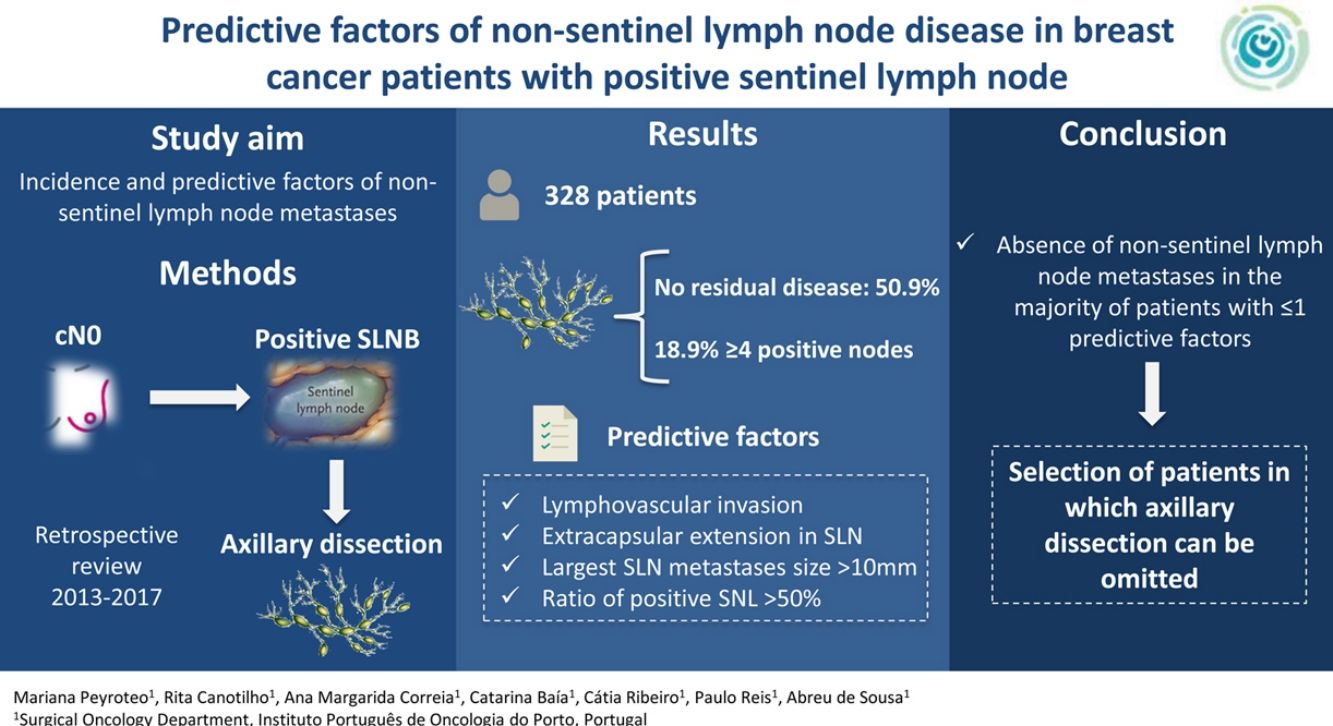
Management of positive sentinel lymph node biopsy (SLNB) in breast cancer remains a matter of debate. Our aim was to evaluate the incidence and identify predictive factors of non-sentinel lymph node metastases.
MethodsRetrospective review of all cN0 breast cancer patients treated between January 2013 and December 2017, with positive SLNB that were submitted to ALND.
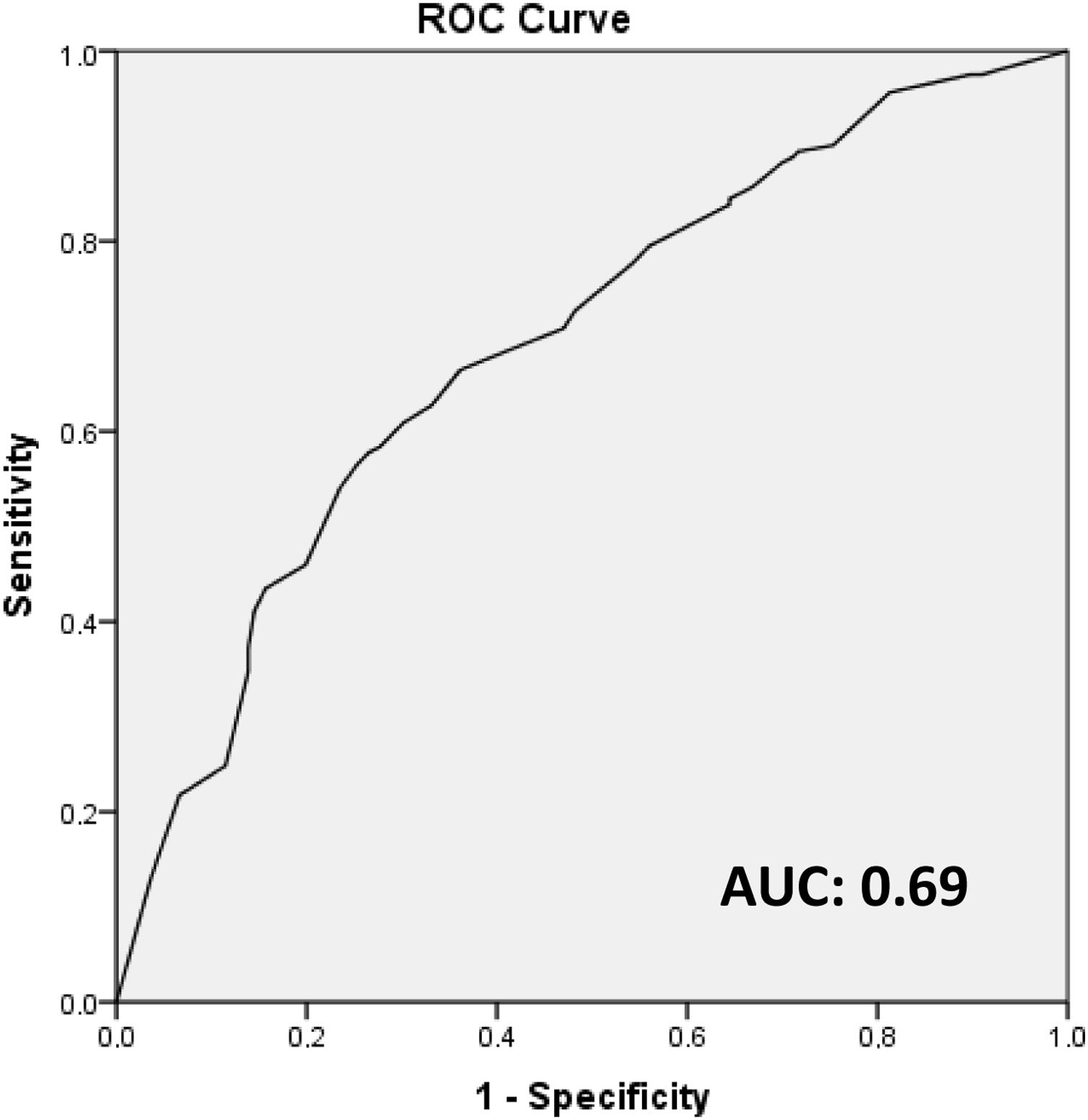
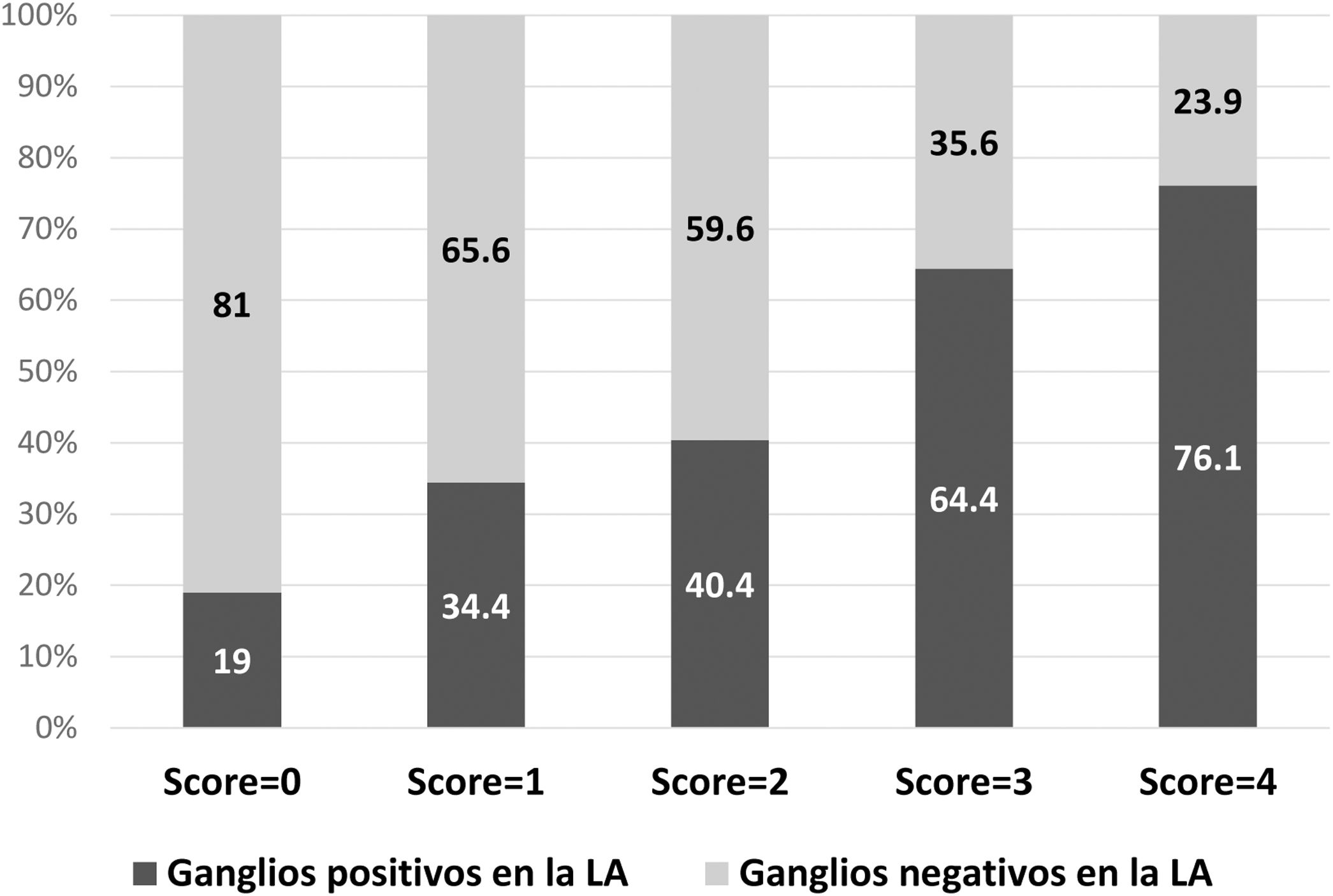
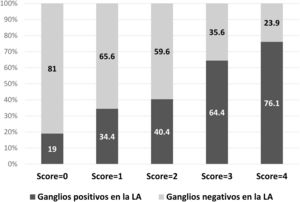
ResultsOf the 328 patients included, the majority of tumors were cT1 or cT2, with lymphovascular invasion in 58.4% of cases. The mean isolated nodes in SLNB was 2.7, with a mean of 1.6 positive nodes, 60.7% with extracapsular extension. Regarding ALND, a mean of 13.9 nodes were isolated, with a mean of 2.1 positive nodes. There was no residual disease in the ALND in 50.9% of patients, with 18.9% having ≥4 positive nodes. In the multivariate analysis, lymphovascular invasion, extracapsular extension in SLN, largest SLN metastases size (>10 mm) and ratio of positive SNL (>50%) were independent predictors of non-sentinel lymph node metastases. These four factors were used to build a non-pondered score to predict the probability of a positive ALND after a positive SLNB. The AUC of the model was 0.69 and 81% of patients with score = 0 and 65.6% with score = 1 had no additional disease in ALND.
ConclusionThe absence of non-sentinel lymph node metastases in the majority of patients with 1–2 positive SLN with low risk score questions the need of ALND in this population. The identified predictive factors may help select patients in which ALND can be omitted.
El manejo del ganglio centinela positivo en cáncer de mama sigue siendo un tema de debate. Nuestro objetivo fue evaluar la incidencia e identificar factores predictivos de metástasis en ganglios no centinela.
MétodosRevisión retrospectiva de los pacientes con cáncer de mama con axila clínicamente negativa (cN0) tratados entre enero de 2013 y diciembre de 2017, con biopsia de ganglio centinela (BGC) positiva a quienes se les realizó linfadenectomía axilar (LA).
ResultadosDe los 328 pacientes incluidos, la mayoría tenía tumores cT1 o cT2, con invasión linfovascular en el 58,4% de casos. La media de ganglios detectados en BGC fue 2,7, con media de 1,6 ganglios positivos, el 60,7% con extensión extracapsular. En LA, una media de 13,9 ganglios fueron detectados, con media de 2,1 ganglios positivos. No se observó metástasis en LA en el 50,9% de los pacientes y el 18,9% tenía ≥ cuatro ganglios positivos. En análisis multivariado, la invasión linfovascular, la extensión extracapsular, la dimensión de mayor metástasis (>10 mm) y la ratio de ganglios centinela positivos (>50%) fueron factores predictivos independientes de metstasis en ganglios no centinela. Estos factores fueron usados para construir un score para predecir la posibilidad de LA positiva después de BGC positiva. El área bajo la curva ROC (AUC) del modelo fue 0,69 y el 81% de los pacientes con score = 0, y el 65,6% con score = 1 no tenían metástasis en la LA.
ConclusiónLa ausencia de metástasis en ganglios no centinela en la mayoría de los casos con uno a dos ganglios positivos en la BGC con score de bajo riesgo cuestiona la necesidad de hacer LA en estos pacientes. Los factores predictivos identificados pueden ayudar a seleccionar pacientes para omitir la LA.
Lymph node disease is an important prognostic factor in breast cancer. Sentinel lymph node biopsy (SLNB) has long been the standard staging technique for patients with clinically negative axillae (cN0)1–4. However, the management of positive sentinel lymph nodes (SLN) remains a subject of debate, since some 40%–70% of patients with positive SLN do not have other metastases in the axillary lymph node dissection (ALND)1,5–11. Due to the evolution of the surgical technique and the multidisciplinary approach to breast cancer, the potential benefit of ALND has been widely questioned, and there is a growing interest in identifying patients in whom ALND can be ruled out12.
The three main studies on the subject have demonstrated the lack of a significant impact on survival when ALND was not performed in selected groups of patients with positive SLNB13–15. Consequently, and especially after the conclusions of the ACOSOG Z0011 trial, the recommendations for ALND in the context of breast-conserving surgery have changed. However, in the context of total mastectomy, ALND remains the standard surgery after a positive SLNB16,17. Despite these results, there is still significant controversy about these studies regarding the representation of patients with tumors and high-risk characteristics18. Thus, in the current context of progressively selecting fewer patients for ALND, it is very important to define the predictive factors for non-sentinel node metastases, so that a more adequate selection can be made.
The objective of this study is to evaluate the incidence and identify the predictive factors of metastasis in non-sentinel nodes in patients with positive SLNB, as well as to construct a score to guide clinical decision-making.
MethodsWe conducted a retrospective review of all cN0 breast cancer patients treated consecutively at our institution between January 2013 and December 2017. The study included all cN0 patients with a positive SLNB who had undergone ALND. Patients with stage IV or recurrent breast cancer, as well as those treated with neoadjuvant therapy, were excluded.
The SLNB was performed with the dual tracer method by injecting patent blue and a radioactive isotope (technetium 99 m). All pathology studies were performed by pathologists at our institution, and all sentinel lymph nodes were examined by 2 mm serial sections stained with hematoxylin-eosin. Immunochemical analysis was carried out occasionally. The extemporaneous examination was not carried out routinely, since the study period included the same period in which the ACOSOG Z0011 criteria13 were adopted in our surgical oncology department, followed by the progressive abandonment of the extemporaneous examination.
Statistical analysisWe collected data for preoperative, surgical procedure and pathological analysis variables. The statistical analysis was performed with SPSS® version 21, and P < .05 was considered statistically significant.
The bivariate analysis was first carried out to examine the association of potential predictive factors (independent variables) with the outcome of interest (dependent variable), which in this case was a positive ALND. This analysis was done with the chi-squared test or Fisher’s exact test, according to the variables. The independent variables analyzed were multifocality/multicentricity, tumor grade, tumor type, biological tumor subtype, tumor size (pT ≤2 cm/>2 cm), lymphovascular invasion, extracapsular extension in the sentinel lymph node, mean size of the largest sentinel node metastasis (≤10 mm/>10 mm), total number of positive sentinel lymph nodes (>1), and ratio of positive lymph nodes for the total number of sentinel nodes (≤50%/>50%).
Subsequently, the multivariate analysis was performed, using the statistically significant variables in the univariate analysis, with a logistic regression model, reporting odds ratio and 95% confidence intervals. After the independent predictive factors were identified with the multivariate analysis, an unweighted score was constructed (with one point attributed for each factor) to predict the probability of a positive ALND after a positive SLNB. The discriminative power of the model was evaluated with the area under the receiver operating curve (AUC), which varies between 0 and one (one indicates perfect discriminating power, and 0.5 is no better than determination by chance)19.
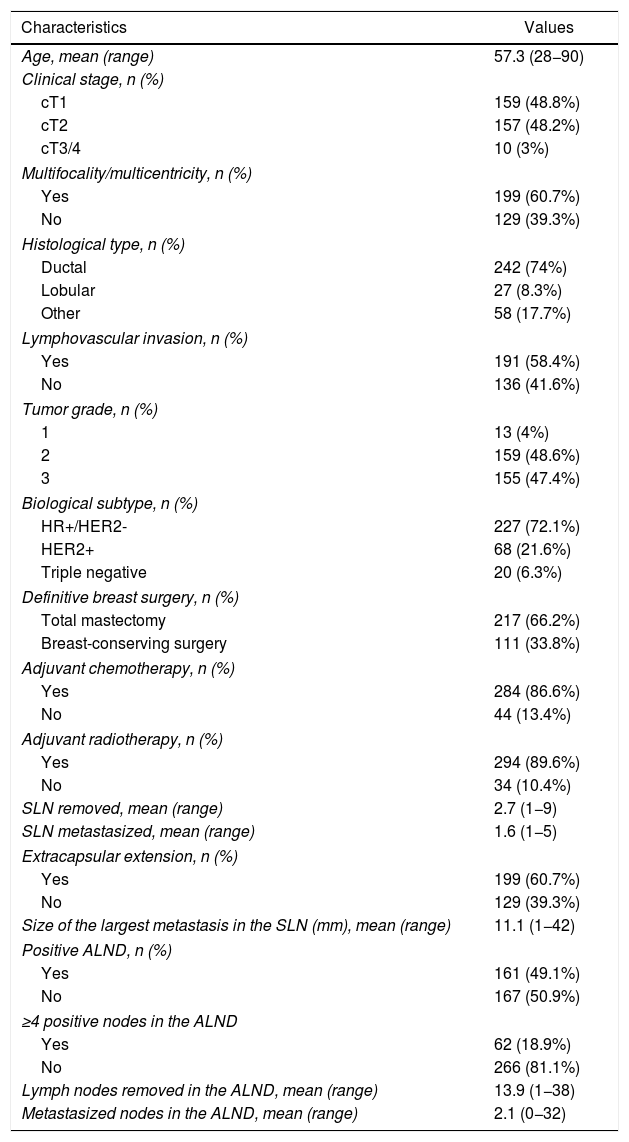
ResultsA total of 328 patients were included in the study, 99.1% (n = 325) of whom were female. Mean age was 57.3 years (range 28–90).
The descriptive characteristics of the study group are shown in Table 1.
Descriptive characteristics of the study group.
| Characteristics | Values |
|---|---|
| Age, mean (range) | 57.3 (28−90) |
| Clinical stage, n (%) | |
| cT1 | 159 (48.8%) |
| cT2 | 157 (48.2%) |
| cT3/4 | 10 (3%) |
| Multifocality/multicentricity, n (%) | |
| Yes | 199 (60.7%) |
| No | 129 (39.3%) |
| Histological type, n (%) | |
| Ductal | 242 (74%) |
| Lobular | 27 (8.3%) |
| Other | 58 (17.7%) |
| Lymphovascular invasion, n (%) | |
| Yes | 191 (58.4%) |
| No | 136 (41.6%) |
| Tumor grade, n (%) | |
| 1 | 13 (4%) |
| 2 | 159 (48.6%) |
| 3 | 155 (47.4%) |
| Biological subtype, n (%) | |
| HR+/HER2- | 227 (72.1%) |
| HER2+ | 68 (21.6%) |
| Triple negative | 20 (6.3%) |
| Definitive breast surgery, n (%) | |
| Total mastectomy | 217 (66.2%) |
| Breast-conserving surgery | 111 (33.8%) |
| Adjuvant chemotherapy, n (%) | |
| Yes | 284 (86.6%) |
| No | 44 (13.4%) |
| Adjuvant radiotherapy, n (%) | |
| Yes | 294 (89.6%) |
| No | 34 (10.4%) |
| SLN removed, mean (range) | 2.7 (1−9) |
| SLN metastasized, mean (range) | 1.6 (1−5) |
| Extracapsular extension, n (%) | |
| Yes | 199 (60.7%) |
| No | 129 (39.3%) |
| Size of the largest metastasis in the SLN (mm), mean (range) | 11.1 (1−42) |
| Positive ALND, n (%) | |
| Yes | 161 (49.1%) |
| No | 167 (50.9%) |
| ≥4 positive nodes in the ALND | |
| Yes | 62 (18.9%) |
| No | 266 (81.1%) |
| Lymph nodes removed in the ALND, mean (range) | 13.9 (1−38) |
| Metastasized nodes in the ALND, mean (range) | 2.1 (0−32) |
SLN: sentinel lymph node; ALND: axillary lymph node dissection; HR: hormone receptors.
Most tumors were stage cT1 (48.8%; n = 159) or cT2 (48.2%; n = 157), and 60.7% (n = 199) were classified as multicentric or multifocal.
The definitive breast surgery was mastectomy in 66.2% (n = 217) of the cases; adjuvant chemotherapy was performed in 86.6% (n = 284) of the patients, and adjuvant radiotherapy in 89.6% (n = 294).
Regarding the histopathological variables, 74% (n = 242) of the tumors were classified as ductal, and the majority were grade II (48.6%; n = 159) or grade III (47.4%; n = 155). Lymphovascular invasion was observed in 58.4% (n = 191) of the tumors. The most frequent biological subtype was hormone receptor (HR) positive/HER2 negative in 72.1% (n = 227) of the patients, followed by HER2 positive in 21.6% (n = 68) and triple negative in 6.3% (n = 20).
As for the SLN, the mean number of lymph nodes removed was 2.7, with a mean of 1.6 positive lymph nodes. Extracapsular extension was found in 60.7% (n = 199) of the sentinel nodes, and the mean size of the largest sentinel node metastasis was 11.1 mm. Regarding ALND, a mean of 13.9 lymph nodes were detected, with a mean of 2.1 positive lymph nodes. Metastases in the ALND were observed in 49.1% (n = 161) of the patients, and 18.9% had ≥4 positive nodes.
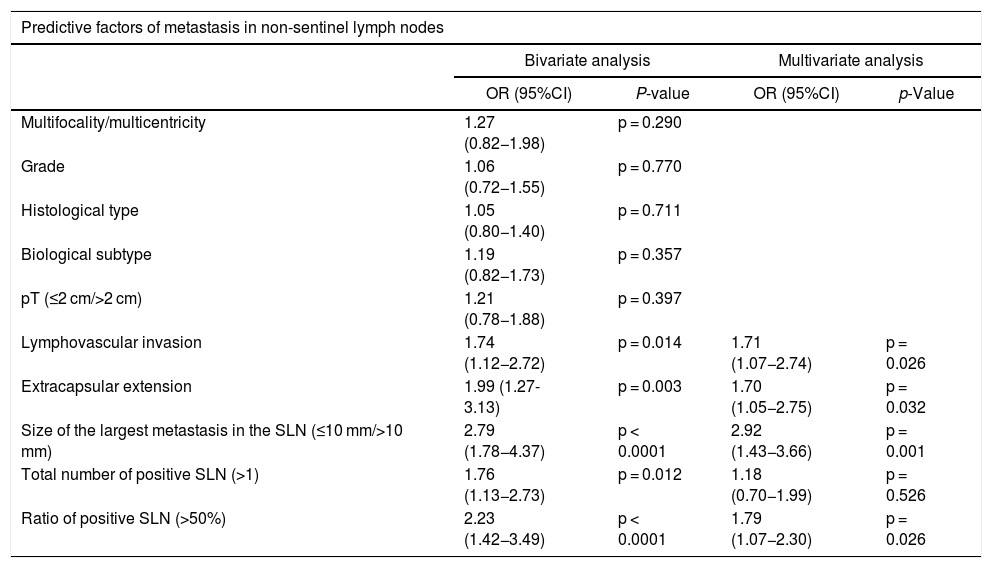
Statistically significant predictive factors of non-sentinel node metastasis in the bivariate analysis were lymphovascular invasion, extracapsular extension in the sentinel node, size of the largest sentinel lymph node metastasis (>10 mm), total number of positive sentinel nodes (>1) and the ratio of positive sentinel lymph nodes (>50%) (Table 2). In the multivariate analysis, all factors except the total number of positive sentinel nodes were independent predictors of non-sentinel node metastasis (Table 2).
Bivariate and multivariate analysis of predictive factors of metastasis in non-sentinel lymph nodes.
| Predictive factors of metastasis in non-sentinel lymph nodes | ||||
|---|---|---|---|---|
| Bivariate analysis | Multivariate analysis | |||
| OR (95%CI) | P-value | OR (95%CI) | p-Value | |
| Multifocality/multicentricity | 1.27 (0.82−1.98) | p = 0.290 | ||
| Grade | 1.06 (0.72−1.55) | p = 0.770 | ||
| Histological type | 1.05 (0.80−1.40) | p = 0.711 | ||
| Biological subtype | 1.19 (0.82−1.73) | p = 0.357 | ||
| pT (≤2 cm/>2 cm) | 1.21 (0.78−1.88) | p = 0.397 | ||
| Lymphovascular invasion | 1.74 (1.12−2.72) | p = 0.014 | 1.71 (1.07−2.74) | p = 0.026 |
| Extracapsular extension | 1.99 (1.27-3.13) | p = 0.003 | 1.70 (1.05−2.75) | p = 0.032 |
| Size of the largest metastasis in the SLN (≤10 mm/>10 mm) | 2.79 (1.78−4.37) | p < 0.0001 | 2.92 (1.43−3.66) | p = 0.001 |
| Total number of positive SLN (>1) | 1.76 (1.13−2.73) | p = 0.012 | 1.18 (0.70−1.99) | p = 0.526 |
| Ratio of positive SLN (>50%) | 2.23 (1.42−3.49) | p < 0.0001 | 1.79 (1.07−2.30) | p = 0.026 |
CI: confidence interval; SLN: sentinel lymph node; OR: odds ratio.
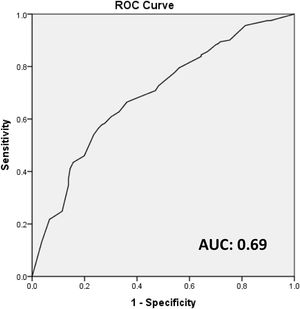
These four independent factors (lymphovascular invasion, extracapsular extension in the sentinel node, size of the largest sentinel node metastasis [>10 mm] and the percentage of positive sentinel lymph nodes [>50%]) were used to construct an unweighted score (attributing one point to each factor) to predict the probability of a positive ALND after a positive SLN biopsy. The AUC of the model was 0.69 (Fig. 1).
The probability of a positive ALND according to the model is described in Fig. 2. In 81% of the patients with a score = 0 and in 65.6% with a score = 1, no additional metastases were detected in the ALND.
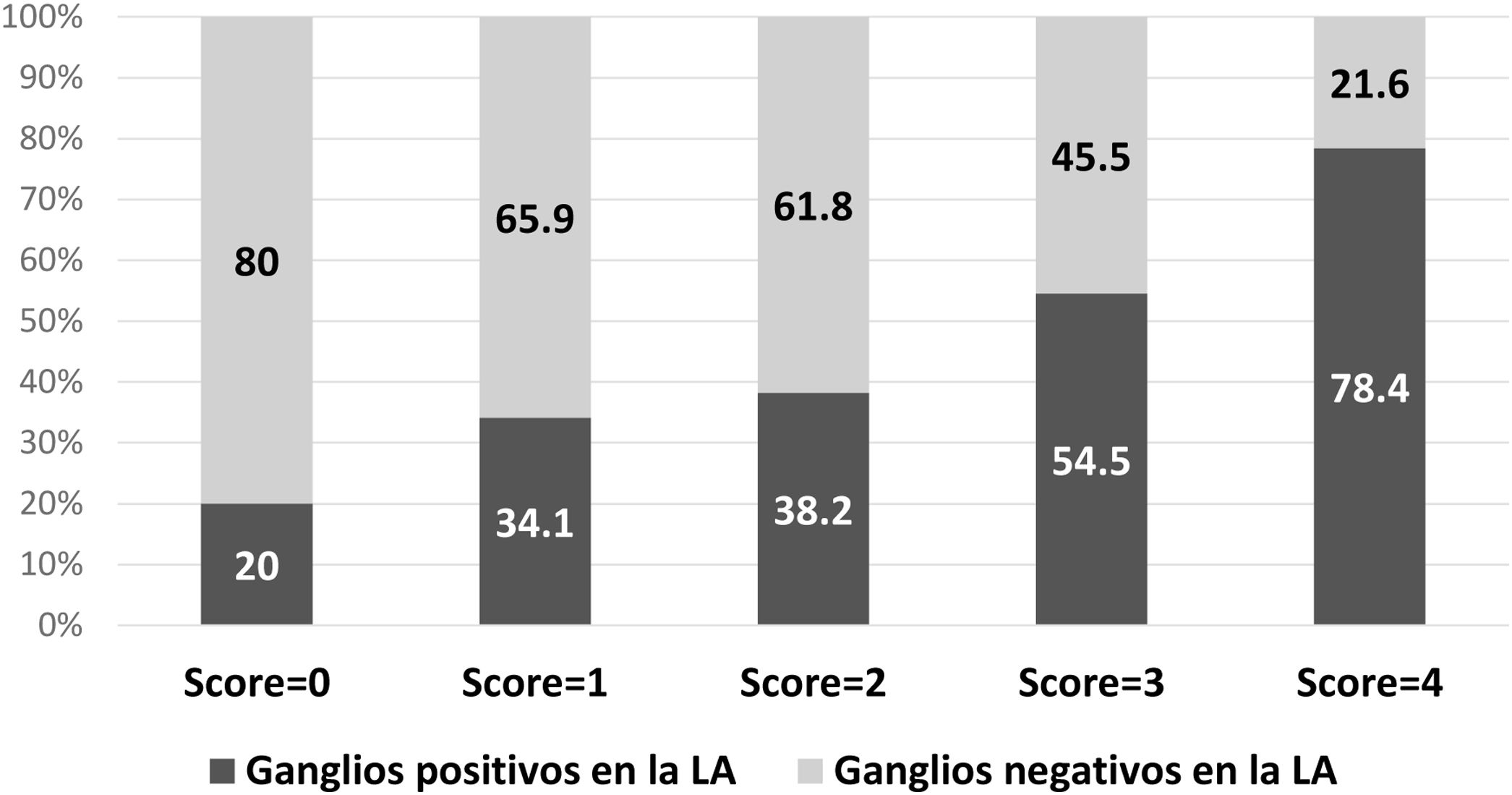
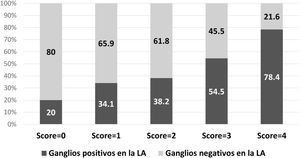
The same model was applied only in patients treated with mastectomy (n = 217), with an AUC of 0.68. The probability of a positive ALND according to the model is described in Fig. 3.
DiscussionThe objective of this study was to evaluate the incidence of metastases in non-sentinel nodes in patients with positive SLN, as well as to identify the predictive factors for metastasis. No metastases were detected in the ALND in 50.9% (n = 167) of the patients, and only 18.9% had ≥4 positive nodes. The independent predictive factors identified were lymphovascular invasion, extracapsular extension in the sentinel node, size of the largest sentinel node metastasis >10 mm, and the ratio of positive sentinel lymph nodes >50%. Based on these factors, a predictive model was constructed in which 81% of the patients with a score = 0 and 65.6% of the patients with a score = 1 did not have metastases in the ALND.
Several studies have previously reported on the incidence of non-sentinel lymph node metastases, with a variable percentage between 17% and 53%10,20. In the most recent studies, the incidence was 27% in ACOSOG Z001113 and 32% in the AMAROS trial14. In our study, a higher percentage of non-sentinel node metastases was detected (49.1%), which may be due to the fact that there was a higher incidence of high-risk factors, specifically lymphovascular invasion in 58.4% of patients and extracapsular extension in the sentinel lymph node in 60.7%. This may also depend on a possible selection bias for ALND, with higher percentages of these risk factors in this group, compared to our overall patient population. On the other hand, even in these cases selected by ALND, 50.9% did not have non-sentinel node metastases, which clearly emphasizes the need for refining the selection criteria.
Despite this incidence of non-sentinel node metastases, the potential survival benefit of ALND in selected groups has not been demonstrated in previous articles13,21–23. In contrast, several research studies have emphasized the morbidity associated with ALND compared to SLNB, namely higher rates of lymphedema, arm/shoulder pain, and sensory and motor complaints in the upper limb23–25. In our study group, 89.6% of the patients underwent adjuvant radiotherapy (after ALND), which raises questions about a double axillary treatment with doubtful oncological benefit and cumulative morbidity.
Previous studies have reported on predictive factors for metastasis in non-sentinel lymph nodes6,26,27, and several predictive models have been constructed28. Out of the predictive factors identified in our study, the ratio of positive lymph nodes in the SLNB, size of the metastases in the sentinel lymph node and lymphovascular invasion were previously represented in some way in most of the predictive models8,29–32. Unlike the aforementioned models, extracapsular extension in the sentinel lymph node was an independent predictive factor in our study group. Extracapsular extension is defined as the growth of tumor cells through the lymph node capsule, which was previously proposed as a factor for a poor prognosis in breast cancer33. The omission of this factor in previous predictive models may be related to the absence of its consistent inclusion in the pathological anatomy reports, as well as the lack of an exact definition of the extent of the invasion. This emphasizes the need for the standardization of extracapsular extension reporting in order to facilitate further studies as well as its inclusion in future models34.
Predictive nomograms are essential to guide clinical practice. This is especially true for the selection criteria for ALND, given the current context in which progressively fewer patients are subjected to it. Although various models have been described28, their validation in independent populations is not so simple35–38, which may explain the variety of models available and, at the same time, the lack of one model being adopted universally. The absence of validation may continue to be related to the variability of the interpretation of the sentinel node concept and the pathological analysis35; meanwhile, the models degrade when applied to different patient populations29. This should encourage different institutions to build a model based on their specificities, which was one of our objectives in this study.
Patients undergoing mastectomy should be especially considered in this context, since ALND remains the standard surgery after positive SLN biopsy. In our study, a high percentage of patients undergoing mastectomy (66.2%) was identified. This datum can be explained by several factors: first, this was the definitive surgery and many of these people have had one or more previous attempts at breast-conserving surgery; furthermore, a high percentage of multifocal/multicentric tumors was detected (60.7%); lastly, the study period includes the transition from the adoption of ACOSOG Z011 criteria13 in our department and, of course, many patients treated with breast-preserving surgery did not have ALND and were not included in the study. Even though ALND remains the standard surgery in these cases, previous studies have reported no advantages in survival, describing similar survival and recurrence rates between ALND and SLNB39,40. Furthermore, although there are no experimental studies on this topic, mainly due to problems in patient recruitment, clinical practice is changing and favoring SLNB in these cases41. When we applied our score to patients who had undergone mastectomy in our study group, 80% had a score = 0, and 65.9% with a score = 1 did not have metastases in non-sentinel nodes, which emphasizes the need to change clinical practices.
Lastly, it is necessary to mention the limitations of our study, which is a single-center, retrospective analysis. Furthermore, there is a possible selection bias as the ACSOG Z001113 criteria were adopted during the study period, and a survival analysis was not performed. Finally, the constructed score must be validated prospectively in an independent patient population to assess its actual predictive power.
In conclusion, despite the fact that our study group had a high percentage of risk factors, most of the patients did not have additional metastases in the ALND after a positive SLN, especially the cases with a score ≤1. This calls into question the need and the oncological benefit of conducting ALND in these patients. The identified predictive factors can help select patients in whom ALND could be omitted after a positive SLN, and the clinician can include this information in the discussion of the advantages and disadvantages of ALND in order to make the best decision.
FundingThis study has received no funding of any kind.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Peyroteo M, Canotilho R, Correia AM, Baía C, Ribeiro C, Reis P, et al. Factores predictivos de metástasis en ganglios no centinela en el cáncer de mama con ganglio centinela positivo. Cir Esp. 2022;100:81–87.