Colorectal cancer is the third most common cancer worldwide both in men and women. Around one-third of patients with cancer will suffer from anxiety or depression symptoms. The aim of this study was to evaluate the effectiveness of a Mindfulness-based stress reduction intervention through a mobile application (“En Calma en el Quirófano”).
MethodThis study is a multicenter, single-blind (evaluator), controlled, randomised trial that compares the effectiveness of a mindfulness training through a mobile application (intervention group) and treatment as usual (control group) in three different moments (T0 or baseline, T1 or hospital discharge and T2 or one month after surgery). Anxiety and depression symptoms (HADS), quality of life (WHOQOL), pain, (VAS) and satisfaction (CSQ) were assessed.
ResultsIn all, there were 270 referred patients. Among them, 39 and 43 were assigned to the intervention and control groups respectively. 82 patients were analyzed: 39 patients used the app, and 43 patients continued with the treatment as usual. There were no significant changes between groups and time. We observed a slight trend in which intervention group had less depression and anxiety symptoms since T0 and T2 (B = −0.2; 95% CI between 8.8 and 9.2).
ConclusionsThe sample of this study had a high mean age (65 years old), and low levels of anxiety and depression and medium levels of pre-surgery quality of life in baseline. These factors could have influenced limiting the effectiveness of the app. Prospective research lines should focus on evaluating the effectiveness of mobile applications for younger patients with surgical pathologies.
El cáncer colorrectal representa el tercer cáncer con mayor incidencia en ambos sexos. Un tercio de los pacientes con cáncer experimentan sintomatología ansiosa o depresiva. El objetivo de este estudio fue evaluar la eficacia de una intervención de reducción de estrés basada en Mindfulness a través de una aplicación móvil (“En Calma en el Quirófano”)
MétodoEs un ensayo controlado, aleatorizado, con evaluador ciego y multicéntrico, que compara la eficacia de una aplicación de entrenamiento en mindfulness para móviles (rama experimental) con tratamiento habitual (rama control), en tres tiempos de medida (T0 o línea base, T1 o alta a domicilio, T2 o un mes tras cirugía). Se evaluó la sintomatología ansiosa y depresiva (HADS), la calidad de vida (WHOQOL), Escala de Dolor (EVA), y escala de satisfacción (CSQ).
ResultadosHubo un total de 270 derivaciones. Fueron analizados 82 personas: 39 personas utilizaron la APP, y 43 continuaron su tratamiento habitual. No hubo cambios significativos entre grupos ni tiempos de medida. Se observó una ligera tendencia en la que el grupo experimental tuvo menos síntomas de depresión y ansiedad entre T0 y T2 (B = −0,2; IC 95% entre 8,8 y 9,2).
ConclusionesNuestra población mostraba una edad media alta (65 años) y niveles bajos de ansiedad y depresión, y niveles medios de calidad de vida en T0. Estos factores pudieron interactuar limitando la eficacia de la app. Nuevas líneas de investigación tienen que ir dirigidas a evaluar la eficacia de las Apps para pacientes con patologías quirúrgicas en poblaciones más jóvenes.
Colorectal cancer is the cancer with the third highest rate in both sexes1. It has been estimated that in Spain the total number of people diagnosed with cancer in 2021 amounted to 276,239 and the most frequently diagnosed were colon and rectal cancers2.
A third of patients with cancer are subject to symptoms of anxiety or depression and it has been proven that these symptoms reduce health and quality of life in cancer patients3–7.
People awaiting cancer surgery have high levels of anxiety8,9, and evidence shows that interventions prior to surgery can have an impact on postoperative recovery9. Involving people in the surgical process alleviates some of the emotional distress surrounding the anticipation of surgery and the process afterwards10.
Psychological interventions based on stress reduction in cancer patients improve psychosocial variables such as quality of life11,12. For stress reduction, there are programmes based on mindfulness13. Mindfulness is defined as the intention to pay full attention, moment by moment, to one's own experiences, without judgement14. The great potential for dissemination of mobile applications and their easy accessibility can be an alternative to face-to-face interventions15, an option that is unique in the COVID-19 pandemic situation, as recommended by medical scientific societies16,17.
In the oncology field, there are numerous applications for people with cancer that seek to provide information, record symptoms or moods, among other functions18. There are studies aimed at cancer survivors that evaluate anxious and depressive symptomatology with mobile applications based on education and rehabilitation of anxiety, depression and quality of life vida19, and others based on cognitive-behavioural stress management interventions20.
The aim of this study was to evaluate the efficacy of an intervention based on mindfulness through an app (Calm in the operating room) to reduce anxious-depressive symptoms and improve quality of life in patients recently diagnosed with colorectal cancer awaiting surgery.
MethodThis was a randomised, controlled, evaluator-blinded, multicentre trial comparing the effect of an application of mindfulness training (experimental arm) with treatment as usual (control arm). The main outcome variable was anxious and depressive symptomatology measured by the Hospital Anxiety and Depression Scale (HADS) questionnaire one month after hospital discharge.
Two groups were compared: an experimental group, using the Calm in the Operating Room app, and a control group or treatment as usual. These 2 groups were compared at 3 assessment points (baseline or T0, discharge from hospital or T1 and one month after discharge from hospital or T2).
Eligible participants were all persons newly diagnosed with colorectal cancer from 2 public general hospitals in Madrid (Spain) from April 2019 to March 2020 awaiting surgery. There were a total of 270 people. Inclusion criteria were: ≥18 years; being on the waiting list for colorectal cancer surgery and signing the informed consent. Exclusion criteria were: having a diagnosis of severe mental disorder according to DSM-5 with acute episode at the time of selection or having difficulty using the apps.
This study adhered to the principles of the Declaration of Helsinki, SPIRIT 201321. The ethics committee of both general hospitals in Madrid (Spain) approved this clinical trial (identifiers 5219 and 19,057). The protocol was prospectively registered in January 2019 in clinicaltrials (clinicaltrials.gov identifier NCT04184557), following the 2010 CONSORT statement22.
Study participants completed a sociodemographic-clinical questionnaire (T0) and a battery of instruments (at T0, T1 and T2).
At the beginning of the study the following information was collected: gender, age, civil status and main carer.
The main variable was studied with the HADS23 questionnaire, which is a self-report tool consisting of a depression subscale (7 items), an anxiety subscale (7 items) and a global score (14 items). Higher scores represent higher reported symptomatology. The psychometric properties of the Spanish adaptation obtained a Cronbach’s test scores of .86 (anxiety) and .86 (depression)24. For the oncology population25, in the anxiety subscale the score was .90 and for the depression subscale it was .84.
For the secondary variables, the following questionnaires were used:
The WHOQOL-BREF (The WHOQOL Group, 1998)26, which is a self-report composed of 26 items. Item 1 measures general quality of life, item 2 measures satisfaction with health and the remaining 24 items (items 3 to 26) are grouped into 4 domains: physical health, psychological health, social relationships and environmental health. Higher scores mean higher levels of self-perceived quality of life26. For the Spanish population27 it shows an internal consistency measured by Cronbach's score of .90.
The CSQ-8 (satisfaction questionnaire)28, Spanish version by Martínez et al.29 is a self-report composed of 8 questions. Satisfaction is directly related to the sum of the scores; the maximum score is 32 points. In the Spanish population,28 the instrument has an internal consistency measured by Cronbach's test of .91.
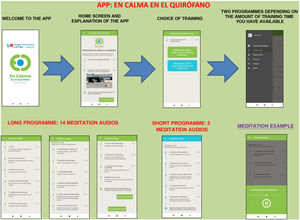
The Calm in the Operating Room app shows a brief and simple set of mindfulness exercises designed by professionals accredited as teachers of mindfulness programmes.
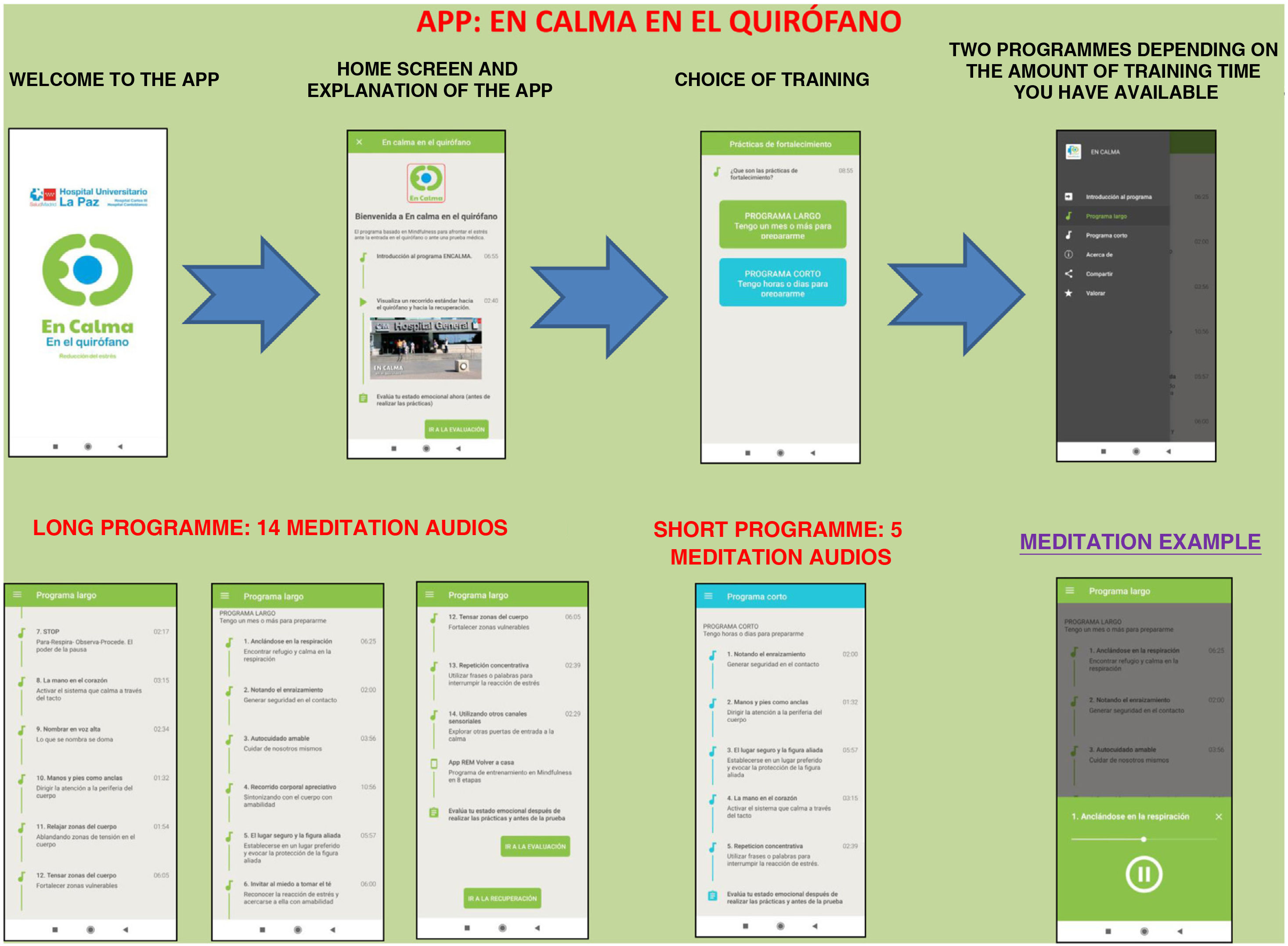
The app includes a brief introduction to the Calm Down programme and a video of the surgical hospital context. Two training programmes are shown: the long programme (surgery scheduled in 15 days or a month) and the short programme (surgery scheduled in a few hours or days). They can be downloaded free of charge (Fig. 1).
People randomised to this group followed treatment as usual, which did not include any protocolised mental health intervention, although patients could be in psychiatric or psychological treatment on their own.
The baseline assessment was conducted 15 days before surgery (T0), the post-treatment assessment at discharge (T1) and the follow-up assessment one month after discharge (T2). At baseline (T0), they signed the informed consent form and completed the baseline assessment. They were randomly assigned to the intervention group (1:1), regardless of their responses. The sequence was obtained through TeamMaker software and no restrictions were applied.
Assessments were conducted by one of the research assistants, who was blinded to treatment assignment. This procedure was repeated at each randomisation.
There are no previous studies on the efficacy of mindfulness training programmes through an app for the improvement of psychological distress in surgical patients. In order to find statistically significant differences between the experimental and control arms, and anticipating a loss of around 15%, the required number of participants will be approximately 88 people, for a significance level of .05, a statistical power of .80 and a moderate effect size (.50).
Statistical analysisNominal and ordinal variables were represented as frequencies and percentages, and quantitative variables as means and standard deviations. To test the hypothesis that persons assigned to the experimental arm would report fewer symptoms of anxiety and depression than persons assigned to the control arm at the end of the study, we conducted a modified intention-to-treat analysis without missing value imputation. We used a generalised linear model for Poisson-type distributions to explore the interactive effect of group (experimental vs. control) and time of measurement (T0, T1 and T2) on the HADS total score. Secondary analyses consisted of successive generalised linear models for the HADS anxiety and depression subscales, and general linear models for the WHOQOL-BREF subscales. Hospital centre was included as a fixed factor in all models. Results were expressed in terms of regression coefficients (B) and 95% confidence intervals. We performed 2 types of non-prespecified sensitivity analyses to confirm whether estimators were similar when: a) the same models were used after multiple imputation of missing values and b) generalised estimating equations were used, rather than generalised or generalised linear models.
Participants who had no information recorded at the 3 time points (lost questionnaires, incomplete questionnaires, death, etc.) were excluded.
All analyses were done in R Studio for Mac (version 1.2.5042), using the packages summarytools, dplyr, ggploT2, ggpubr, mice, gee and geepack.
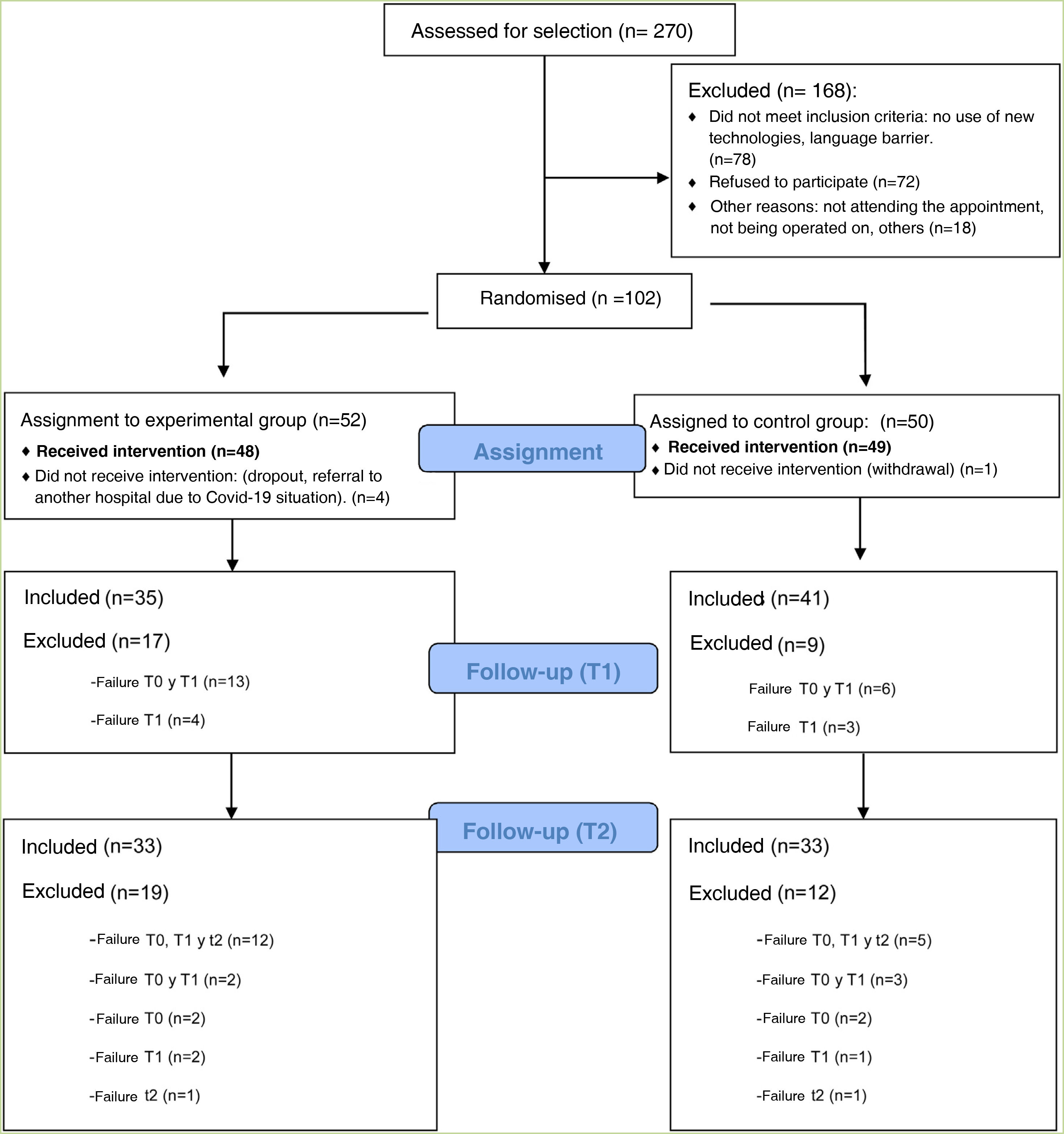
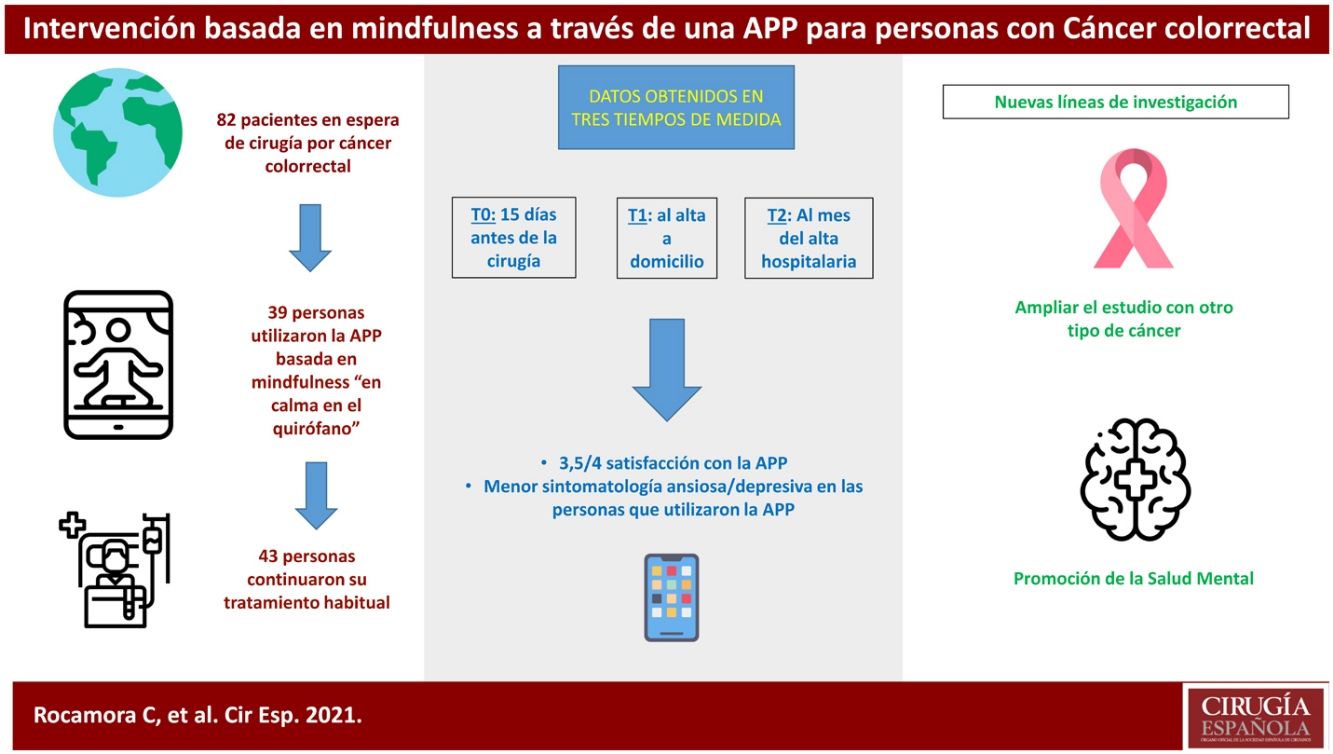
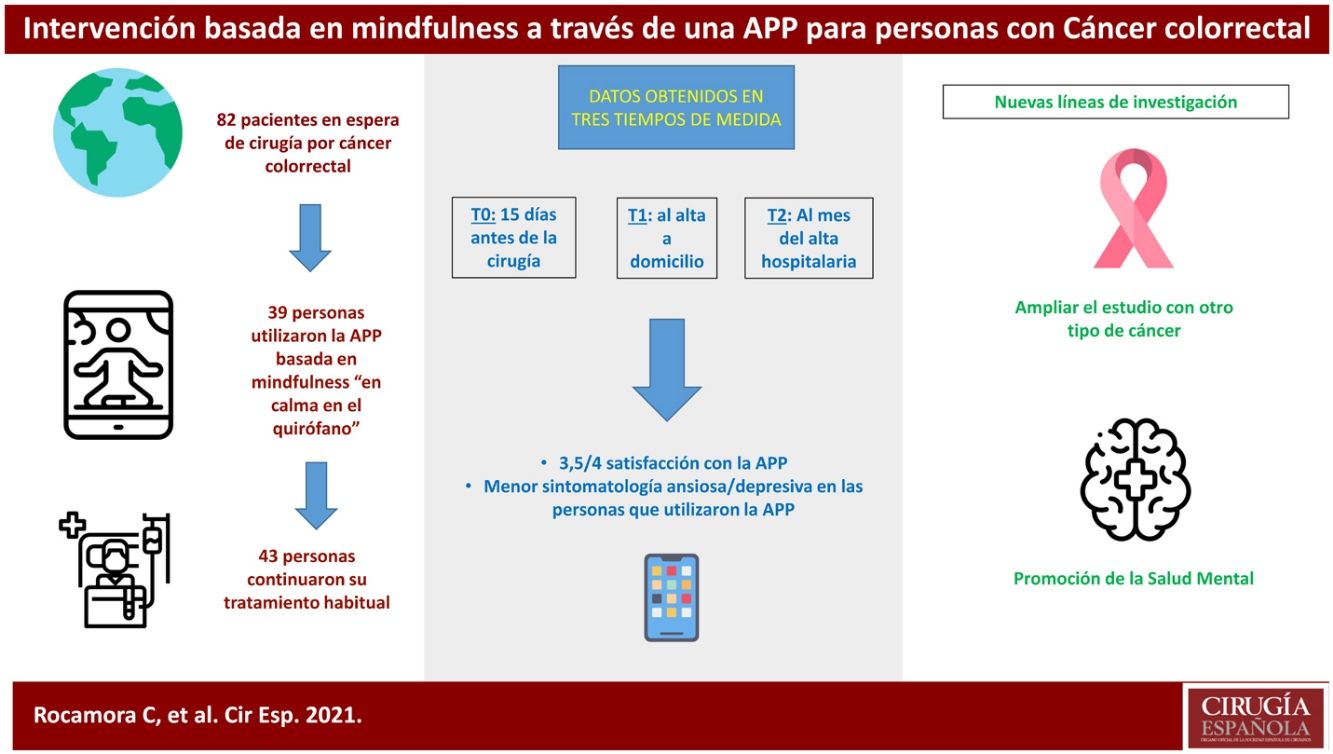
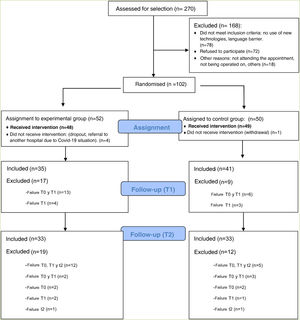
ResultsThis clinical trial explored whether there were differences between the experimental and control groups on the demographic variables of age and sex, and on the outcome variables HADS and WHOQOL, and found that both groups were equivalent. There were a total of 270 people awaiting surgery. 62% were excluded from the study as they did not meet the inclusion criteria: 46% did not use apps, 42% declined to participate. Of the total sample, 102 people met the inclusion criteria. Of this total, data from subjects who did not complete the questionnaires were not studied: they dropped out of the study, or were referred due to COVID, or lost questionnaires, etc. Finally, 82 people were analysed: 39 people used the Calm in the Operating Room app and 43 continued their usual treatment. Fig. 2 shows the flow chart.
The majority of participants were male (64%), with a mean age of 65 years (Table 1). The mean age of patients who did not participate was 71 years, almost 70% were male.
Socio-demographic and clinical characteristics of participants at the start of the study.
| App groupCalm(n = 39) | Control G. (n = 43) | Total (n = 82) | |
|---|---|---|---|
| Age in years, mean | 63.7 | 66.4 | 65.1 |
| Centre, n (%) | |||
| Hospital La Paz | 27 (69.2) | 31 (72.1) | 58 (70.7) |
| Hospital 12 de Octubre | 12 (30.8) | 12 (27.9) | 24 (29.3) |
| Sex n (%) | |||
| Women | 15 (38.5) | 14 (32.6) | 29 (35.4) |
| Men | 24 (61.5) | 29 (67.4) | 53 (64.6) |
| Civil status n (%) | |||
| Married | 33 (84.6) | 35 (81.4) | 68 (82.9) |
| Divorced | 3 (7.7) | 4 (9.3) | 7 (8.5) |
| Widowed | 2 (5.1) | 3 (7) | 5 (6.1) |
| Single | 1 (2.6) | 1 (2.3) | 2 (2.4) |
| Main carer | |||
| Husband/wife | 32 (82.1) | 35 (81.4) | 67 (81.7) |
| Son/daughter | 5 (12.8) | 6 (14) | 11 (13.4) |
| Friend | 1 (2.6) | 2 (4.6) | 3 (3.7) |
| Others | 1 (2.6) | 1 (1.2) | |
| Pain scale (VAS), mean | |||
| T0 | 1 | 1 | 1 |
| T1 | 3 | 2 | 1 |
| T2 | 1 | 1 | 1 |
Of the participants who used the app, 18 completed an evaluation, based on intervals of use, practice and minutes of practice. More than 75% of respondents had used both programmes. Almost 40% had listened to the entire short programme and more than 30% to the long one. Forty-four percent reported use between 1 and 4 weeks, and 22% reported use of more than 4 weeks. Fifty per cent expressed a practice of between 2 and 3 days per week, and 38% more than 4 days per week. Finally, 61% of people indicated that they used the app for between 10 and 30 min.
There were no significant changes between groups or measurement times, with 95% confidence intervals. In the app group, a slight difference was observed in the total HADS scale, with a reduction before surgery and after one month of hospital discharge in the mean of 1.5 with a standard deviation of 1.1 (B = −.2, 95% CI: 8.8–9.2).
On the HADS sub-variables (anxiety and depression), the analysis showed no statistically significant changes. On the anxiety scale (HADS-A), the experimental group decreased by more than one point between the means before surgery versus one month after hospital discharge (T0 = 7.5; T2 = 6.4).
In the linear regression analysis on quality of life, the results showed no significant changes between groups.
In the satisfaction scale (CSQ), administered to the experimental group after completion of the study, a mean score of 3.5 points (out of 4) was observed.
DiscussionThis study explored the efficacy of an app-based intervention for patients on the waiting list for colorectal cancer surgery. The results show that there are no significant differences between the groups at the different measurement times.
The experimental group obtained a difference between their means on the HADS scale and the time of measurement of 1.5 points. These data are along the lines of those found in the clinical trial of Thalén-Lindström et al.30 with oncology patients, where they obtained a difference between their HADS means of 2 points in the experimental group.
In our study, participants did not report elevated levels of anxiety or depression before entering the operating theatre. In the study by Greer et al.31, comparing an app-based intervention based on cognitive behavioural therapy vs. a health education programme, they observed between-group differences in HADS-A only in people who showed an initially elevated HADS-A31.
In our study, the HADS-A scale shows a trend of decreasing values between T0 and T2 in the experimental group. In addition, the HADS-A scale shows higher mean scores than the depression scale (HADS-D) (Table 2). These data are in line with the study by Park et al.32, in which they conducted a cross-sectional follow-up of women with metastatic breast cancer, which showed a higher percentage of anxiety symptoms (mean 7.9) and to a lesser extent depressive symptoms (mean 4.4).
Descriptive analysis of the mean and standard deviation of the main variables at the three measurement times, the effects of the estimator, confidence interval and p-value.
| Visit | app Group calm | Usual treatment | Total | B(Se) | Confidence interval | p | |
|---|---|---|---|---|---|---|---|
| Main Variable | |||||||
| Total (HADS), M (SD)Depression (HADS-D)Anxiety (HADS-A) | T0T1T2T0T1T2T0T1T2 | 11.4 (7.3)11.7 (6.6)9.9 (6.2)3.8 (3.6)4.9 (3.9)3.5 (3.1)7.5 (4.3)6.9 (3.5)6.4 (3.9) | 8.1 (4.9)9.3 (6.4)8.2 (7.2)2.5 (2.3)4.1 (3.4)3.0 (3.6)5.6 (3.5)5.2 (3.4)5.2 (4.3) | 9.7 (6.3)10.4 (6.6)9.0 (6.8)3.1 (3.0)4.5 (3.6)3.3 (3.3)6.5 (4.0)6.0 (3.5)5.8 (4.2) | −.1 (.1)−.2 (.1)−.3 (.2)−.3 (.2)−.0 (.1)−.1 (.1) | 1.2 and 10.68.8 and 9.24.1 and 4.92.9 and 3.75.8 and 6.25.6 and 6 | .31.14.14.14.88.45 |
| Secondary Variable | |||||||
| WHOQOL physical health WHOQOL psychological health WHOQOL Social relationsWHOQOL Environment | T0T1T2T0T1T2T0T1T2T0T1T2 | 14.7 (2.6)12.8 (3.2)13.9 (2.9)14.5 (2.3)14.1 (2.7)14.5 (2.5)14.5 (2.5)13.7 (2.2)14.6 (2.5)15.1 (2.1)14.8 (2.2)15.1 (2.4) | 15.1 (2.7)12.9 (2.5)14.3 (2.5)15.5 (2.4)14.7 (2.8)15.2 (2.7)15.2 (2.7)14.3 (2.8)14.5 (3.1)15.7 (2.5)15.2 (2.2)15.4 (2) | 14.9 (2.6)12.9 (2.8)14.1 (2.7)15.0 (2.4)14.4 (2.7)14.9 (2.6)14.9 (2.6)14.0 (2.5)14.5 (2.8)15.4 (2.3)15.0 (2.2)15.3 (2.2) | .2 (.9)−.0 (.9).3 (.8).2 (.8).1 (.9).8 (.9).2 (.7).3 (.7) | 11.1 and 14.712.3 and 15.912.8 and 1613.3 and 16.512.2 and 15.812.7 and 16.313.6 and 16.414 and 16.7 | .84.10.68.85.92.37.83.67 |
Results obtained after free imputation by chained equations (MICE).
HADS: Hospital Anxiety and Depression Scale, WHOQOL: Quality of Life Scale.
No significant differences in quality of life were found between the two groups. Some studies show that quality of life in patients who have undergone colorectal cancer surgery improves after the sixth day of the intervention33, even returning to the levels prior to one month after surgery34. The literature reports that quality of life in the first years after colorectal cancer treatment is similar to or better than what is expected in the population35. In another study, older age was found to be significantly associated with higher levels of well-being36. The population in our study had a mean age of 65 years, which may explain the lack of significant differences in quality of life.
On the satisfaction scale, we scored 3.5 out of 4 points, with high acceptability and satisfaction of use.
Our study has some strengths: it is a randomised clinical trial and, protecting external validity, all people on the surgical waiting list for colorectal cancer were offered participation: 270 people.
Randomisation (10–15 days prior to surgery), blinded outcome assessment and free app were included.
In terms of limitations, 62% of the total sample did not meet the inclusion criteria: 82 subjects were analysed compared to the estimated 88 in the sample. Among others, age may be limiting the use of the app, as concluded by another study, which shows that older adults (aged 50 and over) are less likely to use smartphones than younger adults (aged 18–29)37.
In this randomised controlled trial comparing a mindfulness-based intervention using the Calm in the Operating Room app with treatment as usual, no significant post-surgery differences were found in anxiety, depression and quality of life prior to surgery in a population diagnosed with colorectal cancer.
Our population showed a high mean age (65 years) and low levels of anxiety and depression, as well as average levels of pre-surgery quality of life. These factors may have acted to limit the app’s potential demonstration of efficacy under more adverse conditions. Even so, a high score was obtained on the satisfaction scale (CSQ), which shows the operability of these new technologies in complex situations, such as waiting for colorectal cancer surgery.
New lines of research should be aimed at evaluating the effectiveness of apps for patients with surgical diseases in younger populations.
Conflict of interestsThe authors have no conflict of interests to declare.