Zollinger–Ellison syndrome (Z–E) is characterized by gastrin-secreting tumors, responsible for causing refractory and recurrent peptic ulcers in the gastrointestinal tract. The optimal approach and the extension of tumor resection remains the subject of debate.
MethodsDuring the period February 2005 and February 2014, 6 patients with Z–E underwent surgery, 4 men and 2 women with a median age 46.8 years (22–61). Two patients were affected with multiple endocrine neoplasia type-1 (MEN-1). Fasting gastrin levels greater than 200pg/ml (NV: <100) was diagnostic. Radiologic imaging to localize the lesion included octreoscan 6/6, computer tomography (CT) 6/6, and endoscopic ultrasonography (EUS) 1/6.
ResultsThe octreoscan was positive in 5 patients. The CT localized the tumor in the pancreas in 2 patients, in the duodenum in 3 patients (1 confirmed by EUS) and between the common bile duct and vena cava in one patient. The laparoscopic approach was used in 4 patients, 2 patients converted to open surgery. The following surgical techniques were performed: 2 pylorus-preserving pancreatico-duodenectomy (PPPD), one spleen-preserving distal pancreatectomy, one duodenal nodular resection, 1 segmental duodenectomy and one extrapancreatic nodular resection. Pathological studies showed lymph nodes metástasis in 2 patients with pancreatic gastrinomas, and in one patient with duodenal gastrinoma. The median follow-up was 76.83 months (5–108) and all patients presented normal fasting gastrin levels.
ConclusionsSurgery may offer a cure in patients with Z–E. The laparoscopic approach remains limited to selected cases.
El síndrome de Zollinger–Ellison (Z–E) está caracterizado por tumores productores de gastrina responsables de la aparición de úlceras recurrentes en el tracto gastrointestinal. El abordaje quirúrgico y la extensión de la resección tumoral son todavía controvertidos.
MétodosDe febrero de 2005 a febrero de 2014 se intervino a 6 pacientes con Z–E, 4 hombres y 2 mujeres, con una mediana de edad 46,8 años (22–61). Dos pacientes presentaban una neoplasia endocrina múltiple-1 (NEM-1). El diagnóstico se estableció por la determinación de gastrina basal en ayunas >200pg/ml (VN <100). Para el diagnóstico de localización se utilizó el octreoscan (6/6), la tomografía axial computarizada (TAC) (6/6) y la ultrasonografía endoscópica (USE) (1/6).
ResultadosEl octreoscan fue positivo en 5 pacientes. La TAC localizó el tumor en todos los pacientes: páncreas (2), duodeno (3, uno confirmado por USE), entre el conducto biliar y la vena cava (uno). El abordaje laparoscópico se utilizó en 4 pacientes, 2 pacientes fueron convertidos a cirugía abierta. Entre las técnicas quirúrgicas se realizaron: 2 duodenopancreatectomías cefálicas con preservación pilórica (DPCPP), una pancreatectomía distal con preservación esplénica, una resección nodular duodenal, una resección duodenal segmentaria y una resección nodular extrapancreática. La anatomía patológica demostró metástasis linfáticas en 2 pacientes con gastrinomas pancreáticos y en un paciente con gastrinoma duodenal. La estancia hospitalaria mediana fue 11,3 días (10–14). Durante el período de seguimiento clínico, con una mediana de 76,83 meses (5–108), todos los pacientes presentaron una gastrina en ayunas normal.
ConclusionesLa cirugía puede ofrecer la curación en pacientes con Z–E. El abordaje laparoscópico permanece limitado a casos seleccionados.
Gastrinomas are uncommon endocrine tumors, with an incidence of 0.5–1/1000000 inhabitants/year, and the second in frequency, preceded by insulinomas. Their incidence is greater in men than in women, and mean age at presentation is between 45 and 50.1 The clinical manifestations of these tumors are associated with gastrin hypersecretion, causing an elevation of gastric acid that leads to the appearance of Zollinger–Ellison syndrome. In turn, this produces gastroduodenal and jejunal ulcers and altered gastrointestinal motility, causing diarrhea in up to 70% of cases.2 Gastrinomas can appear sporadically (70%) or as part of multiple endocrine neoplasia, type 1 (MEN-1) syndrome.3 Although these tumors grow slowly, they are malignant in 60%–70% of cases, and 25% of cases progress quickly.4
The diagnosis and localization of gastrinomas has changed favorably in recent years with the use of computed tomography (CT), endoscopic ultrasound (EUS) and especially scintigraphy through the injection of octreotide, a somatostatin analog that binds with tumor somatostatin receptors.5 This latter test has high diagnostic sensitivity when tumors are larger than 2cm (96%), but it drops to 30% when tumors are smaller than 1cm.5 The diagnosis of gastrinomas requires demonstrating elevated fasting gastrin levels and, when in doubt, this elevation becomes more evident with the injection of secretin.6,7
The control of the disease involves the administration of proton pump inhibitors. However, it is surgery that provides a cure in up to 40% of patients with sporadic gastrinomas and, in cases of gastrinomas associated with MEN-1, the prevention of malignant transformation.8 The choice of surgical technique is controversial with regards to either open or laparoscopic surgery and the extension of the surgery, which is either conservative or radical.
The aim of this study is to analyze the immediate and long-term results of surgery in patients with sporadic gastrinomas and in patients with gastrinomas associated with MEN-1.
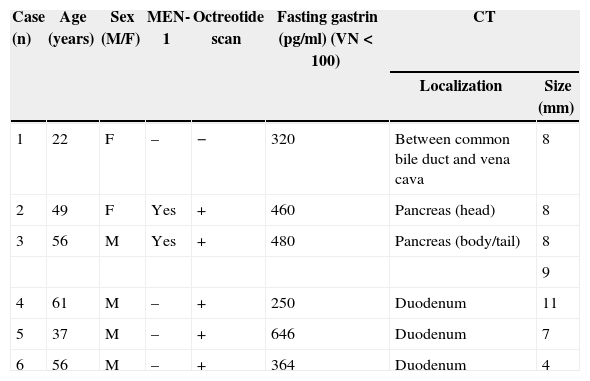
Patients and MethodsFrom February 2005 to February 2014, 6 patients (4 men and 2 women) were diagnosed with Zollinger–Ellison syndrome and treated surgically. Mean age was 46.8 (22–61). Patients had experienced gastrointestinal discomfort, and multiple gastric or duodenal ulcers were detected by gastroscopy. Demographic data of the diagnosed patients are presented in Table 1. One patient (case 5) was treated surgically due to perforation of a duodenal ulcer, and another patient (case 6) had upper gastrointestinal bleeding due to erosive esophagitis. Two patients had a family history of MEN-1: the father of one patient (case 2) had died due to a malignant gastrinoma with hepatic metastasis; the other patient (case 3) had undergone subtotal parathyroidectomy due to primary hyperparathyroidism. Over the last year, this latter patient had experienced episodes of acute recurring pancreatitis with hyperamylasemia.
Demographic Data of the Patients and Diagnostic Methods Used.
| Case (n) | Age (years) | Sex (M/F) | MEN-1 | Octreotide scan | Fasting gastrin (pg/ml) (VN<100) | CT | |
|---|---|---|---|---|---|---|---|
| Localization | Size (mm) | ||||||
| 1 | 22 | F | – | − | 320 | Between common bile duct and vena cava | 8 |
| 2 | 49 | F | Yes | + | 460 | Pancreas (head) | 8 |
| 3 | 56 | M | Yes | + | 480 | Pancreas (body/tail) | 8 |
| 9 | |||||||
| 4 | 61 | M | – | + | 250 | Duodenum | 11 |
| 5 | 37 | M | – | + | 646 | Duodenum | 7 |
| 6 | 56 | M | – | + | 364 | Duodenum | 4 |
−: negative; +: positive; MEN-1: multiple endocrine neoplasia, type 1; CT: computed tomography.
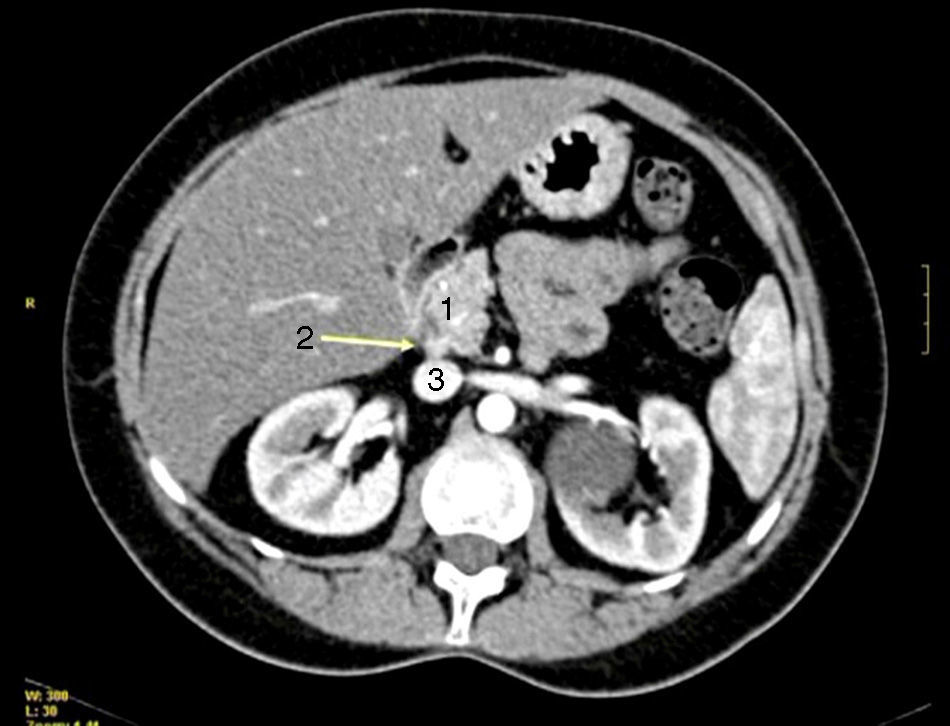
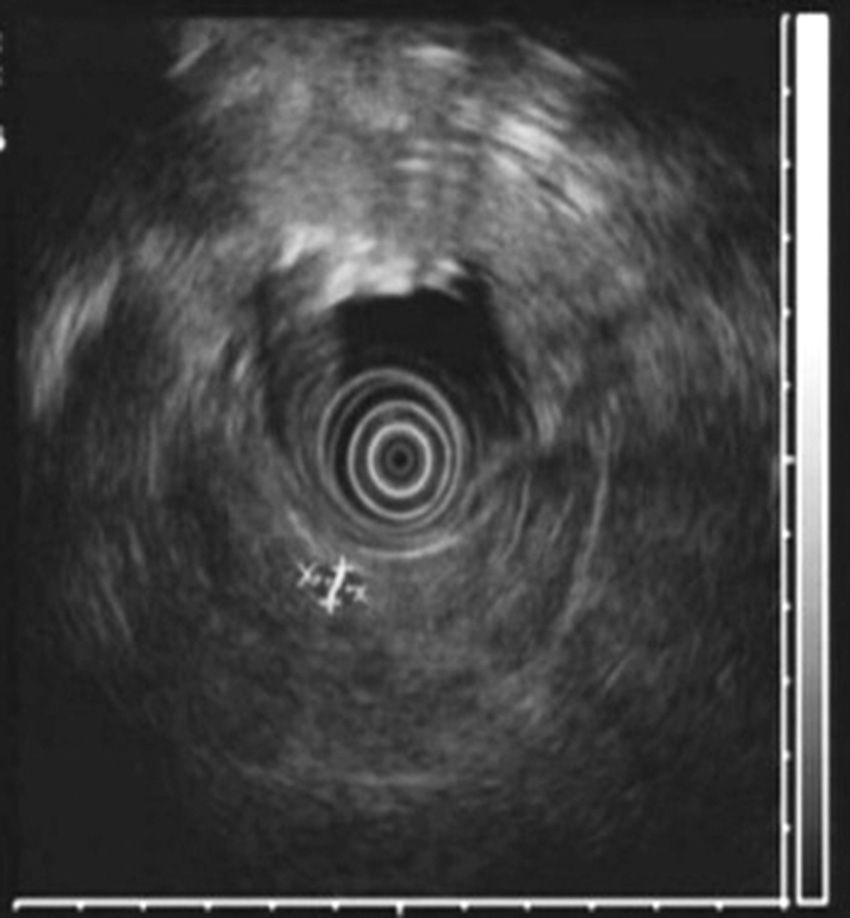
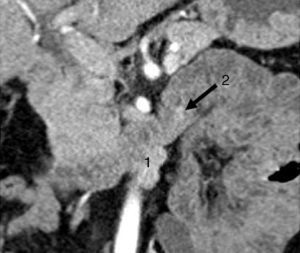
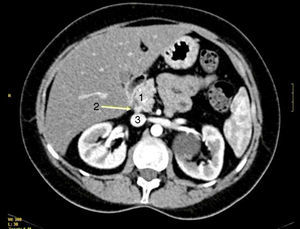
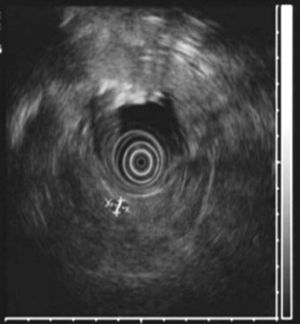
All patients had high fasting gastrin levels >200pg/ml (NV<100), with an average of 420pg/ml (250–646). In 2 patients (cases 1 and 4), a stimulation test with secretin was used, which showed serum gastrin levels of 1000 and 1500pg/ml, respectively. Octreotide scan was positive in 5 patients. CT demonstrated one or several nodules in the region of the head of the pancreas (case 2), the body/tail of the pancreas (case 3), duodenum (cases 4, 5 and 6) (Fig. 1) and between the common bile duct and vena cava (case 1) (Fig. 2). EUS confirmed the diagnosis in case 4 (Fig. 3).
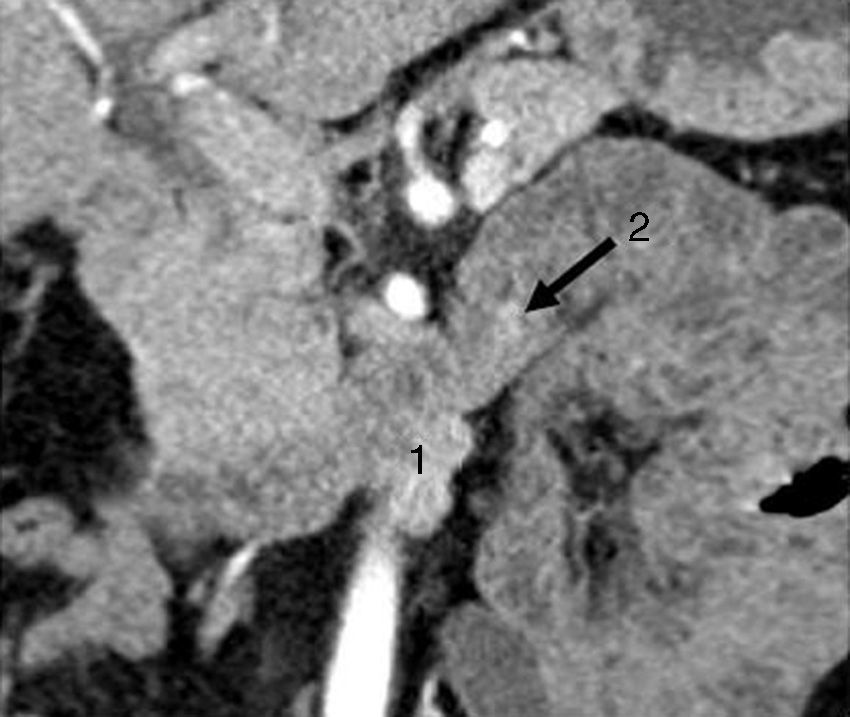
CT scan showing a bilobular, hypervascular mass in arterial phase in the 3rd part of the duodenum (lower edger) that showed caudal and exophytic growth (27mm×11mm) and corresponded with metastatic lymph nodes (1). Approximately 2cm distal to this lesion, there is a second hypervascular image measuring 7mm, which was a gastrinoma of the 3rd portion of the duodenum (2).
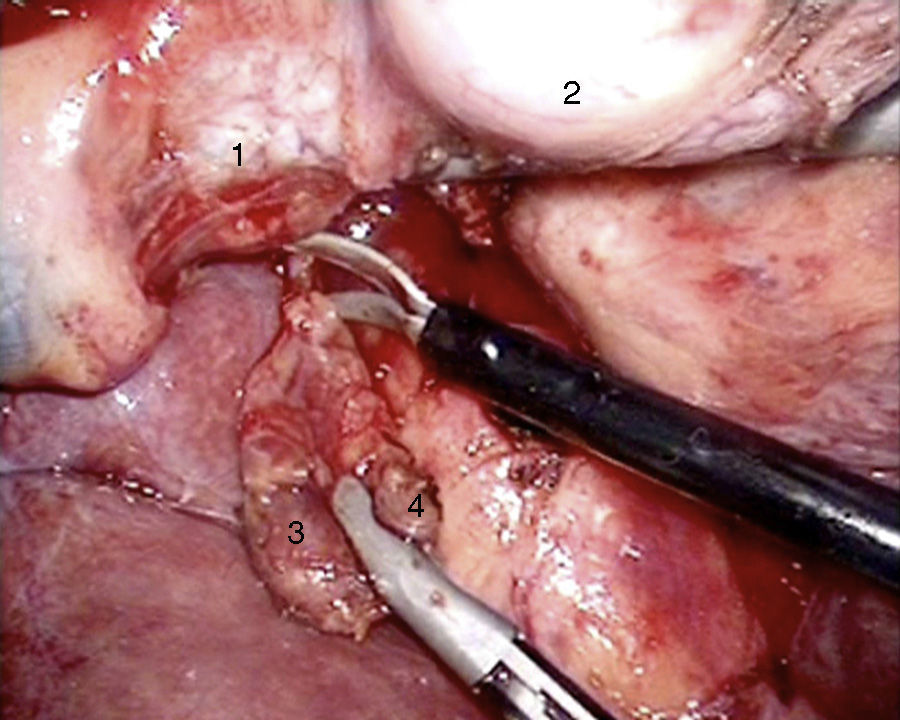
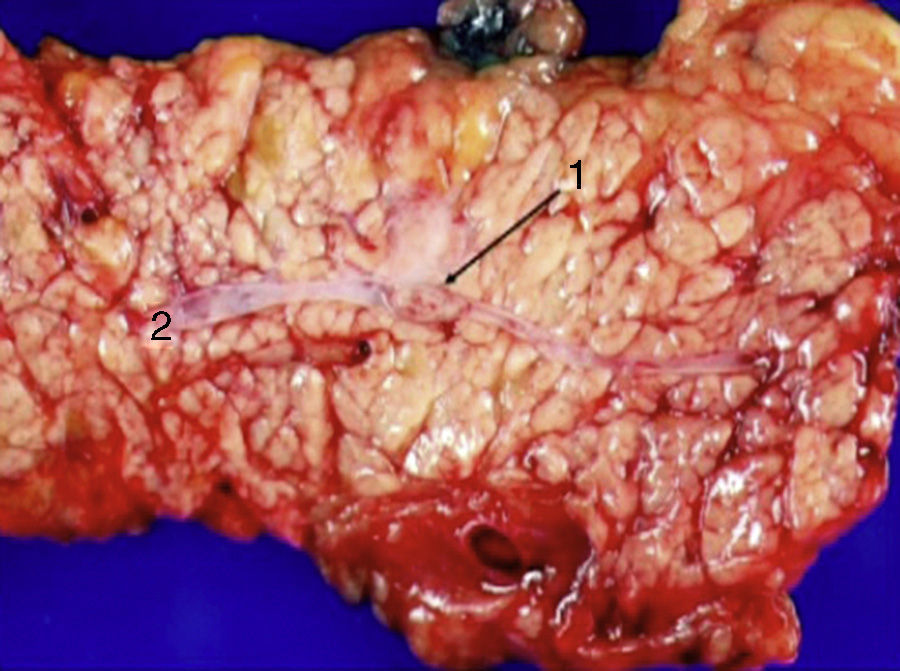
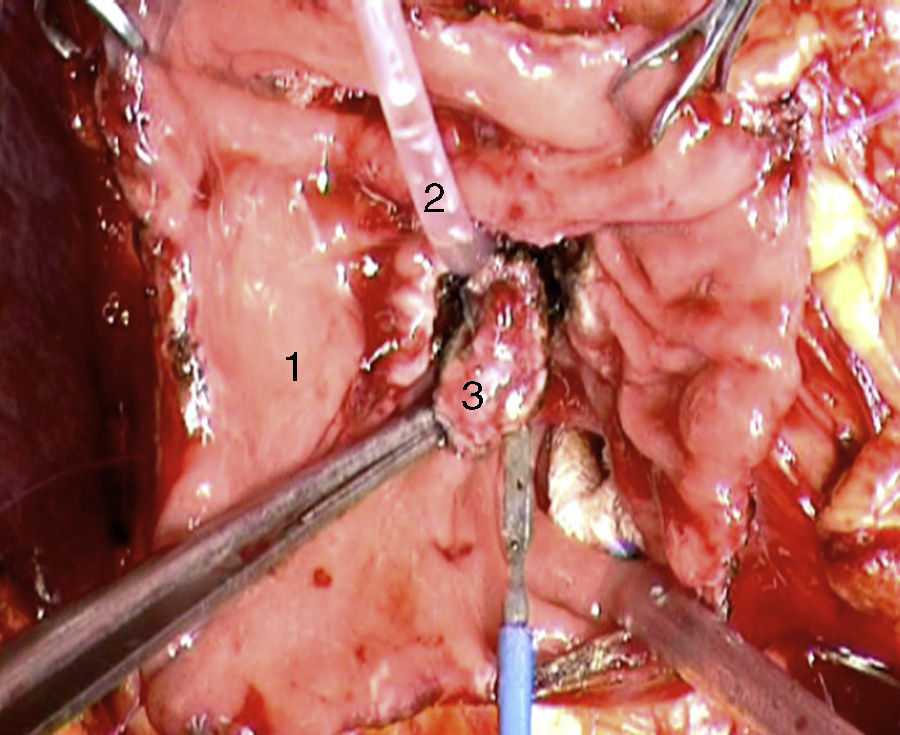
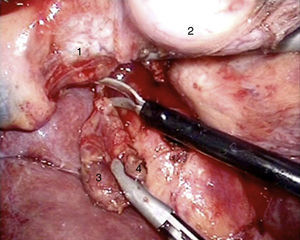
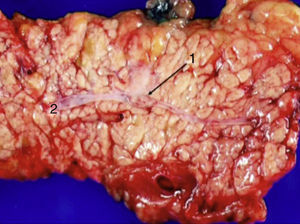
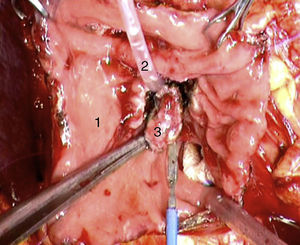
Laparoscopy was performed in 4 patients. In one patient (case 1), a nodule was found on the posterior side of the entry of the common bile duct into the pancreas, which was removed. Intraoperative biopsy confirmed the presence of a gastrinoma in a lymph node and ruled out the presence of metastasis in the regional lymph nodes (Fig. 4). The definitive pathology study confirmed the diagnosis of primary lymphatic gastrinoma. In another patient (case 3), distal pancreatectomy with pylorus preservation was done successfully, following the Warshaw technique. The pathology report demonstrated 2 gastrinomas in the body/tail of the pancreas, one of which was compressing the Wirsung duct, causing distal dilatation (Fig. 5). Two patients required conversion to open surgery. In one patient (case 6), an extrapancreatic nodule was found in the region of the uncinate process of the pancreas; biopsy showed it was a lymph node metastasis. Pylorus-preserving pancreaticoduodenectomy (PPPD) was performed. In the surgical specimen, a 4mm gastrinoma was found in the second portion of the duodenum. In another patient (case 4), intraoperative ultrasound demonstrated an 11mm nodule located on the posterior side of the duodenum adjacent to the duodenal papilla. By means of conversion to open surgery, a longitudinal duodenotomy was completed. A plastic cannula inserted through the papillary orifice in the common bile duct made it possible to extract the gastrinoma, with identification after exiting the pancreatic duct (Fig. 6).
An open approach was initially indicated in the patient (case 2) with gastrinoma associated with MEN-1, and PPPD was done. The pathology study of the resected specimen demonstrated the presence of a pancreatic gastrinoma measuring 8mm and lymphatic metastases in one of the 10 resected lymph nodes. In another patient (case 5) segmental duodenectomy was performed (distal part of the second portion of the duodenum and up to the medial region of the third portion of the duodenum) in conjunction with a peripancreatic lymphadenectomy. The pathology study demonstrated a 7mm duodenal gastrinoma and 2 lymph node metastases.
Patients who were treated by laparoscopy had no complications during the postoperative period and were discharged 11 days after surgery. The 2 patients with PPPD had no further incidences and were discharged 14 days after surgery. Another patient presented a pancreatic fistula that was type B according to the International Study Group of Pancreatic Fistulas (ISGPF) and was discharged 22 days after surgery. Hospitals stays (without complications) were 14 days in the patient with local resection of the periampullary gastrinoma and 10 days in the patient with segmental duodenal resection.
Mean follow-up was 76.83 months (5–108). All patients are currently asymptomatic and have normal fasting gastrin levels. The patients with MEN-1 syndrome (cases 2 and 3) have also presented normal gastrin levels 6 and 7 years after surgery, respectively; one 49-year-old woman with a gastrinoma located in the duodenum/pancreas region, and 56-year-old man with multiple gastrinomas in the body/tail of the pancreas. These patients present a higher long-term risk for disease recurrence. The tumor from case 1 can be considered a primary lymphatic gastrinoma because, 9 years after surgery, there have been no further clinical or biochemical manifestations of the disease, and gastrin levels have remained normal.
DiscussionThe natural history of gastrinomas differs between sporadic gastrinomas and those associated with multiple endocrine neoplasia, type 1.9 Sporadic gastrinomas present lymph node metastases during surgery in approximately 40%–70% of cases, and 20%–40% of patients have unresectable liver metastases. Gastrinomas in patients with MEN-1 are frequently small (some undetectable during radiology studies), multiple, and prone toward metastatic dissemination.10
There are enormous difficulties that arise in gastrinoma surgery, which explains why the technical options are a source of debate.11 Around 30% of patients go into surgery with the diagnosis of occult gastrinoma, but no preoperative localization.11,12 In this situation, the surgeon should focus his/her attention on the so-called “gastrinoma triangle”, which is an anatomical area whose upper limit is the junction of the cystic duct and the common hepatic duct, the lower limit is the meeting point of the 2nd and 3rd duodenal portions, and the medial limit is the neck and body of the dorsal and ventral pancreas.13 Between 65% and 90% of all gastrinomas found in surgery are located in the region of the duodenal segment of the head of the pancreas. Duodenal gastrinomas are more frequently located in the first (56%), second (32%), third (6%) and fourth (6%) part of the duodenum.12,13 In the pancreas, they are most frequently located in the head and in the body (2:1), while in 10% the presentation is in both sites.
The laparoscopic approach in gastrinoma surgery is controversial. Norton and Jensen14 have given 4 reasons to contraindicate minimal access surgery: (1) gastrinomas are located in the duodenum 3–10 times more frequently than in the pancreas; (2) the preoperative localization of duodenal gastrinomas can be difficult (size<1cm); (3) a high percentage of duodenal-pancreatic gastrinomas have associated metastases in the regional lymph nodes (50%–70%); (4) the localization of these tumors in the so-called “gastrinoma triangle” does not make for easy surgery, as it normally associates prolonged operative times and occasionally may require pancreaticoduodenectomy.
Some of these reasons have led to conversion to open surgery in 2 of our patients. In one case, intraoperative ultrasound demonstrated an 11mm tumor in the posterior wall of the duodenum, in close contact with the duodenal papilla. In the other case, during the laparoscopic exploration of the duodenum/pancreas area, an 11mm extrapancreatic tumor was found in the region of the uncinate process of the pancreas, in close contact with the duodenal wall; intraoperative biopsy demonstrated the presence of lymph node metastasis. We decided to perform pancreaticoduodenectomy and observed in the surgical specimen a 4mm tumor in the duodenal region. In our experience, however, laparoscopy was successful in 2 patients: in one, the gastrinoma was located between the bile duct and the vena cava, which turned out to be a primary lymph node gastrinoma; the other patient with MEN-1 presented several gastrinomas in the body/tail of the pancreas, and pylorus-preserving distal pancreatectomy was performed in accordance with the Warshaw technique.
In the literature, there are few cases of gastrinomas in which laparoscopy has been successfully completed, so this type of approach should only be recommended in very select cases.15–17
In sporadic gastrinoma surgery, once the tumor is located, the surgery of choice has also been debated: conservative surgery (enucleation) or tumor resection surgery, with selective lymph node dissection or systematic lymphadenectomy.
Giovinazzo et al.18 have reviewed the results of 20 patients diagnosed with Zollinger–Ellison syndrome in a period of 19 years. Tumor recurrence was observed in 8% of the patients treated with tumor resection, and 100% (4 patients) of those with enucleation. The limited number of patients in this series is not conclusive about the possible benefit of conservative surgery. In addition, there are other factors that have been identified as important in the recurrence of the disease after surgery: female sex, short history of Zollinger–Ellison, high serum levels of fasting gastrin, tumor size >3cm and pancreatic location.
In a retrospective study of 48 patients with sporadic gastrinomas and a follow-up period of up to 21 years, Bartsch et al.4 have analyzed the prognostic factors and the importance of lymphadenectomy to disease prognosis. In one group of patients, regional lymphadenectomy was used selectively, and in another group systematic lymphadenectomy was done, including the removal of pancreatic, pancreaticoduodenal and hepatoduodenal ligament lymph nodes and those situated between the aorta and vena cava. This last group associated a higher percentage of biochemical cure of the disease and demonstrated a more favorable tendency in the survival times, both disease-specific as well as disease-free. In this study, the following poor prognosis factors were observed: pancreatic location, tumor size ≥25mm, Ki-67 more than 5%, preoperative gastrin ≥3000pg/ml and the presence of hepatic metastases.
The definition of a surgical sporadic gastrinoma cure depends on the follow-up time of these patients and the study chosen for follow-up.14,19 The results are very favorable if the time is short and if the disease recurrence analysis is based on radiological detection of tumors. The most reliable diagnostic method is gastrin determination. With these methods, it is estimated that a cure is reached in 40% of patients 10 years after surgery.1,3,19
The existence of a primary gastrinoma in a lymph node has created controversy. This possibility is confirmed by a study that demonstrates the presence of neuroendocrine cells in abdominal lymph nodes.20 In the literature, there are examples of patients who were cured in the short and long term after surgery.21,22 Norton et al.23 analyzed the experience of 176 patients with Zollinger–Ellison syndrome who were treated surgically over a period of 17 years, and they found that 26 patients (15%) followed for 10 years met the criteria of primary lymph node gastrinomas. During this follow-up, 16 patients (12%) remained cured, while in 6 patients the disease reappeared. These authors indicated the difficulty that surgeons may have during a procedure to predict whether a suspicious lymph node is a primary gastrinoma or metastasis of a duodenal or pancreatic gastrinoma. In cases of doubt, the surgeon should continue to search for the primary tumor and to resect and biopsy other peripancreatic lymph node chains. This will help avoid overlooking a gastrinoma that would probably need more extensive surgery. In our patient, we resected the nodule that was detected by radiology examination, which in the end was a primary lymphatic gastrinoma that met the criteria by presenting gastrin normalization immediately after surgery and absence of clinical symptoms for 9 years of follow-up.
The debate continues in gastrinoma surgery when associated with MEN-1.24,25 In these patients, disease recurrence is 95% 3–5 years after surgery. Radical surgery with pancreaticoduodenectomy or subtotal pancreatectomy has not demonstrated greater benefits than other surgeries, which are not as extensive, when tumors have been able to be identified preoperatively. The objective of the operation would be biochemical cure of the disease. Norton et al.19 have observed initial postoperative cure rates after 5 and 10 years of 60, 40 and 34%, respectively. Ellison et al.8 have reported the experience of surgical series that included between 4 and 48 patients, indicating that the biochemical cure rate varied between 0% and 38%. Despite the high recurrence of the disease, surgery should always be indicated whenever possible for the prevention of distant metastasis.25 The mortality rate of these patients in the long term is half that of patients who do not undergo surgery.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: Fernández-Cruz L, Pelegrina A. Cirugía del gastrinoma: Resultados inmediatos y a largo plazo. Cir Esp. 2015;93:390–395.