Laparoscopic resection of the pancreas (LRP) has been implemented to a varying degree because it is technically demanding and requires a long learning curve. In the present study we analyze the risk factors for complications and hospital readmissions in a single center study of 105 consecutive LRPs.
MethodsWe conducted a retrospective study using a prospective database. Data were collected on age, gender, BMI, ASA score, type of surgery, histologic type, operative time, hospital stay, postoperative complications, degree of severity and hospital readmission.
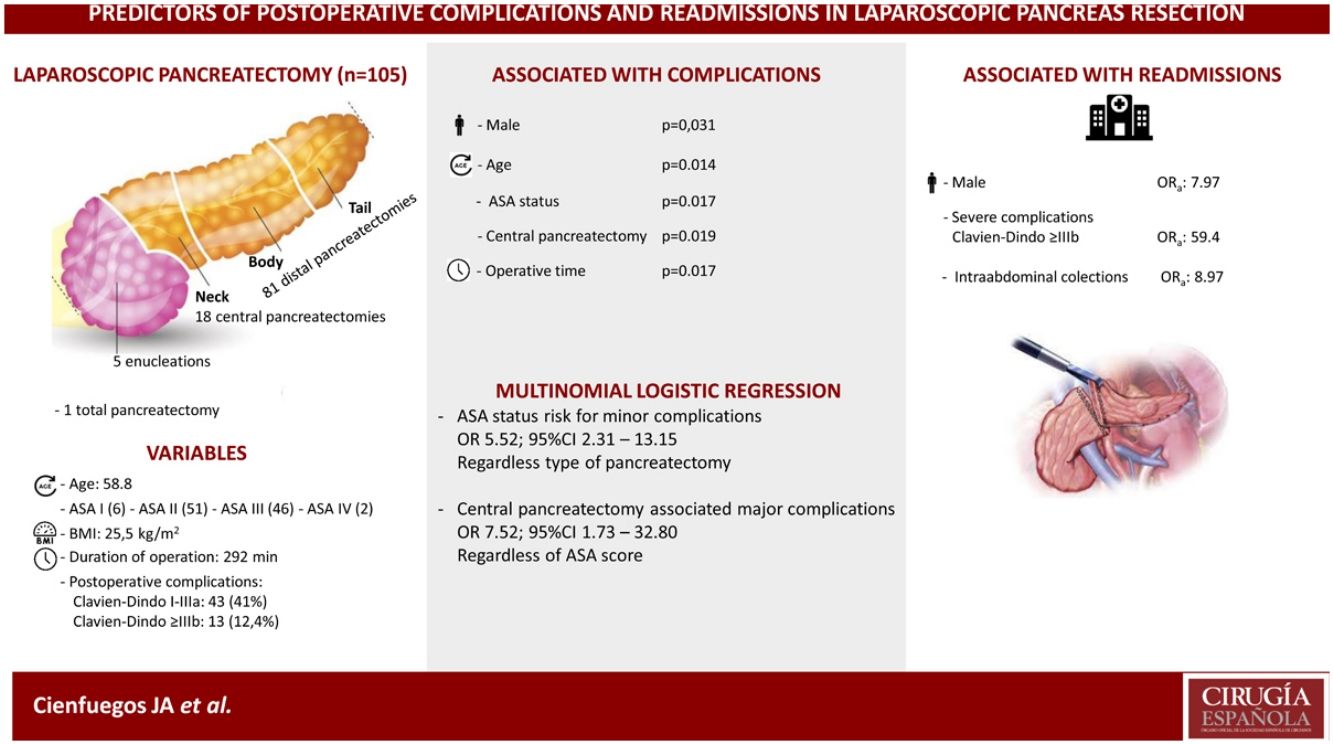
ResultsThe cohort included 105 patients, 63 females and 42 males with a median age and BMI of 58 (53–70) and 25.5 (22,2–27.9) respectively.
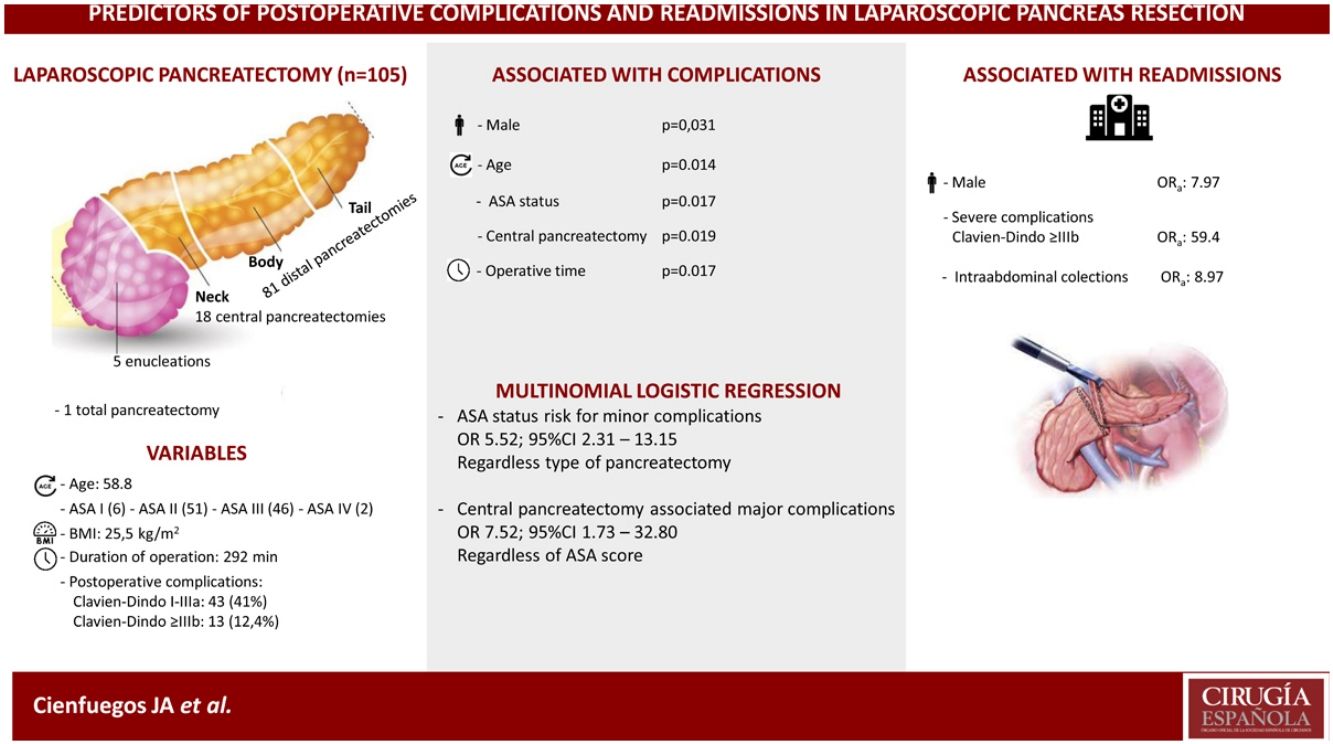
Eighteen (17%) central pancreatectomies, 5 (4.8%) enucleations, 81 (77.6%) distal pancreatectomies and one total pancreatectomy were performed.
Fifty-six patients (53.3%) experienced some type of complication, of which 13 (12.3%) were severe (Clavien-Dindo > IIIb) and 11 (10.5%) patients were readmitted in the first 30 days after surgery.
In the univariate analysis, age, male gender, ASA score, central pancreatectomy and operative time were significantly associated with the development of complications (P <0.05). In the multivariate analysis, male gender (OR 7.97; 95% CI 1.08–58.88)), severe complications (OR 59.40; 95% CI, 7.69–458.99), and the development of intrabdominal collections (OR 8.97; 95% CI, 1.28–63.02)) were associated with hospital readmission.
ConclusionsAge, male gender, ASA score, operative time and central pancreatectomy are associated with a higher incidence of complications. Male gender, severe complications and intraabdominal collections are associated with more hospital readmissions.
Las resecciones laparoscópicas del páncreas (RLP) tienen un grado de implantación muy heterogéneo debido a su dificultad técnica y a exigir una curva de aprendizaje larga. En el presente trabajo estudiamos los factores de riesgo de las complicaciones y de los reingresos en una serie unicéntrica de 105 RLP.
MétodosSe realizó un estudio retrospectivo. Se recogieron la edad, sexo, índice de masa corporal, el grado ASA, tipo de cirugía, tipo histológico, duración de la intervención, estancia hospitalaria, las complicaciones postoperatorias, grado de gravedad y reingreso.
ResultadosLa cohorte comprende 105 pacientes, 63 mujeres y 42 varones, con una mediana de edad y IMC, de 58 (53–70) y 25.5 (22.2–25.5) respectivamente.
Se realizaron 18 (17%) pancreatectomias centrales, 81 (77%) distales, 5 (4.8%) enucleaciones y una total.
56 (53.3%) pacientes sufrieron alguna complicación, 13 (12.3%) fueron graves (Clavien-Dindo > IIIb) y hubo 11 (10.5%) reingresos.
En el análisis univariante, la edad, el sexo masculino, el grado ASA, la pancreatectomía central y el tiempo operatorio se asociaban significativamente con el desarrollo de complicaciones (P < 0.05). En el análisis multivariante, los varones (OR 7.97; 95% IC 1.08–58.8), las complicaciones severas (OR 59.40; 95% IC 7.69–458.9), el desarrollo de colecciones intraabdominales (OR 8.97; 95% IC 1.2–63.0) se asociaban con el reingreso hospitalario.
ConclusionesLa edad, el sexo masculino, el grado ASA, la duración de la intervención y la pancreatectomía central se asocian con mayor incidencia de complicaciones. Los varones, las complicaciones graves, las colecciones intraabdominales se asociaban con más reingresos hospitalarios.
Since its introduction in 1996 by Gagner and Pomp1, laparoscopic resection of the pancreas (LRP) has been widely accepted in the treatment of benign lesions, neuroendocrine tumors and malignant lesions located in the tail of the pancreas2–5.
However, apart from the well-known benefits of minimally invasive resections the technique has been implemented to a varying degree due to its technical difficulty, the high rate of conversions to open surgery and the need for a long learning curve2,6,7. In the case of laparoscopic duodenopancreatectomy (LDP), after two favorable trials 8,9, a recent multi-center trial (LEOPARD-2) had to be suspended due to a higher incidence of postoperative complications in patients undergoing laparoscopy than open surgery10.
The introduction of robot-assisted surgery offers a possible means of overcoming, these limitations and currently there are three trials comparing open, laparoscopic and robot-assisted LDPs2,11.
The objective of this study is to analyze the factors associated with surgical complications and hospital readmissions in a cohort of 105 consecutive laparoscopic resections.
In our study we confirm that laparoscopic techniques meet the requirements of safety, efficacy and efficiency12. Our analysis has allowed us to identify those pre-operative and intra-operative parameters which are associated with post-operative complications and hospital readmission.
MethodsOverview of study designA retrospective study was carried out using data from a prospective database of all consecutive patients undergoing laparoscopic resection of the pancreas between 2000 and 2020 in the Clínica Universidad de Navarra.
The study was conducted following the STROCCS guidelines13. Patients gave their consent to participate in the study which was conducted following the latest version of the Declaration of Helsinki. The study was approved by the Medial Ethics Committee and registered in the National Clinical Trial.org (NCT04935216).
Data collection and outcomesThe following variables were extracted from the patients’ electronic medical records: age, sex, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), American Society of Anesthesiology (ASA) score14 and the type of surgery15 The preoperative diagnosis was confirmed by at least two imaging tests and by endoscopic ultrasonography and fine needle aspiration (FNA).
Duration of the surgery, use of blood products, intraoperative complications, length of hospital stay and the size and histology of the tumor were all recorded. Operative time (minutes) was defined as the time from first incision to final skin closure (the last stich). Length of hospital stay (LOS) was calculated from the day of surgery through and including the day of discharge.
Central pancreatectomy and DP were performed using the techniques described elsewhere16–18. In DP every attempt was made to spare the spleen and the splenic vessels using the technique of Kimura19.
In DP, intraabdominal drainage was avoided and was performed very selectively in CP and enucleations, with the drain being removed on the third postoperative day20.
Two authors, (JAC, L H-P) reviewed all the postoperative complications and classified them using the Clavien-Dindo classification21. Complications were considered major when they were > IIIb. Pancreatic fistula, postoperative bleeding and delay in gastric emptying were defined according to the International Study Group of Pancreatic Surgery (ISGPS)22–24.
Postoperative intraabdominal collection was defined as the accumulation of >5 cm of fluid as indicated by CT scan or US25,26. Symptomatic collections or those associated with increases in inflammatory markers were drained via a nasogastric tube using endoscopic or percutaneous ultrasound27.
Perioperative death was defined as death occurring in hospital or within 30 days of the operation. Readmission was defined as an admission to any hospital for 24 h or more for any reason within 60 days of surgery.
Statistical analysisDescriptive variables are presented as n (percentages) with confidence intervals and quantitative variables as means plus standard deviations or medians plus interquartile ranges where distribution of the data was not normal.
For the comparison of categorical variables, the Pearson Chi-squared test was used with the estimation of exact P values. For the comparison of quantitative variables, student t tests and ANOVA or their non-parametric equivalents the U-Mann–Whitney or Kruskal–Wallis tests were used. Non-conditional multivariate logistic regression was used to determine the independent association between the variables studied and the development of postoperative complications or hospital readmission. Multinomial logistic regression was performed adjusting for significant variables in the bivariate analysis: age, gender, ASA score, type of surgery and length of surgery. Statistical significance was assessed at the 95th percentile. P values <.05 were considered to be statistically significant.
ResultsBetween January 2000 and October 2020, 105 laparoscopic resections of the pancreas were performed, of which 100% were completed by the same surgeon (FR). There were no conversions to open surgery.
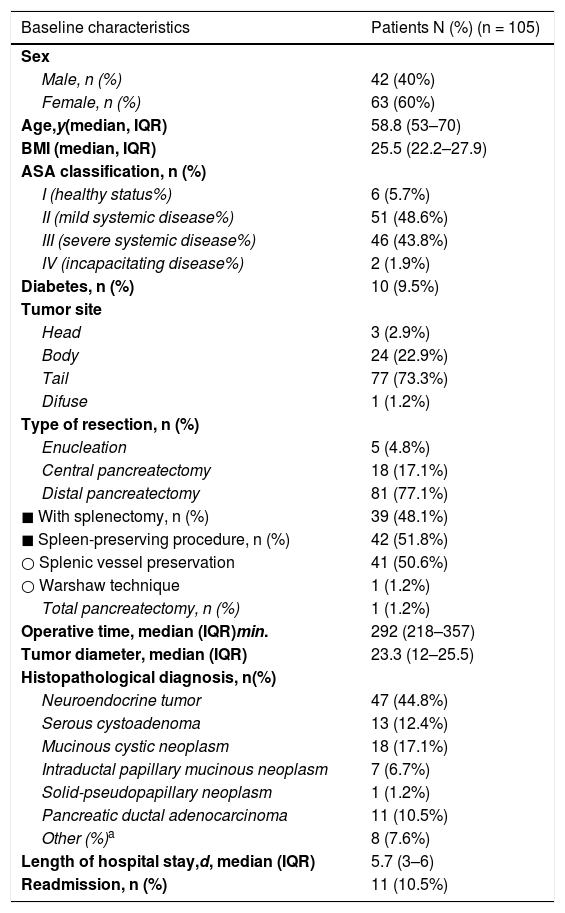
Table 1 summarizes the clinical, surgical and histologic characteristics of the 105 patients.
Baseline demographic and clinicopathologic characteristics of 105 patients who underwent laparoscopic pancreas resection.
| Baseline characteristics | Patients N (%) (n = 105) |
|---|---|
| Sex | |
| Male, n (%) | 42 (40%) |
| Female, n (%) | 63 (60%) |
| Age,y(median, IQR) | 58.8 (53–70) |
| BMI (median, IQR) | 25.5 (22.2–27.9) |
| ASA classification, n (%) | |
| I (healthy status%) | 6 (5.7%) |
| II (mild systemic disease%) | 51 (48.6%) |
| III (severe systemic disease%) | 46 (43.8%) |
| IV (incapacitating disease%) | 2 (1.9%) |
| Diabetes, n (%) | 10 (9.5%) |
| Tumor site | |
| Head | 3 (2.9%) |
| Body | 24 (22.9%) |
| Tail | 77 (73.3%) |
| Difuse | 1 (1.2%) |
| Type of resection, n (%) | |
| Enucleation | 5 (4.8%) |
| Central pancreatectomy | 18 (17.1%) |
| Distal pancreatectomy | 81 (77.1%) |
| ◼ With splenectomy, n (%) | 39 (48.1%) |
| ◼ Spleen-preserving procedure, n (%) | 42 (51.8%) |
| ○ Splenic vessel preservation | 41 (50.6%) |
| ○ Warshaw technique | 1 (1.2%) |
| Total pancreatectomy, n (%) | 1 (1.2%) |
| Operative time, median (IQR)min. | 292 (218–357) |
| Tumor diameter, median (IQR) | 23.3 (12–25.5) |
| Histopathological diagnosis, n(%) | |
| Neuroendocrine tumor | 47 (44.8%) |
| Serous cystoadenoma | 13 (12.4%) |
| Mucinous cystic neoplasm | 18 (17.1%) |
| Intraductal papillary mucinous neoplasm | 7 (6.7%) |
| Solid-pseudopapillary neoplasm | 1 (1.2%) |
| Pancreatic ductal adenocarcinoma | 11 (10.5%) |
| Other (%)a | 8 (7.6%) |
| Length of hospital stay,d, median (IQR) | 5.7 (3–6) |
| Readmission, n (%) | 11 (10.5%) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
Eighty-one (77.1%) DP were performed, of which 41 (50.6%) were carried out with sparing of the spleen and splenic vessels (Kimura procedure) and one case of Warshaw technique. Eighteen (17.1%) CP were performed, 5 enucleations (4.8%) and one total pancreatectomy.
The most frequent indication was neuroendocrine tumors (n = 47; 44.8%), followed by cystic tumors of the pancreas (n = 39; 37.1%). The median operative time and hospital stay was 292 min (range: 218–357) and 5.7 days (range 3–6), respectively.
Abdominal drainage was left in place in 17 (16.1%) cases: 10 (55.6%) in CP, 5 (6.2%) in DP and 1 in enucleations (20%) and total pancreatectomy, respectively.
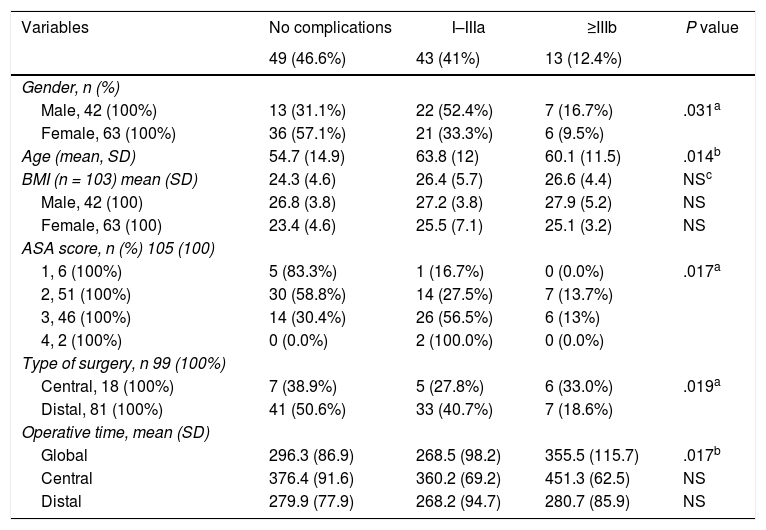
Patient-related factors associated with outcomeOf the 105 patients, 49 (46.6%), experienced some type of complication, of which 43 (41%) were minor or moderate and 13 (12.4%) major. Table 2 shows the analysis of the variables associated with the development of complications. Age, male gender, ASA score, CP and operative time were all significantly associated with a higher incidence of postoperative complications.
Variables associated with postoperative complications.
| Variables | No complications | I–IIIa | ≥IIIb | P value |
|---|---|---|---|---|
| 49 (46.6%) | 43 (41%) | 13 (12.4%) | ||
| Gender, n (%) | ||||
| Male, 42 (100%) | 13 (31.1%) | 22 (52.4%) | 7 (16.7%) | .031a |
| Female, 63 (100%) | 36 (57.1%) | 21 (33.3%) | 6 (9.5%) | |
| Age (mean, SD) | 54.7 (14.9) | 63.8 (12) | 60.1 (11.5) | .014b |
| BMI (n = 103) mean (SD) | 24.3 (4.6) | 26.4 (5.7) | 26.6 (4.4) | NSc |
| Male, 42 (100) | 26.8 (3.8) | 27.2 (3.8) | 27.9 (5.2) | NS |
| Female, 63 (100) | 23.4 (4.6) | 25.5 (7.1) | 25.1 (3.2) | NS |
| ASA score, n (%) 105 (100) | ||||
| 1, 6 (100%) | 5 (83.3%) | 1 (16.7%) | 0 (0.0%) | .017a |
| 2, 51 (100%) | 30 (58.8%) | 14 (27.5%) | 7 (13.7%) | |
| 3, 46 (100%) | 14 (30.4%) | 26 (56.5%) | 6 (13%) | |
| 4, 2 (100%) | 0 (0.0%) | 2 (100.0%) | 0 (0.0%) | |
| Type of surgery, n 99 (100%) | ||||
| Central, 18 (100%) | 7 (38.9%) | 5 (27.8%) | 6 (33.0%) | .019a |
| Distal, 81 (100%) | 41 (50.6%) | 33 (40.7%) | 7 (18.6%) | |
| Operative time, mean (SD) | ||||
| Global | 296.3 (86.9) | 268.5 (98.2) | 355.5 (115.7) | .017b |
| Central | 376.4 (91.6) | 360.2 (69.2) | 451.3 (62.5) | NS |
| Distal | 279.9 (77.9) | 268.2 (94.7) | 280.7 (85.9) | NS |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
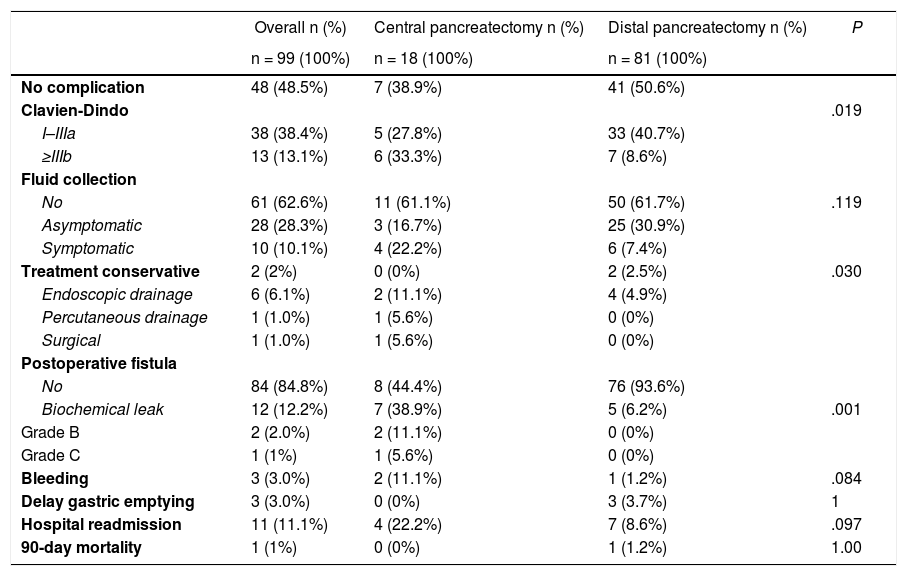
Given that CP was associated with a higher incidence of complications, a comparison was made between CP and DP. Table 3 shows the univariate analysis of the complications from each type of surgery. Grave complications, the development of biochemical leak, pancreatic fistula and the need to drain a postoperative intraabdominal collection were significantly more frequent in CP.
Comparison of the postoperative complications between central and distal laparoscopic pancreatectomy.
| Overall n (%) | Central pancreatectomy n (%) | Distal pancreatectomy n (%) | P | |
|---|---|---|---|---|
| n = 99 (100%) | n = 18 (100%) | n = 81 (100%) | ||
| No complication | 48 (48.5%) | 7 (38.9%) | 41 (50.6%) | |
| Clavien-Dindo | .019 | |||
| I–IIIa | 38 (38.4%) | 5 (27.8%) | 33 (40.7%) | |
| ≥IIIb | 13 (13.1%) | 6 (33.3%) | 7 (8.6%) | |
| Fluid collection | ||||
| No | 61 (62.6%) | 11 (61.1%) | 50 (61.7%) | .119 |
| Asymptomatic | 28 (28.3%) | 3 (16.7%) | 25 (30.9%) | |
| Symptomatic | 10 (10.1%) | 4 (22.2%) | 6 (7.4%) | |
| Treatment conservative | 2 (2%) | 0 (0%) | 2 (2.5%) | .030 |
| Endoscopic drainage | 6 (6.1%) | 2 (11.1%) | 4 (4.9%) | |
| Percutaneous drainage | 1 (1.0%) | 1 (5.6%) | 0 (0%) | |
| Surgical | 1 (1.0%) | 1 (5.6%) | 0 (0%) | |
| Postoperative fistula | ||||
| No | 84 (84.8%) | 8 (44.4%) | 76 (93.6%) | |
| Biochemical leak | 12 (12.2%) | 7 (38.9%) | 5 (6.2%) | .001 |
| Grade B | 2 (2.0%) | 2 (11.1%) | 0 (0%) | |
| Grade C | 1 (1%) | 1 (5.6%) | 0 (0%) | |
| Bleeding | 3 (3.0%) | 2 (11.1%) | 1 (1.2%) | .084 |
| Delay gastric emptying | 3 (3.0%) | 0 (0%) | 3 (3.7%) | 1 |
| Hospital readmission | 11 (11.1%) | 4 (22.2%) | 7 (8.6%) | .097 |
| 90-day mortality | 1 (1%) | 0 (0%) | 1 (1.2%) | 1.00 |
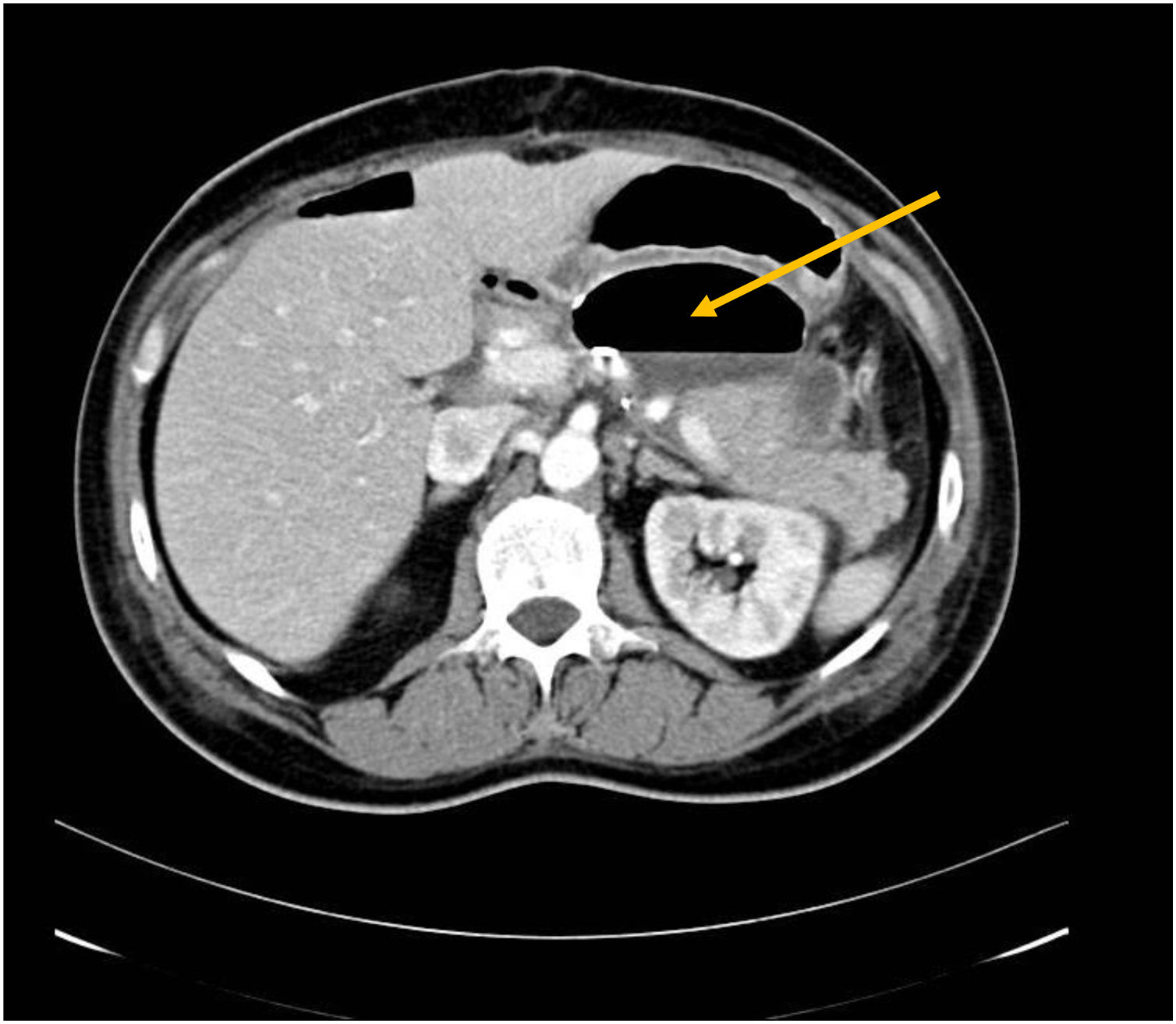
Twelve (11.4%) of the 104 patients (excluding the single case of total pancreatectomy) developed biochemical leak and 3 (2.8%) pancreatic fistula (2 grade B and one grade C). Thirty-eight patients (36.1%) developed intraabdominal fluid collections, of which 28 (3 in CP and 25 in DP) were asymptomatic and resolved spontaneously with conservative treatment. Ten patients developed symptomatic collections of which 8 required drainage: 6 endoscopically (Fig. 1), 1 percutaneously and 1 surgically.
Four patients (3.8%) experienced delay in gastric emptying (grade A) and 3 had extraluminal bleeding in the first 24 h after surgery, of which 1 required repeat laparoscopic surgery.
In multivariate analysis ASA score was a risk factor for minor complications, odds ratio (OR) 5.52 (95% CI 2.31–13.15) independently of type of surgery; and central pancreatectomy was associated with major complications OR 7.52 (95% CI 1.73–32.80) regardless ASA status.
Eleven patients (10.5%) were readmitted in the first 30 days after surgery: 9 for observation and the treatment of intraabdominal collections, one for peripheral venous thrombosis and another with delayed gastric emptying. The single patient death was due to massive bleeding secondary to the endoscopic drainage of a collection performed 10 days after the operation in the patient’s home town.
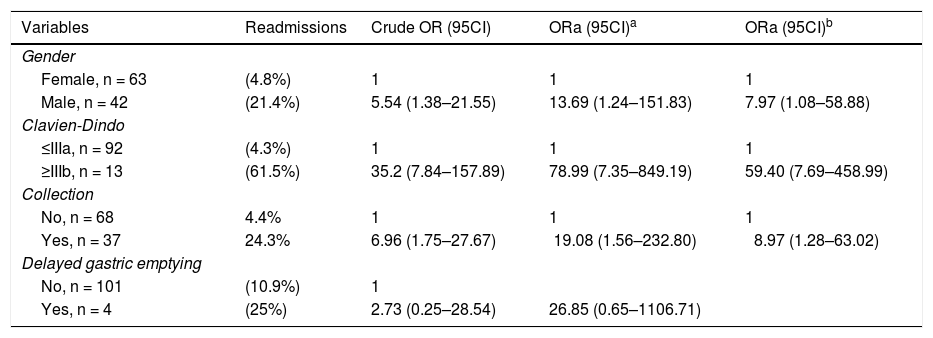
Table 4 shows the multivariate analysis of the variables associated with hospital readmission. Male gender, OR 7.97 (95% CI, 1.08–58.88), grave complications OR 59.40, (95% CI, 7.97–458.99) and intraabdominal fluid collections OR 8.97; (95% CI, 1.28–63.02) were associated with hospital readmission. However, no association was found with delay in gastric emptying.
Risk factors to readmission adjusted logistic regression.
| Variables | Readmissions | Crude OR (95CI) | ORa (95CI)a | ORa (95CI)b |
|---|---|---|---|---|
| Gender | ||||
| Female, n = 63 | (4.8%) | 1 | 1 | 1 |
| Male, n = 42 | (21.4%) | 5.54 (1.38–21.55) | 13.69 (1.24–151.83) | 7.97 (1.08–58.88) |
| Clavien-Dindo | ||||
| ≤IIIa, n = 92 | (4.3%) | 1 | 1 | 1 |
| ≥IIIb, n = 13 | (61.5%) | 35.2 (7.84–157.89) | 78.99 (7.35–849.19) | 59.40 (7.69–458.99) |
| Collection | ||||
| No, n = 68 | 4.4% | 1 | 1 | 1 |
| Yes, n = 37 | 24.3% | 6.96 (1.75–27.67) | 19.08 (1.56–232.80) | 8.97 (1.28–63.02) |
| Delayed gastric emptying | ||||
| No, n = 101 | (10.9%) | 1 | ||
| Yes, n = 4 | (25%) | 2.73 (0.25–28.54) | 26.85 (0.65–1106.71) |
Minimally invasive pancreatic resections have rapidly gained acceptance in the treatment of tumors which are benign or have low-grade malignancy, especially in resections which spare the pancreatic parenchyma: central, distal pancreatectomies and enucleations2,3,5.
In our series, most of the pancreatic resections were central or distal and as such the phenotype of the patients coincides with the published series in this type of resections28,29.
Although the most frequent tumors were neuroendocrine (47.4%), we performed only 5 enucleations (4.8%) given the high incidence of pancreatic fistula reported with this procedure30.
All the surgeries were performed laparoscopically and by the same surgeon (FR), with no conversions to open surgery.
Operative time was slightly longer than that reported in extensive multicenter series and systematic reviews, perhaps due to the greater complexity of the procedures performed (CP, sparing of the spleen and splenic vessels), the systematic use of intraoperative ultrasound and meticulous hemostasis avoiding the use of harmonic scalpels (high-energy sealant devices)18,31. In our series the duration of the operation was not considered a priori a reason to convert to open surgery in spite of the reported association between operative time and postoperative complications32.
The median hospital stay for minimally invasive CP was shorter than that reported in other studies33, while in the minimally invasive DP it was similar to that reported by Van Buren et al and Weber et al.20,34.
Most complications were minor (Clavien-Dindo < IIIb), and we noted that male gender, age, ASA score, type of surgery (CP vs DP) and operative time were significantly associated with the development of postoperative complications14.
Age and ASA score reflect the patient’s baseline performance status and homeostatic ability to respond to the surgery14.
Operative time was also a factor predictive of postoperative complications. Apart from reflecting technical difficulty, operative time has been linked to greater stimulation of the innate immune system, secondary to the greater tissue damage32.
In our series CP was associated with more postoperative complications than DP, which reflects the greater technical complexity of this procedure2,16. Apart from the longer operative time, CP requires a pancreato-enteric anastomosis, with the consequent risk postoperative fistula. The three pancreatic fistulas (2 grade B and one grade C) occurred in CP.
The experience reported in minimally invasive laparoscopic CP is limited to small series35. In our series of minimally invasive CP (18 cases), hospital stay (median of 5 days) and incidence of pancreatic fistula (16.6%) were similar to those reported in series with more than 15 cases, with the limitations cited above36.
In our series there were 3 (2,8%) pancreatic fistulas (2 grade B and one grade C), a number that is lower than that reported in CP (15%–40%)33,36 and DP (15%–20%)20.
In line with previous studies, we avoided systematically leaving abdominal drains in place in DP used them only selectively in CP and removed them in the first three days after surgery20,37.
Apart from the comments above, we believe that one of the factors for the low incidence of pancreatic fistula is sparing the integrity of the arterial vascularization of the remnant pancreas.
Furthermore, most resections were DP (n = 81; 77.1%), which do not require pancreatojejunal anastomosis and only 5 enucleations (4.8%) were performed for the reasons previously mentioned30.
However, we observed 38 pancreatic collections, which were mostly asymptomatic, and which resolved with conservative treatment. We are aware that the advantages of not leaving the drain in place have to weighed against the diagnostic and therapeutic dilemma of postsurgical peripancreatic collections, which may have been considered as “biochemical leaks” had they been drained22.
Twenty-eight patients (28.3%) developed asymptomatic intraabdominal collections: 3 (16.6%) in CP and 25 (30.9%) in minimally invasive DP. The incidence of collections in DP is similar to that reported by other studies in which intraperitoneal drains were not left in place and higher than those reported in studies where drains were systematically left in place20. Of the 31 cases of acute fluid collection (AFC) in DP, 4 (12.9%) patients required endoscopic drainage, a figure similar to that of the studies previously cited20,25.
A great deal of variability in the incidence of AFC has been reported due to the diversity of the criteria for definition, the use of US in the postoperative period and the type of surgery25,37.
In agreement with other authors, we believe that asymptomatic collections should not be drained38 or should be only in the presence of symptoms. This policy requires strict monitoring of patients and the availability of endoscopists skilled in transgastric drainage27. The only death was due to endoscopic drainage of an intraabdominal collection in another hospital.
Given that many of the parameters related to complications are known in the preoperative period, they are of great practical value when it comes to assigning resources (previous experience of the surgeon, estimation of operative time) aimed at reducing complications.
In our series, there were 11 (10%) hospital readmissions, of which 9 were due to the treatment of intraabdominal collections. These figures are similar to those reported by Kamarajah et al.39, although this study refers basically to pancreato-duodenectomies.
In the multivariate analysis, we found three variables that were associated with hospital readmission: male gender, severe complications and intraabdominal collections.
Since the first report of laparoscopic duodenopancreatectomy (LDP) by Gagner and Pomp1 in 1994, minimally invasive resection of the pancreas has progressively become more widely implemented and accepted2,3,5. The well-known advantages of laparoscopic surgery must be weighed against its disadvantages which include its technical difficulty, the high rate of conversions to open surgery and the need for a long learning curve (80–100 cases for LDP, 40 cases for LDP)40,41, as a result of which the degree of implementation of the technique is very varied and the dilemma of concentrating this type of surgery in centers with a high volume of patients arise42,43. Despite recognition of the volume-outcome relationship, in our region pancreas surgery is not centralized42.
Laparoscopic resections are the ideal technique for the treatment of neuroendocrine tumors, cystic tumors and malign tumors located in the tail of the pancreas2,3. However, the use of laparoscopic duodenopancreatectomy (LDP), is controversial as a higher incidence of complications has been recorded in a recent multi-center trial (LEOPARD-2)10,41.
The introduction of robot-assisted surgery has provided a potential means to overcome these limitations and currently three trials comparing open, laparoscopic and robot-assisted LDPs are ongoing2.
LimitationsThere are some limitations that must be taken into account when assessing results as it is a limited series of cases (n = 105) with lesions generally located in the distal pancreas and that all the patients were operated on by the same surgeon, which may bias the comparison with results from other centers.
In addition, the study covers a long period of time in which improvements were made in surgical techniques and perioperative care.
However, as it is a single center study, it avoids the great variability that is observed in large multicenter series both in surgical technique and the early diagnosis and treatment of complications.
ConclusionsCentral and distal resections of the pancreas performed laparoscopically are safe and offer the advantages inherent in minimally invasive surgery.
Male gender, age, ASA score, operative time and central pancreatectomy are associated with the development of postoperative complications. Similarly, male gender, the occurrence of severe complications and intraabdominal fluid collections are associated with higher readmission rates.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author contributionsJAC, L H-P, and FR conceived and designed the research question.
JAC and L H-P prepared the data for analysis.
JAC, L H-P, CEB and FG analyzed the data. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
JAC and L H-P wrote the first draft of the manuscript. All authors provided input on interpretation of results. All authors revised the manuscript critically for important intellectual content and read and approved the final manuscript.
Conflict of interestThe authors are not aware of any affiliations, membership, funding, or financial holdings that might be perceived as affecting the objectivity of this manuscript.