Preoperative short-course radiotherapy with immediate surgery improves local control in patients with rectal cancer. Tumor responses are smaller than those described with radiochemotherapy. Preliminary data associate this lower response to the short period until surgery. The aim of this study is to analyze the response to preoperative short-course radiotherapy and its correlation with the interval to surgery especially analyzing patients with mesorectal fascia involvement.
MethodsA total of 155 patients with locally advanced rectal cancer treated with preoperative radiotherapy (5×5Gy) were retrospectively analyzed. Tumor response in terms of rates of complete pathological response, downstaging, tumor regression grading and status of the circumferential resection margin were quantified.
ResultsThe mean interval from radiotherapy to surgery was 23 days. The rate of complete pathological response was 2.2% and 28% experienced downstaging (stage decreased). No differences between these rates and interval to surgery were detected. Eighty-eight patients had magnetic resonance imaging for staging (in 31 patients the mesorectal fascia was involved). The mean time to surgery in patients with involvement of the fascia and R0 surgery was 27 days and 16 days if R1 (P=.016). The cutoff of 20 days reached the highest probability of achieving a free circumferential resection margin between patients with mesorectal fascia involvement, with no statistically significant differences: RR 3.036 95% CI=(0.691–13.328), P=.06.
ConclusionsAfter preoperative short-course radiotherapy, an interval >20 days enhances the likelihood of achieving a free circumferential resection margin in patients with mesorectal fascia involvement.
La radioterapia preoperatoria de curso corto con cirugía inmediata, mejora el control local del cáncer rectal. Las respuestas que consigue son de menor magnitud que las descritas con radioquimioterapia. Datos preliminares asocian esta menor respuesta al corto periodo hasta la cirugía. El objetivo de este estudio es analizar la respuesta obtenida con el esquema preoperatorio de curso corto y su correlación con el tiempo hasta la cirugía, analizando especialmente a los pacientes con fascia mesorrectal afectada.
MétodosSe analiza retrospectivamente a 155 pacientes tratados con radioterapia preoperatoria (5×5Gy). Se cuantificó la respuesta tumoral en términos de tasas de respuesta completa patológica, reducción del estadio, grado de regresión tumoral y estado del margen de resección circunferencial.
ResultadosEl intervalo medio radioterapia-cirugía fue de 23 días. Se alcanzaron respuestas completas patológicas en el 2,2% y reducción del estadio en el 28%. No se detectaron diferencias entre estas tasas y el intervalo hasta la cirugía. Ochenta y ocho pacientes tenían resonancia de estadificación (31 con fascia mesorrectal comprometida). La media de tiempo hasta la intervención en pacientes con fascia comprometida y cirugía R0 fue de 27 días y si R1 de 16 días (p=0,016). El punto de corte de 20 días alcanzó la mayor probabilidad de lograr un margen circunferencial negativo entre los pacientes con fascia mesorrectal comprometida, aunque sin alcanzar significación estadística: RR 3,036, IC del 95%=0,691–13,328, p=0,06.
ConclusionesTras la radioterapia preoperatoria de curso corto, un intervalo > 20 días potencia la probabilidad de lograr un margen de resección libre en pacientes con fascia mesorrectal comprometida.
Preoperative radiotherapy followed by total excision of the mesorectum continues to be the recommended treatment for locally advanced rectal cancer (LARC).1 Preoperative regimens are less toxic and more effective for reducing local recurrences than postoperative treatment.2,3 There are 2 validated regimens: the so-called long regimen or chemoradiotherapy (CRTx) that administers 45–50.4Gy in 25–28 daily fractions, associated with concomitant chemotherapy and followed by surgery deferred by 4–8 weeks2; and, preoperative short-course radiotherapy (SCRT) that administers 25Gy in 5 fractions, without chemotherapy and immediate surgery in 1–7 days.4 These regimens have been extensively compared.5–7 A recent meta-analysis8 concluded that SCRT with immediate surgery is as effective as CRTx with deferred surgery in terms of overall and disease-free survival rates, local and distant control, and toxicity.8 There is no international consensus about the use of these 2 regimens in the context of LARC. While SCRT is widely implemented in northern Europe,1 CRTx is the most widely used regimen in the US.
SCRT is more convenient for patients and more cost-effective for the Spanish national healthcare system.8 However, even though it has demonstrated similar rates of local control, the tumor reduction that is achieved is lower. A randomized trial5 reported complete pathologic response (cPR) rates of 0.7% after SCRT and 16% with CRTx. This lower rate of cPR has been linked to minimal time intervals between the end of SCRT and interventions.9 Therefore, when tumor reduction is required prior to surgery, all clinical guidelines recommend a long CRTx1 regimen in order to achieve free circumferential resection margins (CRM), which is a predictive factor for local control and survival.3,10 Nonetheless, in daily practice there are 2 patient subgroups (those with low performance status [PS] or comorbidities) that are not candidates for CRTx. The same is true for potentially resectable metastases, in which a 5-week regimen, together with a waiting period before surgery of 4–8 weeks, could allow the metastatic disease to progress. In these subgroups, even when found in resection margins, the multidisciplinary team frequently prescribes SCRT. Progressively, results are being reported that prolonging the time from the end of SCRT until surgery increases the tumor response obtained. Promoting this response is especially relevant in the subgroup of patients with compromised mesorectal fascia (MRF) on magnetic resonance imaging (MRI), which requires tumor reduction to achieve optimal surgical results.11 There are trials currently underway,12 but the optimal time interval before surgery has still not been identified.
The objective of this study is to analyze the local response obtained in the group of patients with LARC, cT3.4 and/or N+ treated at our hospital by means of SCRT and total mesorectal excision, especially analyzing patients with involvement of the MRF on MRI.
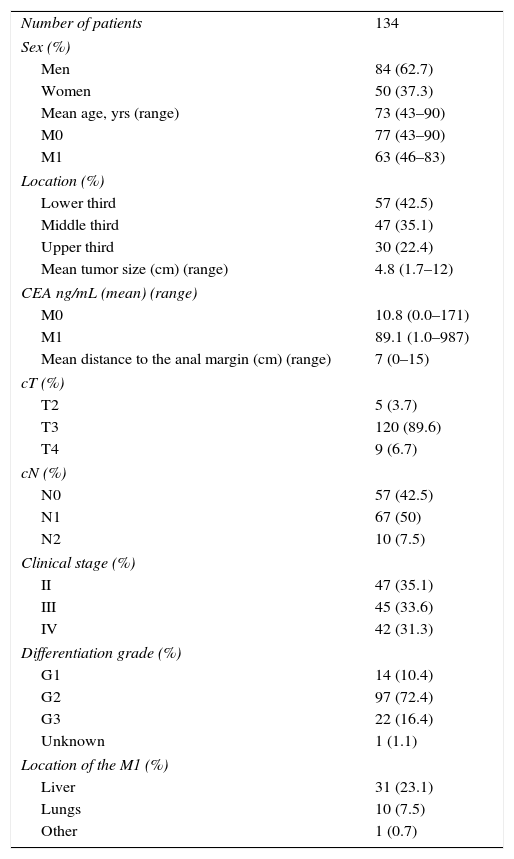
MethodsThis is an observational, retrospective study of 155 patients diagnosed with potentially resectable LARC cT3.4 and/r N+. All patients had histological diagnosis of adenocarcinoma and were treated in the period 1998–June 2015 with SCRT (25Gy in 5 fractions), followed by surgery in a period ≥10 days. Included in the study were 42 patients with potentially resectable synchronous metastasis. Seventeen patients were excluded due to intervention at other hospitals and no available pathology report, and 4 were excluded because they did not undergo surgery. The analysis was done in 134 patients. The extension study included endorectal ultrasound, computed tomography of the thorax/abdomen/pelvis and, since 2007, pelvic MRI. In 31 (23%) patients, positron-emission tomography was used as additional screening for distant disease. Patients were classified by the TNM system (6th Edition), and their clinical characteristics are summarized in Table 1.
Description of the Clinical Characteristics of the Patients.
| Number of patients | 134 |
| Sex (%) | |
| Men | 84 (62.7) |
| Women | 50 (37.3) |
| Mean age, yrs (range) | 73 (43–90) |
| M0 | 77 (43–90) |
| M1 | 63 (46–83) |
| Location (%) | |
| Lower third | 57 (42.5) |
| Middle third | 47 (35.1) |
| Upper third | 30 (22.4) |
| Mean tumor size (cm) (range) | 4.8 (1.7–12) |
| CEA ng/mL (mean) (range) | |
| M0 | 10.8 (0.0–171) |
| M1 | 89.1 (1.0–987) |
| Mean distance to the anal margin (cm) (range) | 7 (0–15) |
| cT (%) | |
| T2 | 5 (3.7) |
| T3 | 120 (89.6) |
| T4 | 9 (6.7) |
| cN (%) | |
| N0 | 57 (42.5) |
| N1 | 67 (50) |
| N2 | 10 (7.5) |
| Clinical stage (%) | |
| II | 47 (35.1) |
| III | 45 (33.6) |
| IV | 42 (31.3) |
| Differentiation grade (%) | |
| G1 | 14 (10.4) |
| G2 | 97 (72.4) |
| G3 | 22 (16.4) |
| Unknown | 1 (1.1) |
| Location of the M1 (%) | |
| Liver | 31 (23.1) |
| Lungs | 10 (7.5) |
| Other | 1 (0.7) |
Compromised MRF was defined as invasion or a tumor ≤1mm from the fascia.
The therapeutic decision was made by a multidisciplinary team. The exact time transpired between radiotherapy and surgery was not protocolized and depended on organizational reasons, not a strict clinical criterion. Surgery was recommended at least 10 days after SCRT, but this was left to the criteria and/or availability of the surgical team. CRM were considered affected if there was invasion or there was a tumor ≤1mm from the margins. The protocol did not include a re-evaluation MRI prior to surgery, although in the last year of recruitment this was done in all patients with compromised MRF on the initial MRI.
The tumor regression grade (TRG) was established by the Mandard system.13 The downstaging effect was established by the comparison of the stages cT, cN and pathological stage, defined as yp stage 0-I (ypT0-2N0M0).
Statistical AnalysisAbsolute and relative frequencies were estimated for the qualitative variables, arithmetic mean, standard deviation, and maximum and minimum values for the quantitative variables. Student's t test was used to compare means. An analysis with different cut-off points in days (15, 20, 25, 30) was created to delimit differences for achieving R0 surgery in MRF+ patients using the estimation of relative risk (RR). Local disease-free survival (LDFS) was calculated with the Kaplan–Meier method, determined from the date of diagnosis until the date of recurrence. The results were compared with the log-rank test. A P value <.05 was considered statistically significant. Data were analyzed with the SPSS v.16 statistical program.
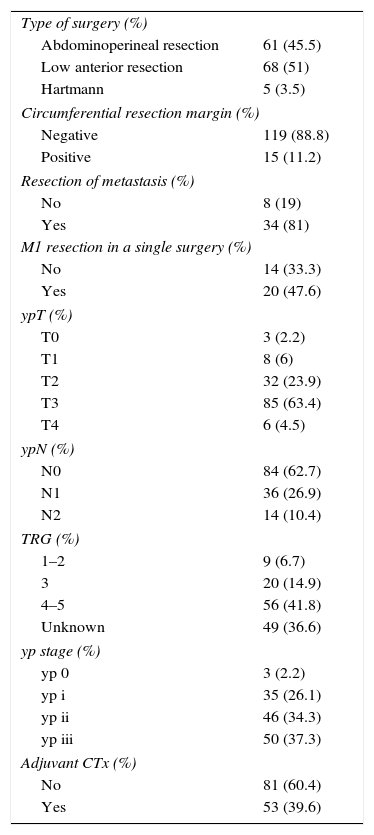
ResultsThe type of surgery conducted and pathological characteristics are shown in Table 2. The mean time transpired from the end of SCRT until surgery was 23 days. 95% of the patients were operated on between 18 and 31 days after SCRT.
Surgery Performed, Pathological Characteristics and Adjuvant CTx.
| Type of surgery (%) | |
| Abdominoperineal resection | 61 (45.5) |
| Low anterior resection | 68 (51) |
| Hartmann | 5 (3.5) |
| Circumferential resection margin (%) | |
| Negative | 119 (88.8) |
| Positive | 15 (11.2) |
| Resection of metastasis (%) | |
| No | 8 (19) |
| Yes | 34 (81) |
| M1 resection in a single surgery (%) | |
| No | 14 (33.3) |
| Yes | 20 (47.6) |
| ypT (%) | |
| T0 | 3 (2.2) |
| T1 | 8 (6) |
| T2 | 32 (23.9) |
| T3 | 85 (63.4) |
| T4 | 6 (4.5) |
| ypN (%) | |
| N0 | 84 (62.7) |
| N1 | 36 (26.9) |
| N2 | 14 (10.4) |
| TRG (%) | |
| 1–2 | 9 (6.7) |
| 3 | 20 (14.9) |
| 4–5 | 56 (41.8) |
| Unknown | 49 (36.6) |
| yp stage (%) | |
| yp 0 | 3 (2.2) |
| yp i | 35 (26.1) |
| yp ii | 46 (34.3) |
| yp iii | 50 (37.3) |
| Adjuvant CTx (%) | |
| No | 81 (60.4) |
| Yes | 53 (39.6) |
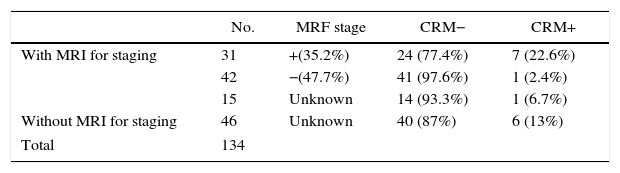
In 88 patients (65.7%), MRI results for staging were available, 31 of which (35.2%) had compromised MRF. Free CRM were achieved in 77.4% (Table 3).
Availability of MRI, MRF Stage at Diagnosis and CRM Result.
| No. | MRF stage | CRM− | CRM+ | |
|---|---|---|---|---|
| With MRI for staging | 31 | +(35.2%) | 24 (77.4%) | 7 (22.6%) |
| 42 | −(47.7%) | 41 (97.6%) | 1 (2.4%) | |
| 15 | Unknown | 14 (93.3%) | 1 (6.7%) | |
| Without MRI for staging | 46 | Unknown | 40 (87%) | 6 (13%) |
| Total | 134 | |||
MRF, mesorectal fascia; CRM, circumferential resection margin; MRI, magnetic resonance.
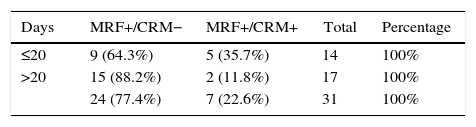
The mean time until intervention in patients with MRF+ and R0 was 27 days (95% underwent surgery 19–35 days later). In those with MRF+ and R1 surgery, the mean was 16 days (95% operated on between days 10–21), and these differences were statistically significant (P=.016).
The 20-day cut-off point had the most significant probability for achieving free CRM in MRF+ patients. 64.3% of the patients who had undergone surgery ≤20 days achieved free CRM, a percentage that reached 88.2% in the group with intervals longer than 20 days.
The “force of association” between the variables “CRM state” and “number of days” obtained an RR of 3.036, 95% CI=0.691–13.328, P=.06.
The cPR rate was 2.2% (3 cases), and so few events limited the statistical analysis. The reduction in tumor stage (yp stage 0-I) was reached in 38 patients (28%), and no significant differences were observed in terms of the number of days transpired until surgery (P=.519). The correlation between TRG and the number of days until surgery was negative (P=.852).
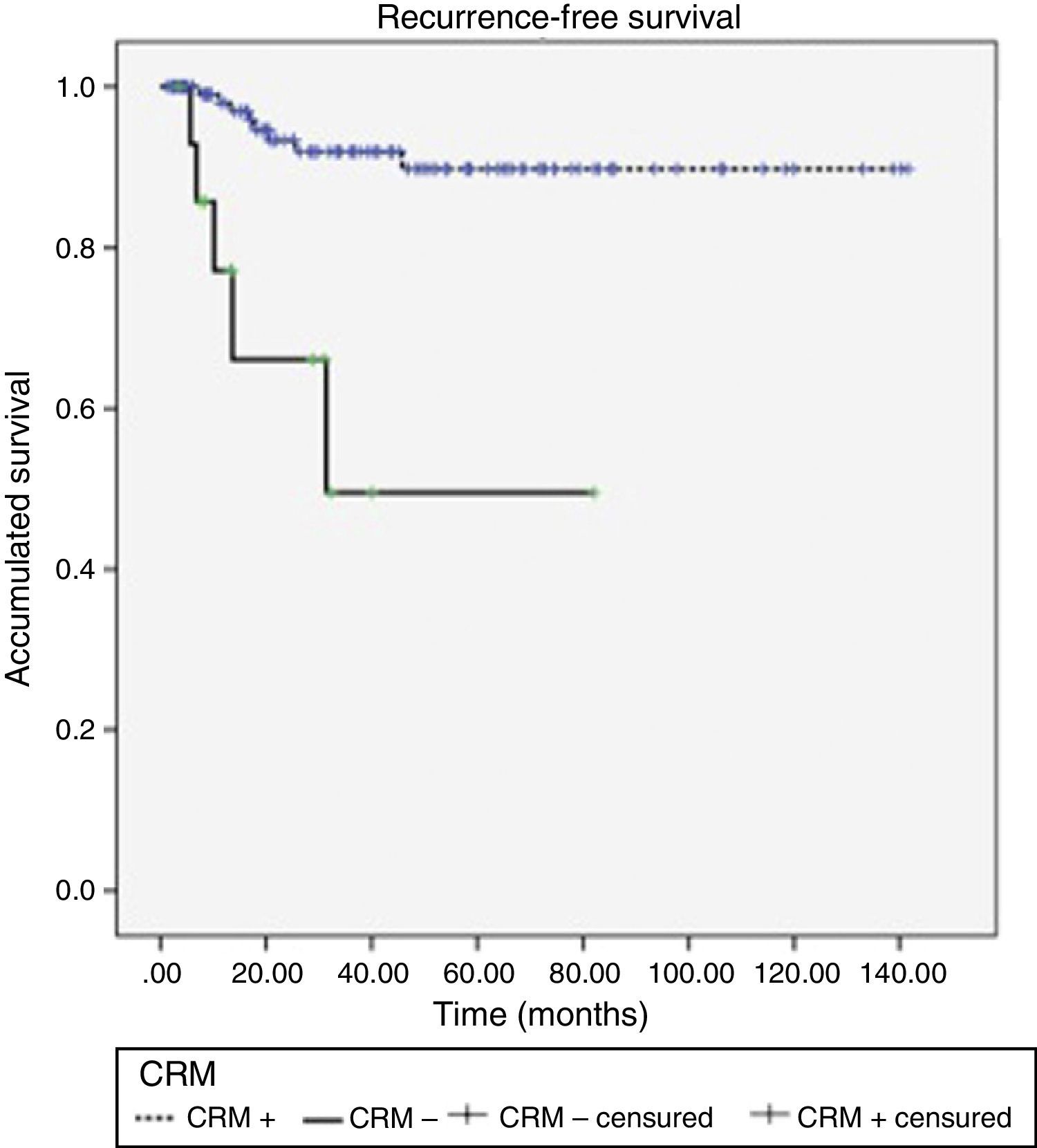
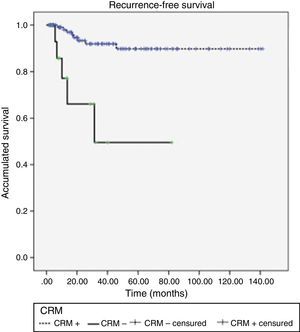
Acute rectal and urinary toxicity (G2 and G3) were detected in 5.5%. With a mean follow-up of 32 months, 13 patients (9.7%) presented local recurrence, 5 of which (38.5%) had had CRM+. LDFS after 1, 2 and 3 years were 95.6, 90.4 and 87.8%, respectively, presenting differences according to CRM stage (P<.0001) (Fig. 1).
DiscussionOur results confirm the overall association between tumor response and time transpired before surgery. However, if the response is quantified as negativization of the CRM in patients with MRF+, there is only an observed tendency to achieve R0 resections if the surgery is deferred, with maximum results in our study for a period equal or greater to 20 days (P=.06). It is likely that the low number of cases has limited the statistical power (contingency table [Table 4]).
Preoperative long-course CRTx is considered the standard treatment for marginally resectable tumors, but a therapeutic alternative is required for patients with poor PS and/or comorbidities.
The general belief that no tumor response is observed with this regimen is due to the short time interval for surgery (1–7 days) stipulated in the initial design.14 Preliminary data from a randomized trial12 show that, by delaying surgery after SCRT, a significant reduction in tumor size is achieved.
In our study, the mean interval before surgery in patients with MRF+ in whom R0 was possible was 27 days, which presents significant differences with the mean delay of 16 days of those patients with R1 resection.
The main series12,15–18 that analyze the response to SCRT according to time transpired before surgery quantify the response by cPR rates, reduction in stage (yp stage 0-I) or TRG. They initiated their recruitment before the findings of the Mercury Study11 introduced the requirement of an MRI study to plan the therapeutic treatment, and they do not analyze the response according to the initial MRF involvement. In fact, in our series, which initiated recruitment in 1998, 34% of the patients did not have an MRI available.
The state of the MRF on MRI predicts the involvement of the CRM,11 and this is a known predictor for local control and survival.3,10
MRI has equally demonstrated its usefulness for assessing CRM state19 after preoperative radiotherapy. It is especially recommended in cases of MRF+ for evaluating the response to preoperative treatment and not subjecting patients to a potentially suboptimal surgery. The CRM CR07 trial3 has already shown evidence that a CRM+ is a predictor for relapse and survival, and that adding postoperative therapy does not reduce this risk.
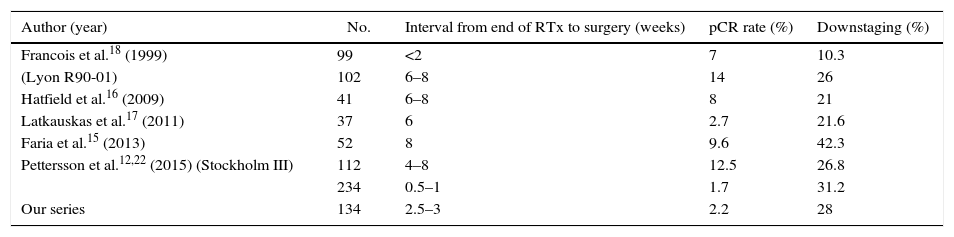
Currently, the experimental branch of the RAPIDO trial,20 which intensifies preoperative therapy, includes SCRT followed by 6 full cycles of chemotherapy (capecitabine-oxaliplatin). These regimens will be an alternative for metastatic patients with MRF+ that is considered potentially resectable,21 but regimens of that intensity will continue to be advised against for patients with low PS or comorbidities. Prolonging the time to surgery after SCRT is a therapeutic alternative for this subgroup. Table 5 summarizes the SCRT-to-surgery interval in the main series detected, the downstaging and the cPR rate obtained. Comparatively, our cPR rate (2.2%) is one of the lowest, but it is also that which least prolonged time before surgery. Two randomized trials have demonstrated the link between cPR and the interval before surgery. Stockholm III12,22 obtains 1.7% with immediate surgery (1–7 days) and 12.5% if the interval is prolonged to 28–56 days (4–8 weeks) P<.001. The Lyon R90-0118 reports a downstaging of 10% if the interval is 2 weeks, which ascends to 26% if it is prolonged to 6–8 weeks. The highest rates of cPR have been described with intervals of 4–8 weeks (Table 5). Therefore, the analysis of the literature makes us reconsider the classic short interval of the initial design with the 5×5 regimen and recommends longer periods until surgery, similar to what is established in the CRTx regimen.
Selection of Series With Short-Course RTx.
| Author (year) | No. | Interval from end of RTx to surgery (weeks) | pCR rate (%) | Downstaging (%) |
|---|---|---|---|---|
| Francois et al.18 (1999) | 99 | <2 | 7 | 10.3 |
| (Lyon R90-01) | 102 | 6–8 | 14 | 26 |
| Hatfield et al.16 (2009) | 41 | 6–8 | 8 | 21 |
| Latkauskas et al.17 (2011) | 37 | 6 | 2.7 | 21.6 |
| Faria et al.15 (2013) | 52 | 8 | 9.6 | 42.3 |
| Pettersson et al.12,22 (2015) (Stockholm III) | 112 | 4–8 | 12.5 | 26.8 |
| 234 | 0.5–1 | 1.7 | 31.2 | |
| Our series | 134 | 2.5–3 | 2.2 | 28 |
pCR, complete pathological response; RTx, radiotherapy.
The optimal time interval between preoperative radiotherapy and surgery is still an unresolved question. The tumor regression process requires time, and this has been quantified by Dhadda et al.,23 who demonstrated that, in addition to time, initial tumor size also plays a role (on average, a tumor requires 14 days to reduce its size by half).
The prolongation of the time interval before surgery should be balanced with the probability for tumor repopulation and the appearance of fibrotic phenomena. Some articles indicate that delaying surgery for 8–11 weeks after the end of radiotherapy does not increase surgical morbidity or mortality, although it does prolong the duration of the procedure.24,25
This study presents the limitations of a retrospective analysis, with heterogeneity in the time interval before surgery and where 38% of the patients did not have an MRI prior to treatment. Our findings enable us to conclude that patients with compromised MRF, if treated with SCRT, require a minimum interval before surgery of 20 days to increase the probabilities of achieving free CRM.
Authors’ ContributionAll the authors have read and approve of the manuscript and meet the requirements for authorship.
Likewise, all the authors endorse the authenticity of the data as well as the proper methods used to complete this study.
Amalia Palacios Eito: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Sonia García Cabezas: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Milagrosa Rodríguez Liñán: data collection, critical review and approval of the final version.
Ana M. Otero Romero: data collection, critical review and approval of the final version.
Carmen M. Bueno Serrano: data collection, critical review and approval of the final version.
José Gómez Barbadillo: data collection, critical review and approval of the final version.
Amalia Palacios Eito and Sonia García Cabezas have equally participated in the production of the manuscript.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: García-Cabezas S, Rodríguez-Liñán M, Otero-Romero AM, Bueno-Serrano CM, Gómez-Barbadillo J, Palacios-Eito A. Respuesta al tratamiento e intervalo de tiempo hasta la cirugía con radioterapia preoperatoria de curso corto en el cáncer de recto. Cir Esp. 2016;94:460–466.
This manuscript has not been previously presented, either partially or in its entirety, at any medical conference