors did not find clear differences between prone compared with lateral decubitus; however, they highlighted the methodological limitations and small sample size. The advantages of prone decubitus are the possibility of performing non-selective ventilation with venous shunt reduction and better maintenance of functional residual capacity, the reduction in the incidence of postoperative atelectasis, and reduced pulmonary manipulation.
The mortality rate related to respiratory complications was reported in four studies (Table 2). Despite the small number of fatal cases, the frequency of mortality due to respiratory causes reflects the severity of these complications.
Other factors related to patient characteristics and perioperative care were added to the constraints posed by the evaluation of effect of the type of surgical technique on post-esophagectomy respiratory morbidity. Bakhos et al.6 analysed one series of 220 patients and emphasised the paucity of data on preoperative respiratory function (only 30% of all cases), the absence of postoperative fluid therapy protocols, and the heterogeneity of the extubation criteria.
The recent SR by Blencowe et al.33 illustrated the aforementioned evaluation difficulties when discussing how to describe the short-term clinical results of esophagectomies. Of the 122 papers analysed, 63% did not include definitions of the described complications, and only one study defined all complications. The classification based on severity was described in varying proportions by 25%. As for specific complications, 57% of studies described pneumonia; 18% of studies defined it using 16 different definitions. The authors concluded that the communication of post-esophagectomy morbidity data is inconsistent, presents significant methodological flaws, and suggests the need to create a working group to define a set of standard criteria for the proper collection of these data.
ConclusionsOur meta-analysis did not find differences in post-esophagectomy respiratory complications between thoracotomies and thoracoscopies. However, the methodological limitations related to the retrospective nature of most of the included studies, and the heterogeneity in the definition and description of respiratory complications limit our ability to interpret these data. The recently published results of the only prospective randomised study that compared open esophagectomies with MIE suggest the superiority of the latter with regard to the incidence of early postoperative pulmonary infections, which contradicts the overall results of the current analysis. The extensive heterogeneity regarding how to define and communicate post-esophagectomy complications, the biases in the methodologies, and the low quality of the other studies analysed do not clarify the uncertainty. Future studies must provide better evidence and improve methodological quality.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Niky Mocanu S, Balagué Ponz MC, Targarona Soler EM, Roque Figuls M, Trias Folch M. La influencia del tipo de abordaje torácico sobre el desarrollo de complicaciones respiratorias tras la esofagectomía. Cir Esp. 2013;91:563–573.