To challenge the risk factors described in Tokyo Guidelines in Acute Calculous Cholecystitis.
MethodsRetrospective single center cohort study with 963 patients with Acute Cholecystitis during a period of 5 years. Some 725 patients with a “pure” Acute Calculous Cholecystitis were selected. The analysis included 166 variables encompassing all risk factors described in Tokyo Guidelines. The Propensity Score Matching method selected two subgroups of patients with equal comorbidities, to compare the severe complications rate according to the initial treatment (Surgical vs Non-Surgical). We analyzed the Failure-to-rescue as a quality indicator in the treatment of Acute Calculous Cholecystitis.
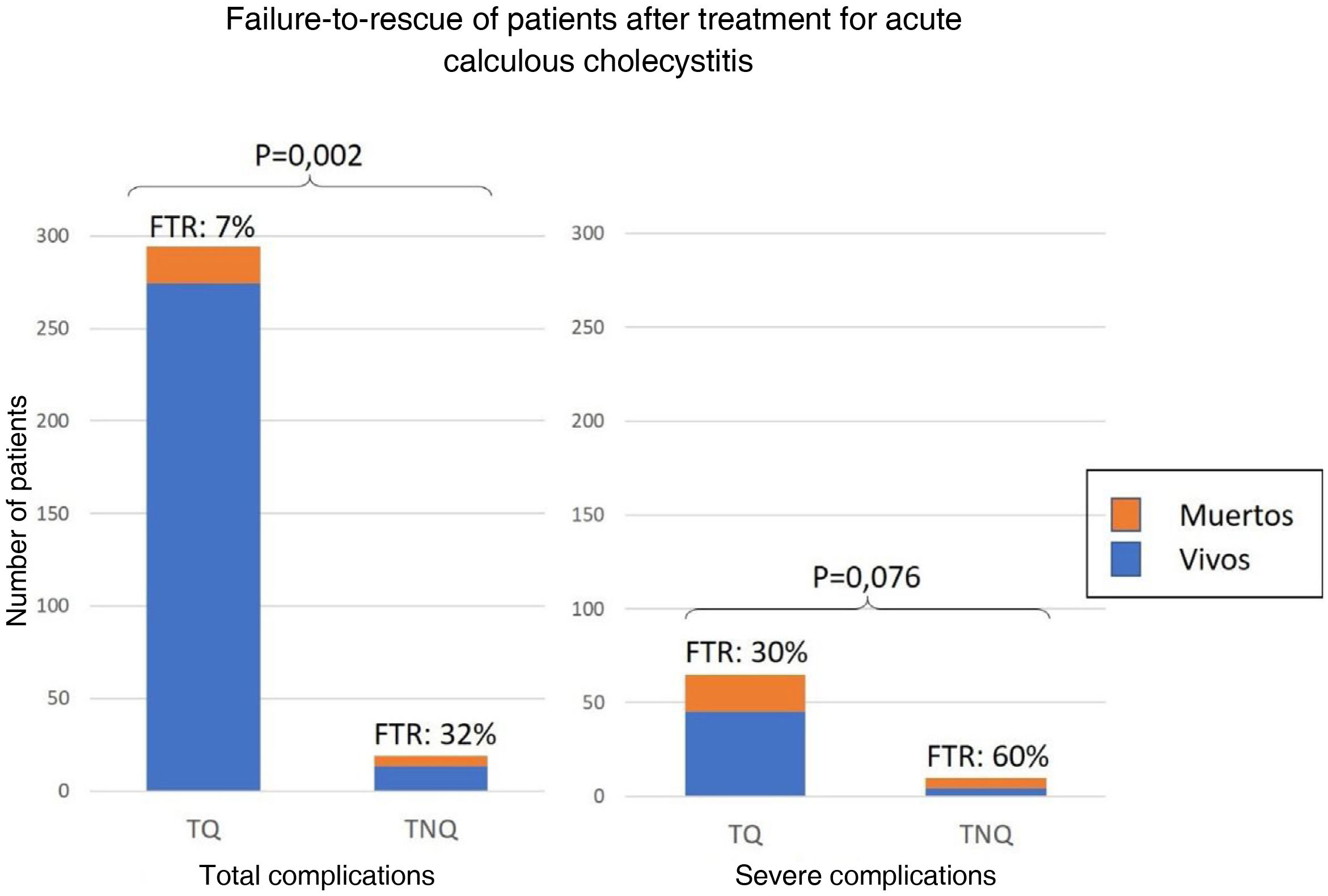
Resultsthe median age was 69 years (IQR 53-80). 85.1% of the patients were ASA II or III. The grade of the Acute Calculous Cholecystitis was mild in a 21%, moderate in 39% and severe in 40% of the patients. Cholecystectomy was performed in 95% of the patients. The overall complications rate was 43% and the mortality was 3.6%. The Logistic Regression model isolated 3 risk factor for severe complication: ASA > II, cancer without metastases and moderate to severe renal disease. The Failure-to-Rescue (8%) was higher in patients with non-surgical treatment (32% vs. 7%; P = 0.002). After Propensity Score Matching, the number of severe complications was similar between Surgical and Non-Surgical treatment groups (48.5% vs 62.5%; P = 0.21).
Conclusionsthe recommended treatment for Acute Calculous Cholecystitis is the Laparoscopic Cholecystectomy. Only three risk factors from the Tokyo Guidelines list appeared as independent predictors of severe complications. The failure-to-rescue is higher in non-surgically treated patients.
Analizar los factores de riesgo de complicaciones para Colecistitis Aguda Litiásica confrontándolos a las Tokyo Guidelines.
MétodoEstudio retrospectivo de 963 pacientes con Colecistitis Aguda durante 5 años. Se seleccionaron 725 pacientes con Colecistitis Aguda Litiásica “pura”, y analizaron 166 variables mediante regresión logística, incluyendo todos los factores de riesgo de las Tokyo Guidelines. Mediante el Propensity Score Matching, se seleccionaron subpoblaciones comparables de 75 pacientes y se analizaron las complicaciones según el tratamiento realizado (Quirúrgico/No-Quirúrgico) y se utilizó el fallo en el rescate como indicador de calidad del tratamiento en la Colecistitis Aguda Litiásica.
ResultadosLa mediana de edad fue de 69 años (RIQ 53-80). La mayoría de los pacientes fueron ASA II ó III (85.1%). El 21% de las colecistitis fueron leves, el 39% moderadas y el 40% graves. Se colecistectomizó al 95% de los pacientes. El 43% de los pacientes se complicaron y la mortalidad fue del 3,6%. Los factores de riesgo independientes para complicaciones graves fueron ASA > II, tumor sólido sin metástasis e insuficiencia renal. El fallo en el rescate (8%) fue mayor en los no operados (32% vs. 7%; P = 0,002). Tras realizar el Propensity Score Matching, la tasa de complicaciones graves fueron comparables entre operados y no operados (48,5% vs 62,5%; P = 0,21).
ConclusionesLa colecistectomía precoz es el tratamiento preferente para la Colecistitis Aguda Litiásica. Sólo tres de los factores de las Tokyo Guidelines son variables independientes para predecir complicaciones graves. El fallo en el rescate es mayor en los pacientes no intervenidos quirúrgicamente.
Early laparoscopic cholecystectomy is the treatment of choice for acute calculous cholecystitis (ACC). Patients at high surgical risk have high complication (8%-20%)1–4 and mortality (.6%-6%) rates.4–7 This morbidity and mortality, especially in the most severe and/or fragile patients, has prompted several proposals for non-surgical treatment (NST). The key lies in answering the question: Who are the patients at the highest surgical risk?
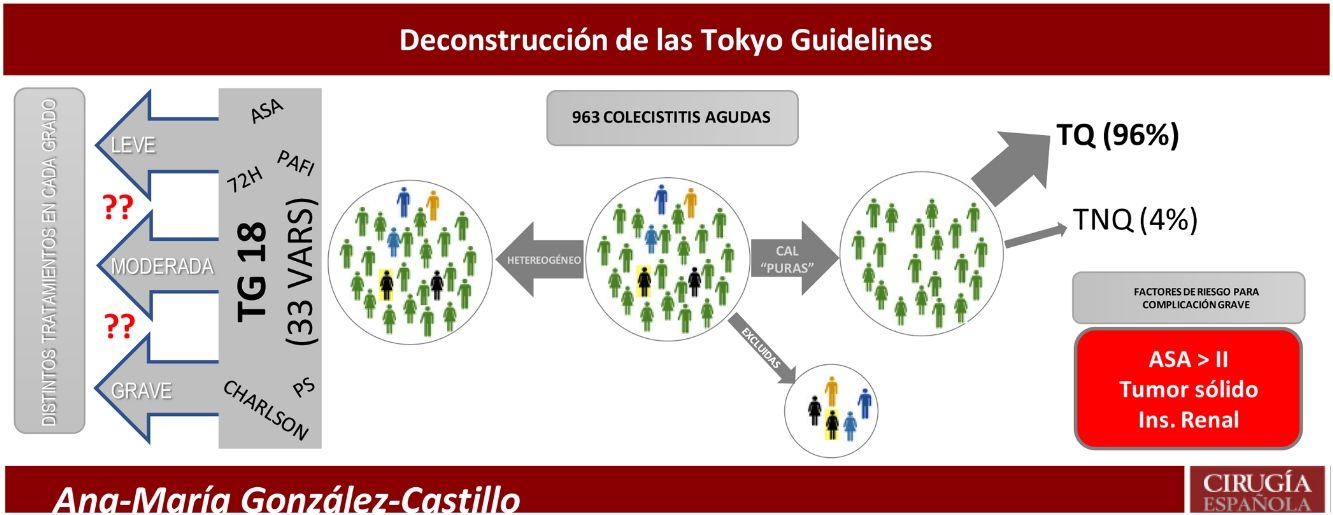
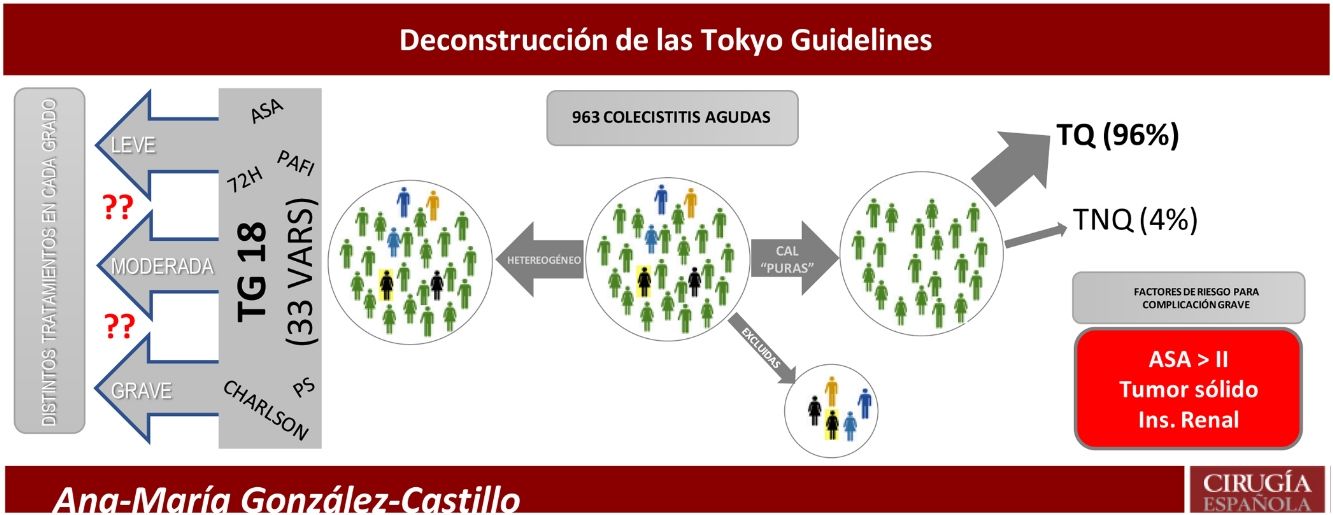
Despite the plethora of recent publications on ACC, there remains controversy about the best therapeutic strategy for the most severe patients. This is due to small populations, diagnostic heterogeneity, inappropriate methodology, and varying definitions of high surgical risk. To identify the severe patient, several general risk scales have been extrapolated and applied to ACC, such as APACHE,8 ASA,9,10 P-POSSUM,10,11 Charlson Comorbidity Index (CCI),12 AAST,13 Clinical Frailty Scale,14 and multiorgan dysfunction.15 Successive versions of the Tokyo Guidelines7 combine several of these scales to classify patients into three groups. This strategy compromises accuracy and leads to confusion in identifying patients who should not undergo early cholecystectomy.
The aim of the present study was to analyse in detail all the risk factors described in the TG18 to identify a reduced set of specific prognostic factors for complications in ACC.
Patients and methodsA retrospective clinical study, from January 2011 to December 2016, in a university hospital with a specialist surgical emergency unit. Study candidates were 963 consecutive patients diagnosed with acute cholecystitis.
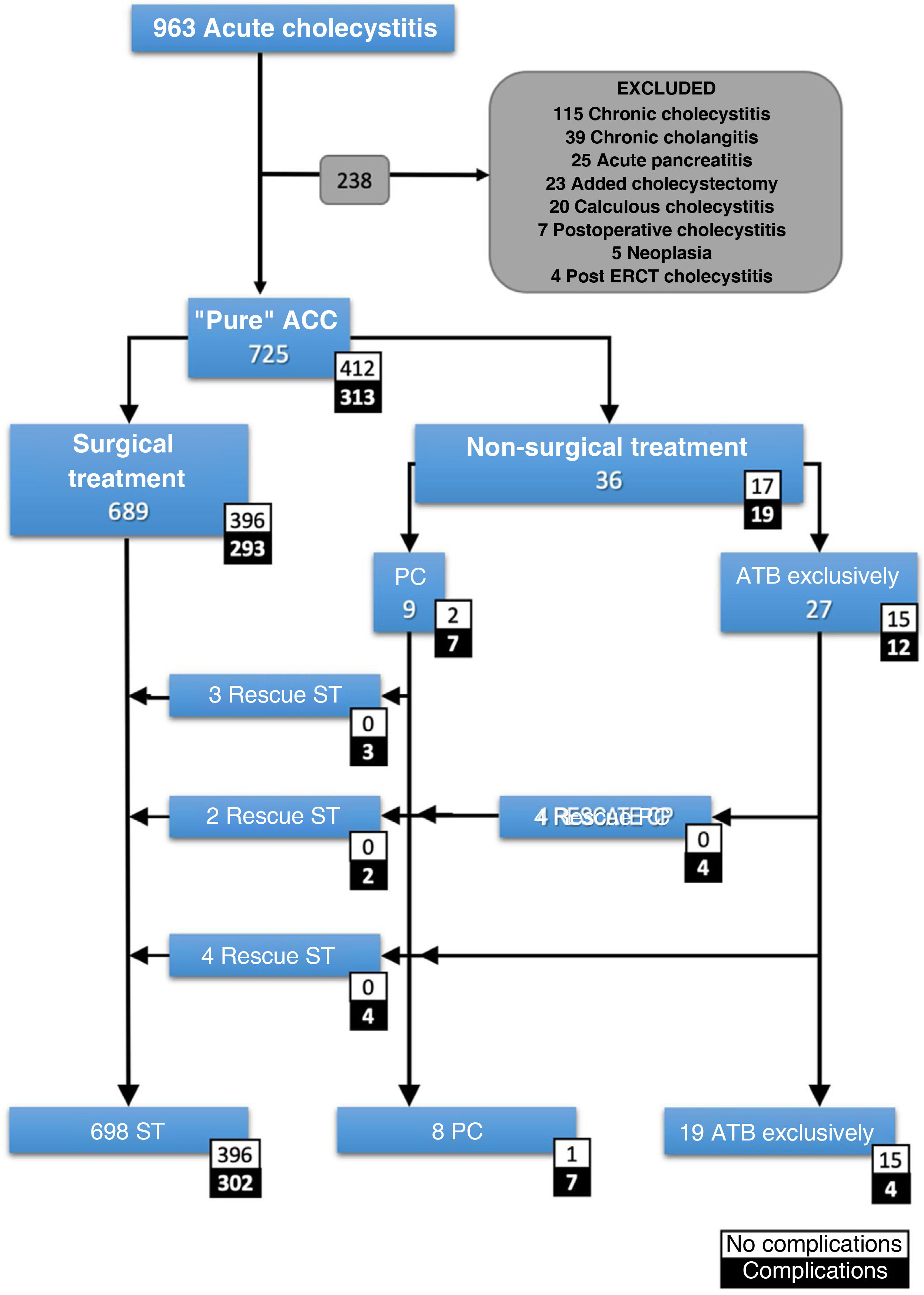
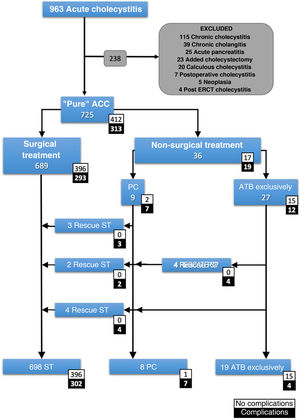
Inclusion and exclusion criteriaAll patients with ACC with a clinical diagnosis according to the Tokyo Guidelines 2018 (TG18), or with a diagnosis of ACC on histopathological report were included. The case definition was a patient with "pure acute cholecystitis". Patients with any other concomitant diagnosis that could influence prognosis were excluded (Fig. 1).
Patient count.
ACC: acute calculous cholecystitis; Added cholecystectomy: cholecystectomy performed during a different emergency surgical procedure; ATB: antibiotic; ERCP: endoscopic retrograde cholangiopancreatography; PC: percutaneous cholecystostomy; Postoperative cholecystitis: cholecystitis diagnosed in the postoperative period of a different surgical procedure; ST: surgical treatment.
The prospectively maintained database in FileMaker v.12 (Mountainview, CA, USA) was used including demographics, type of intervention, hospital stay, and complications. The registry was retrospectively completed with laboratory data, microbiology, antibiotic therapy, severity according to TG18 (Table 1), CCI and surgical risk according to ASA. Initial treatment was surgical (ST: cholecystectomy by laparoscopy or laparotomy) or non-surgical (NST: percutaneous cholecystostomy, PC, or exclusively antibiotics). The primary endpoint was the rate of serious complications (Clavien-Dindo16 > IIIa). Mortality at 30 days and total complication rate were the secondary study variables. Follow-up was 30 days in case of hospital discharge in under that period. Otherwise, follow-up was until hospital discharge regardless of length of stay.
Differences between patients with severe complications vs. no complications in pre-treatment and post-treatment variables.
| Variable | Odds ratiob | 95% CI | p |
|---|---|---|---|
| Sex (M vs. F) | 1.08 | .67-1.75 | .411 |
| Classification of severity according to TG18d | |||
| II (Moderate) | 2.75 | .92-8.19 | .043 |
| III (Severe) | 7.67 | 2.72-21.67 | .001 |
| ASA scorec | |||
| II | 1.03 | 1.01-1.04 | .150 |
| III | 1.26 | 1.18-1.34 | .001 |
| IV | 1,62 | 1.24-2.11 | .001 |
| TG18 P/F ratio <300 | .27 | .55-1.38 | .091 |
| TG18 Oliguria (diuresis <.5 mL/kg/h) | 12.34 | 6.39-23.81 | .001 |
| TG18 Local inflammation | 2.25 | 1.2-4.19 | .012 |
| TG18 Elevated white blood count>18000/mm3 | 2.13 | 1.2-3.6 | .005 |
| TG18 INR> 1.5 | 3.2 | 1.8-5.5 | .001 |
| TG18 Renal failure (creatinine>2 mg) | 8.7 | 5.1-14.99 | .001 |
| TG18 Obtundation | 1.56 | .9-2.71 | .077 |
| TG18 Cardiovascular dysfunction (amines) | 14.02 | 6.49-30.3 | .001 |
| TG18 Murphy’s sign | .65 | .40-1.05 | .052 |
| TG18 Palpable RUQ mass | 1.28 | .75-2.18 | .225 |
| TG18 Tachypnoea (> 20 bpm) | 3.78 | 2.06-6.94 | .001 |
| TG18 Duration of symptoms> 72h | 1.24 | .76-2.03 | .227 |
| Systemic inflammatory response syndrome | 2.47 | 1.5-4.06 | .001 |
| Severe complicationsa | p | ||
|---|---|---|---|
| Yes | No | ||
| N = 75 (10.3%) | N = 650 (89.7%) | ||
| Charlson Comorbidity Index | 2 (3) | 0 (2) | .001 |
| Age (years) | 81 (10) | 67 (28) | .001 |
| Bilirubin (mg/dL) | 1.35 (1.69) | .8 (1.02) | .002 |
| Creatinine (gr/dL) | 1.36 (1.11) | .78 (.38) | .001 |
| Alkaline phosphatase (IU/L) | 120 (153) | 91 (70) | .001 |
| Gamma-glutamyl-transferase IU/L) | 129 (124) | 117 (298) | .317 |
| Glutamyl oxaloacetic transaminase (IU/L) | 39 (132) | 26 (39) | .005 |
| INR | 1.4 (.36) | 1.17 (.26) | .001 |
| Lactate (g/dL) | 2 (2.45) | 1.4 (1) | .008 |
| Temperature (°C) | 36.7 (1.4) | 36.2 (1.1) | .289 |
| Elevated white blood count > 18000/mm3 | 14.23 (8.6) | 13.7 (6.6) | .016 |
| Partial oxygen pressure (mmHg) | 99 (5) | 99 (1) | .010 |
| CRP (g/dL) | 23.35 (20.7) | 13.6 (26) | .110 |
| Platelets (1000/mm3) | 164 (152) | 212 (126) | .210 |
| Treatment | ||||
|---|---|---|---|---|
| N (%) | OR | 95% CI | P | |
| Cholecystectomy | 689 (95) | .27 | .12-.58 | .002 |
| Rescue cholecystectomy | 9 (1.2) | 12 | 3.14-45.8 | .001 |
| Laparotomy | 75 (10) | 12.44 | 7.08-21.87 | .001 |
| Laparoscopy conversion | 93 (13) | 2.33 | 1.28-4.23 | .006 |
| Additional procedures | 164 (23) | 2.41 | 1.44-4.03 | .001 |
| Cholecystostomy | 9 (1.2) | 1.4 | .28-7.3 | .486 |
| Rescue cholecystostomy | 4 (0.5) | 3 | .3-24.91 | .305 |
| Antibiotic therapy only | 27 (3.7) | .23 | .04-1.31 | .087 |
| Post-treatment | |||
|---|---|---|---|
| Severe complications | P | ||
| Yes | No | ||
| N = 75 (10.3%) | N = 412 (89.7%) | ||
| Hospital stay (days). | 17 (18) | 3 (3) | .001 |
bpm: breaths per minute; CRP: C-reactive protein; P/F ratio: Pa02/Fi02; PT-INR: Prothrombin Time International Normalized Ratio.
ASA score 1 was used as control group to calculate the odds ratio for the remaining groups; ASA score V was not included in the analysis because there was only 1 patient in this group.
2018 Tokyo Guidelines classification43 TG I was use as a control group to calculate the odds ratio of the remaining groups.
All patients received intravenous antibiotic therapy according to local protocol. PC was performed under ultrasound guidance, inserting an 8 Fr. catheter (SKATER™, Argon Medical Devices, Rochester, NY, USA) via transhepatic or transperitoneal route, at the radiologist's discretion.
All patients were initially proposed surgical treatment. Non-surgical initial treatment was only applied if ST was formally contraindicated by anaesthesiology and resuscitation, or if the patient or their legal representative refused surgical intervention. The indication for percutaneous cholecystostomy was at the discretion of the attending surgeon, often supported by an in-depth discussion in the daily clinical session.
Laparoscopic cholecystectomy was performed according to the French technique. The indication for open approach, by right subcostal laparotomy, was restricted to patients with haemodynamic instability and under treatment with vasoactive amines at the time of surgery. Culture of vesicular bile and peritoneal exudate was obtained in 67% of cases.
Statistical analysisThe recommendations of Strengthening the Reporting of Observational studies in Epidemiology (STROBE) were followed.17 The Kolmogorov-Smirnov test was used to assess the normality of the distribution of the quantitative variables: none of the variables were normally distributed and therefore their values are expressed as median and interquartile range (IQR).25–75 To evaluate the significance of the differences between the means of the variables between groups, the nonparametric U-Man-Whitney tests were used for two groups, and the Kruskal-Wallis test for three groups. The association between qualitative variables for comparisons between groups was assessed with the χ2 test or Fisher’s exact test, as indicated. The increased risk of an event associated with a variable was expressed by its odds ratio (OR) and 95% confidence interval.
A predictive model of serious complications was created using the multivariate technique of binary logistic regression, with stepwise progressive conditional entry with an F-to-enter of .5, without altering the baseline admission conditions; variables with non-significant differences in the univariate analysis were rejected. The different productive methods were compared by means of their receiver-operating characteristic (ROC) curves.
Failure-to-Rescue (FTR)18,19 was evaluated as an indicator of the overall quality of care for patients with ACC, as described in 1992 by Silber et al.20 This is the proportion of mortality in patients with complications. To offset the asymmetry between treatment rates, propensity score matching21 was used to match two groups of similar severity according to the risk variables identified in the logistic regression.
Ethical and legal aspectsThis study has been registered in Clinical Trials (NCT05135299).
The study protocol was approved by the CEIC of the Hospital del Mar and classified as a non-clinical study, in accordance with the provisions of Organic Law 15/1999, of December 13, 1999, on the Protection of Personal Data (BOE 14/12/1999, no. 248). All data collected in the clinical database have been completely anonymized and all files are stored encrypted.
ResultsA total of 725 patients with pure ACC were selected (Fig. 1). The median age was 69 years (IQR: 53-80) and 26% were older than 80 years. Most patients were classified as ASA II (52.3%) or ASA III (32.8%). Of these, 689 underwent surgery and 36 (5%) opted for non-surgical treatment. The median number of days of symptoms before admission was 3 days (IQR: 2-5). In the NST group, 27 (75%) were treated exclusively with antibiotics and 9 (25%) were indicated for PC. The unfavourable course of some patients led to 4 further PCs, making a total of 13 PCs, and 9 patients required ST. Finally, 698 (96.2%) cholecystectomies were performed.
According to the TG18 classification, 21% were mild, 39% were moderate, and 40% were severe. The surgical approach to ACC was via laparoscopy in 89% (conversion rate of 13%).
ComplicationsComplications occurred in 43% of the patients. Complications in the entire sample were classified according to Clavien-Dindo as mild in 238 patients (76%) (grade I to IIIa) and severe in 75 (24%).
Patients with complications were 13 years older and had double CCI score. Patients with higher ASA scores suffered more complications (Table 1), whereas the percentages of complications in the mild and moderate ACC groups were similar (32% vs. 36%). Patients with severe ACC (TG18) suffered a significantly higher percentage of complications compared with mild and moderate (55.5% vs. 21%; p = .001). In the intention-to-treat analysis, patients undergoing ST or NST had a similar number of complications (53% vs. 42%; p = .3).
Those undergoing open cholecystectomy (11%) had 47% more complications than those undergoing the laparoscopic route (85% vs. 38%; p = .001), and converted laparoscopic cholecystectomy had 33% more complications than unconverted (72 vs. 39%; p = .001).
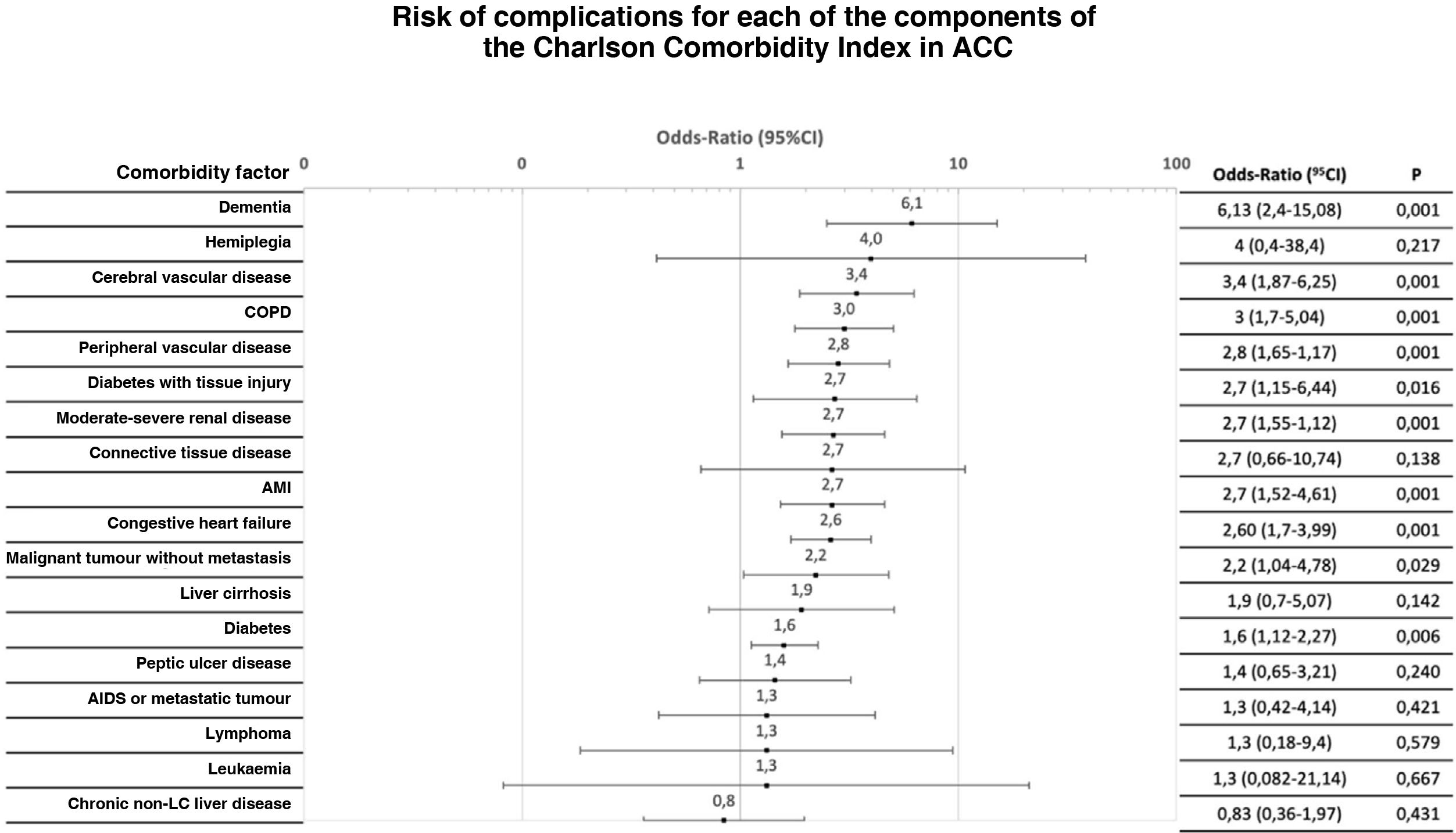
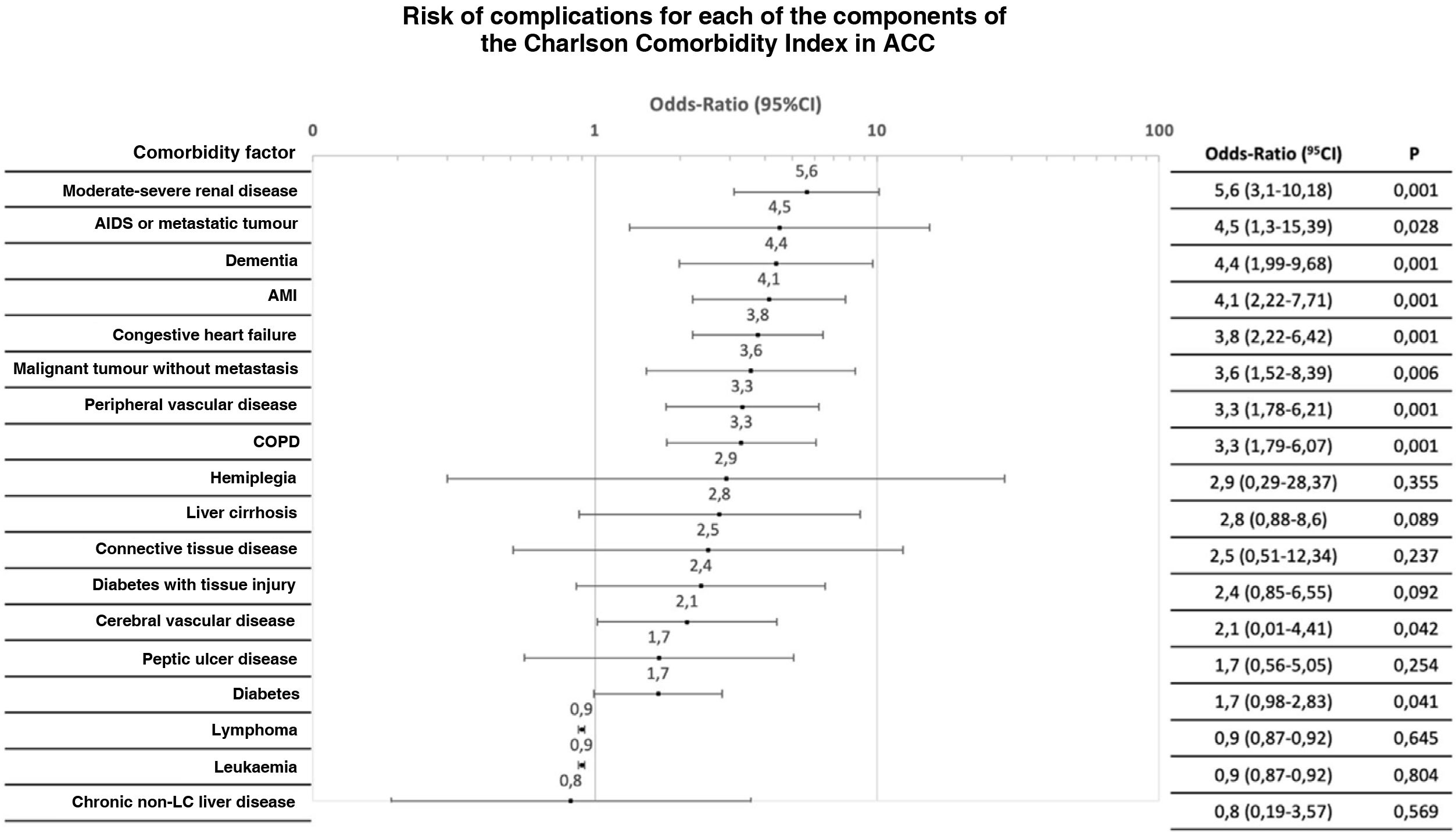
CCI constituent factors were analysed individually for any type of complication. The OR for complications varied up to an order of magnitude for different antecedents (Figs. 2 and 3).
Distribution of the risk of severe complication (Clavien-Dindo > IIIa) after initial treatment for each of the components of the Charlson Comorbidity Index.
ACC: Acute calculous cholecystitis; AMI: Acute myocardial infarction; CI: Confidence Interval; COPD: Chronic obstructive pulmonary disease; LC: Liver cirrhosis.
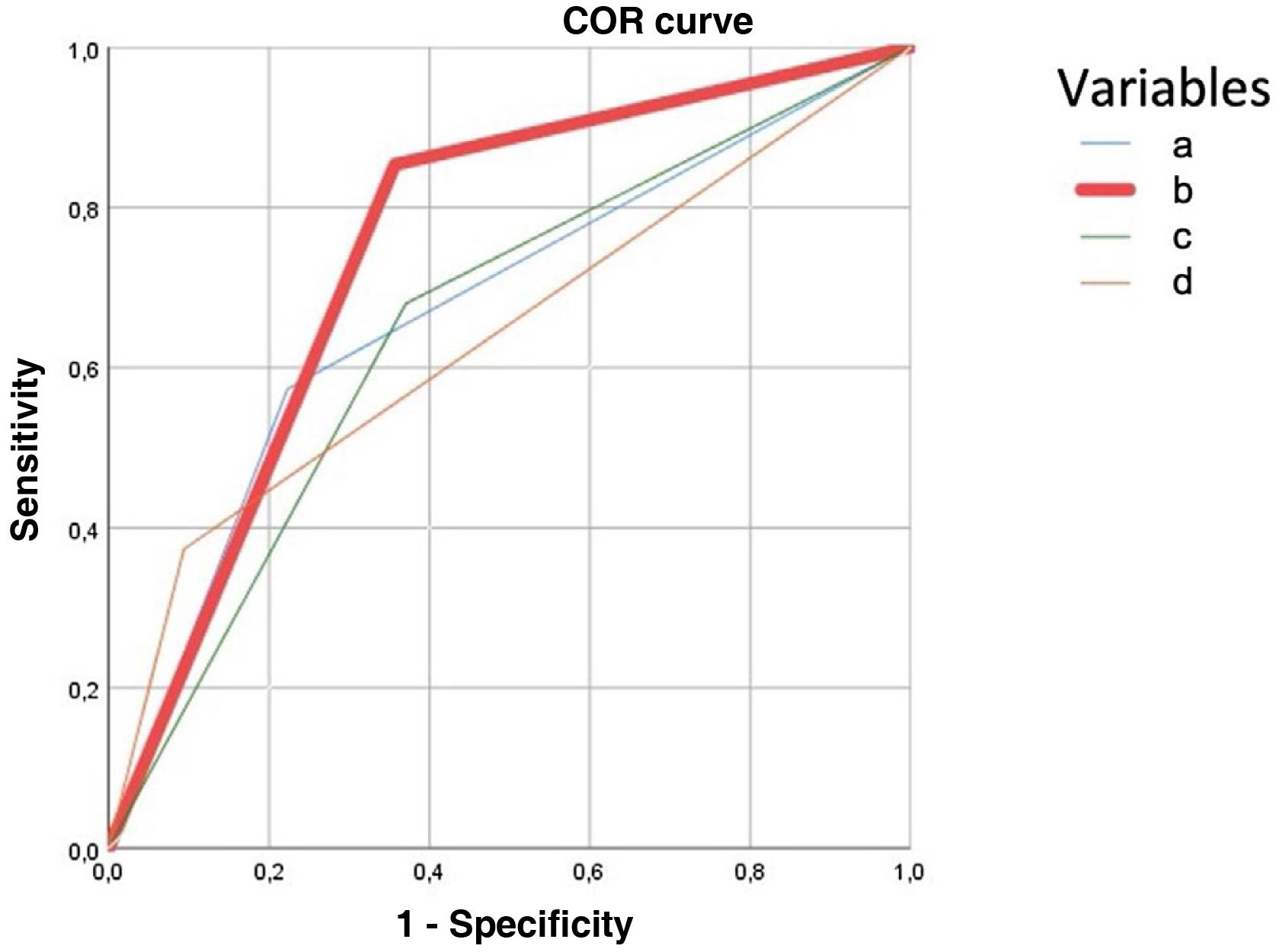
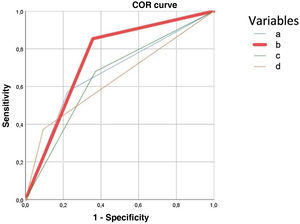
The predictive model for major complications (Clavien-Dindo ≥ IIIb) retained ASA III-IV, diagnosis of solid tumour without metastasis, and renal failure to predict 90.1% of complications. The presence of any of the three severe complication variables obtained a higher area under the ROC curve (75%) than age> 80 years (67%), CCI > 5 (66%), and severe ACC (65%) according to TG18, illustrated in Fig. 4.
ROC curve to predict severe complications after treatment of acute calculous cholecystitis. It is constructed with the variables obtained in multivariate analysis, for the variable age 80 years or over, Charlson Comorbidity Index (CCI) score greater than 5 and severe acute calculous cholecystitis (ACC). Each of the curves corresponds to:
Age 80 years or over: AUC .675 (95CI: .60-.74).
Predictive model curve: presence of any of the variables that are predictors of severe complications in ACC (ASA > 2; any current malignant tumour; moderate-severe renal failure) AUC: .75 (95CI: .7-.8).
TG18 severe ACC curve: patients classified with severe ACC according to TG18, AUC .65 (95CI: .56-.71).
CCI curve> 5: AUC .66 (95CI: .56-.71).
For any type of complication, the predictive model retained ASA III-IV, dementia, COPD, chronic renal failure, and INR > 1.5 as independent variables for any type of complication after indicating initial treatment, and thus correctly predicted 69.3% of patients with complications.
Using the risk factors identified in the multivariate analysis (ASA > 2, any malignant tumour, and renal failure), according to propensity score matching, two groups of 75 patients with similar severity (75 severe complications and 75 without severe complications) were selected in which ST (134) had a similar rate of severe complications as NST (16) (48.5% vs. 62.5%; p = .214). Mortality in ST was 15% vs. 21% in NST p = .5.
MortalityMortality in the series was 3.6%. Patients who died within 30 days of treatment were 20 years older and had higher ASA and CCI scores. Mortality in severe ACC was 9 times higher than in mild ACC. NST resulted in 6 times higher mortality than ST. No significant differences were found between patients treated exclusively with antibiotics and those indicated for PC (15% vs. 22%; p = .62). Open cholecystectomy had a mortality of 20% vs. 1% for laparoscopic cholecystectomy (p = .001). In patients who were surgically rescued due to a torpid course of ACC with NST, mortality was 11%.
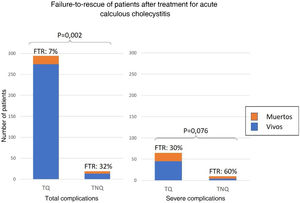
Failure to rescueOf the 313 patients with complications, the FTR was 8% and was significantly higher in those with NST (32% vs. 7%; p = .002). Of the 75 with severe complications, FTR after NST was twice as high as after ST (60% vs. 30% p = .076) (Fig. 5).
DiscussionIn this retrospective review of acute cholecystitis treated mostly with laparoscopic cholecystectomy without adhering to TG18 recommendations, we identified that not all risk factors collected under the TG18 have the same weight in predicting complications.
Strict patient selection is a distinctive feature of this study. A total of 238 patients were excluded, obtaining a homogeneous cohort with "pure" ACC, thus avoiding the distortion of other concomitant diagnoses of the prognosis.22,23 Some articles, source articles of the TG18, analyse heterogeneous cohorts mixing different aetiologies of acute cholecystitis1,6,22,23 or only exclude chronic cholecystitis24 creating statistical noise for the analysis. Some articles attempt a fine selection of patients,25 but this essential and precise selection has not yet been published.
This study is the result of a meticulous registry in an Emergency Unit where the NST, recommended by TG18, has hardly been used. NST was only used in 5% of patients, although 40% of patients had severe ACC, other expert groups in ACC also adopted this approach.3,13,26–28
The rate of severe ACC in our cohort was higher (30%) than in other series (5%-19%),1,28–30 probably due to the higher proportion of respiratory and neurological dysfunction and INR ≥ 1.5 in our patients.
The percentage of complications (43%) was higher than in multicentre studies.1,8,11 The rates of serious complications in this series were 24% for NST and 7% for ST, higher than those reported in the literature. Three factors should probably be considered to explain this high complication rate. First, by discarding patients cholecystectomized in the emergency operating room, but without "pure" acute cholecystitis, we reduce the denominator of complications and increase their rate. It also reflects the high percentage of severe ACC in our reference population, as attested by the high CCI score of the patients in this study. Finally, it is plausible that the effect of honest and thorough prospective recording of complications in the regulated audits performed in our department increases the number of complications, especially non-severe complications, in this series. The age of the patients who suffered complications was higher than that of the uncomplicated cases. It is likely that the application of frailty scales, together with the preoperative optimization of some patients, will drastically reduce this percentage. Nevertheless, it has recently been reported that early recurrence in elderly patients who did not undergo surgery for the initial episode is higher and more severe than the initial episode.31
Complications in the mild and moderate ACC groups (32% and 36%) were similar and significantly lower than in severe ACC (55%). Current clinical algorithms32 could probably benefit from simplifying the classification of ACC into 2 groups: mild and severe.
PSM has been used occasionally to compare similar groups in ACC studies.33,34 In this series, the difference in complication rate between NST and ST of similar severity was only 14%, probably reflecting that many of the complications are not directly related to surgical treatment, as is the case in other studies.35
ACC mortality in TG18 is <1%.7 In this study, mortality was substantially higher (3.6%) than in recent multicentre studies (.6%-13.5%).1,29,36,37 In our series, 5/26 deaths occurred more than 30 days after treatment; without them, mortality would have been 2.8%. If we were to include the diagnoses of "persistent hepatic colic" and "ACC after ERCP" as do many studies, we would report a misleadingly low mortality of 1.8% and yet similar to the international literature.4,38
It is fundamental to identify the high-risk patient in ACC algorithms. Some authors agree with the TG18 and consider any patient with organ dysfunction as high risk.6 ASA-III/IV patients are considered high risk39 and have high morbidity.1,40 However, in a prospective observational study, Gonzalez-Muñoz et al. found that the degree of cholecystitis and the P-POSSUM score were the only two predictors of mortality in ACC, unlike age or ASA or CCI scores.11
Although the TG18 do not exclude cholecystectomy as initial treatment in group III (severe) ACC, they restrict it to "advanced centres" (without defining advanced centre) and also to patients with "good performance status". The inclusion of this last condition, extrapolated from cancer patients, is surprising as it does not specify which of the numerous performance statuses they are referring to. In any case, more than 40% of the ACC in this series would be classified as high risk and probably would not have undergone early cholecystectomy. Nevertheless, we consider that an ASA>2 and severe ACC (TG18) do not always contraindicate ST.
In some studies, age appears as an independent risk factor,41 but by itself does not increase surgical risk. Low functional capacity and comorbidity constitute the greatest risk of morbidity in this and other studies.42 The application of frailty indices and a multidisciplinary approach could contribute to better clinical judgment.
We have different generic scales that predict the risk of complications (APACHE II>15,8 CCI>6,1 and P-POSSUM>4010,11), none of them specific to ACC and, therefore, none particularly accurate or better than another.
NST patients had a 6-fold higher mortality rate than patients treated with ST. The nine patients who had an unfavourable outcome with antibiotics and underwent surgery had high mortality (11%) and more than 50% of the complications. A multicentre observational study compared 4 strategies (intravenous antibiotic, cholecystostomy as a bridge to surgery, as definitive treatment, and early cholecystectomy). Their results support early cholecystectomy as superior to any other option.40 Surgical treatment is the first option for ACC, even in high-risk patients, as demonstrated by the CHOCOLATE trial, which compares early cholecystectomy vs. PC in patients with APACHE scores between 7 and 15 in unselected ACC.8
The complexity of the outcome of NST is shown in Fig. 1: there are patients who are only treated with antibiotics, others in whom PC is initially indicated, and others with an episodic course through each of the therapeutic options until they undergo surgery. In each non-randomized study, there are patients who are initially non-surgical (analysis by intention to treat) who migrate from one treatment to another during their course (analysis by protocol). This undoubtedly is helping maintain uncertainty about the best treatment for high-risk patients.
FTR has been proposed as a quality indicator in surgery.18,19 The high FTR of the NST group (32%) is probably due to the high frailty of the patients in whom the therapeutic effort was limited.
Limitations of the studyThe main limitation of the study is its retrospective and single-centre nature. Conversely, the low NST rate gives this cohort more validity in determining risk factors for postoperative complications, especially in severe ACC.1,8 However, the low NST rate may contribute to selection bias.
The patients in this series had evolved disease and thus had a high number of complications. Nevertheless, we believe that this characteristic provides more robustness to the analysis.
Patient follow-up was 30 days after diagnosis or surgery. It would be interesting to perform a follow-up at one year, as other complications would probably arise.11
The prevalence of some risk factors in our study, such as tachypnoea, P/F ratio<300, leukaemia, lymphoma, and HIV is low, and the results obtained may not be extrapolable to other populations.
ConclusionsEarly cholecystectomy is the recommended treatment for ACC. Classification of ACC would be more practical if we used only 2 groups: mild and severe. Of all the risk factors expressed in the TG18, those that carry the highest risk of severe complications in patients with ACC are ASA>II, diagnosis of solid tumour without metastasis, and renal failure. Patients who did not undergo surgical intervention and suffered any complication have greater mortality.
Conflict of interestsThe authors have no conflict of interests to declare.