Irreversible electroporation is a novel technique growing in popularity over the last years among the ablative modalities. Its unique action mechanism produces irreversible nanopores in the membrane of the cell leading to apoptosis; therefore irreversible electroporation can be used to ablate substantial volumes of tissue without the undesirable thermal effects as the “heat sink effect”. Moreover the extracellular matrix is left unperturbed, thus sparing the structural architecture of surrounding structures such as bile ducts and blood vessels. In the last years its use has been widespread in both liver and pancreatic ablation. Irreversible electroporation has shown its safety with however some caution, feasibility and favorable outcomes in clinical settings such as unresectable locally advanced disease in which the surgical and therapeutic options are very limited.
La electroporación irreversible es una entidad que ha ganado en popularidad entre las técnicas de ablación tumoral en los últimos años. Debido a su innovador mecanismo de acción, ya que induce poros en la membrana celular que conducen a la apoptosis celular, ha demostrado su capacidad para destruir el tejido sin presentar los efectos indeseables de la ablación térmica tales como el «heat sink effect». Asimismo mantiene la integridad de la matriz extracelular, por lo que estructuras como los vasos sanguíneos y los ductos biliares no son afectadas por la electroporación irreversible. Su utilización se ha ido extendiendo en los últimos años tanto en la ablación hepática como en la pancreática mostrando su seguridad y resultados prometedores en escenarios clínicos en los que la cirugía en el momento actual no puede ofrecer otras opciones terapéuticas.
Irreversible electroporation (IRE) is a new ablative technique that has gained popularity in the last decade. In contrast with the traditional paradigm of thermal issue ablation, IRE owes its growing popularity to its mechanism of action, which does not entail thermal damage.1 The application of thermal pulses on a specific frequency and voltage generates nanopores in the lipid structure of the cell plasma membrane that alter the electrical gradient between the intra- and extracellular medium, providing free diffusion of molecules.2,3 If this intense electrical field is maintained over a sufficient period of time, cell damage is irreversible, leading to cell death due to apoptosis.
Radiofrequency and microwave ablation base their mechanism of action on thermal tissue destruction by coagulative necrosis. After a certain temperature, protein denaturalization is triggered, and thus, irreversible structural damage that leads to cell death. Although the experience with these ablative techniques is very extensive, most authors agree that the size of the lesion and proximity to the large vessels are their limitations.4 The mechanism of cell death in IRE is very attractive compared to the spectrum of conventional ablative techniques, whose largest limitation lies in the heat sink effect that occurs in proximity to the blood vessels. The blood flow dissipates heat at this level, causing a refrigeration effect on the tissue treated and, consequently, incomplete tumor ablation.
Another advantage of IRE is its capability to preserve both the architecture of the extracellular matrix of the connective tissue as well as the integrity of the blood vessels,5,6 which, in the case of the liver, allows for ablations to be done close to the hepatic artery, portal vein and hepatic ducts without causing structural damage.
Given the boom in IRE over the last 10 years, the aim of this article is to provide a global vision on the principles and indications of IRE in different clinical scenarios of HBP surgery, as well as to review the oncological results available to date.
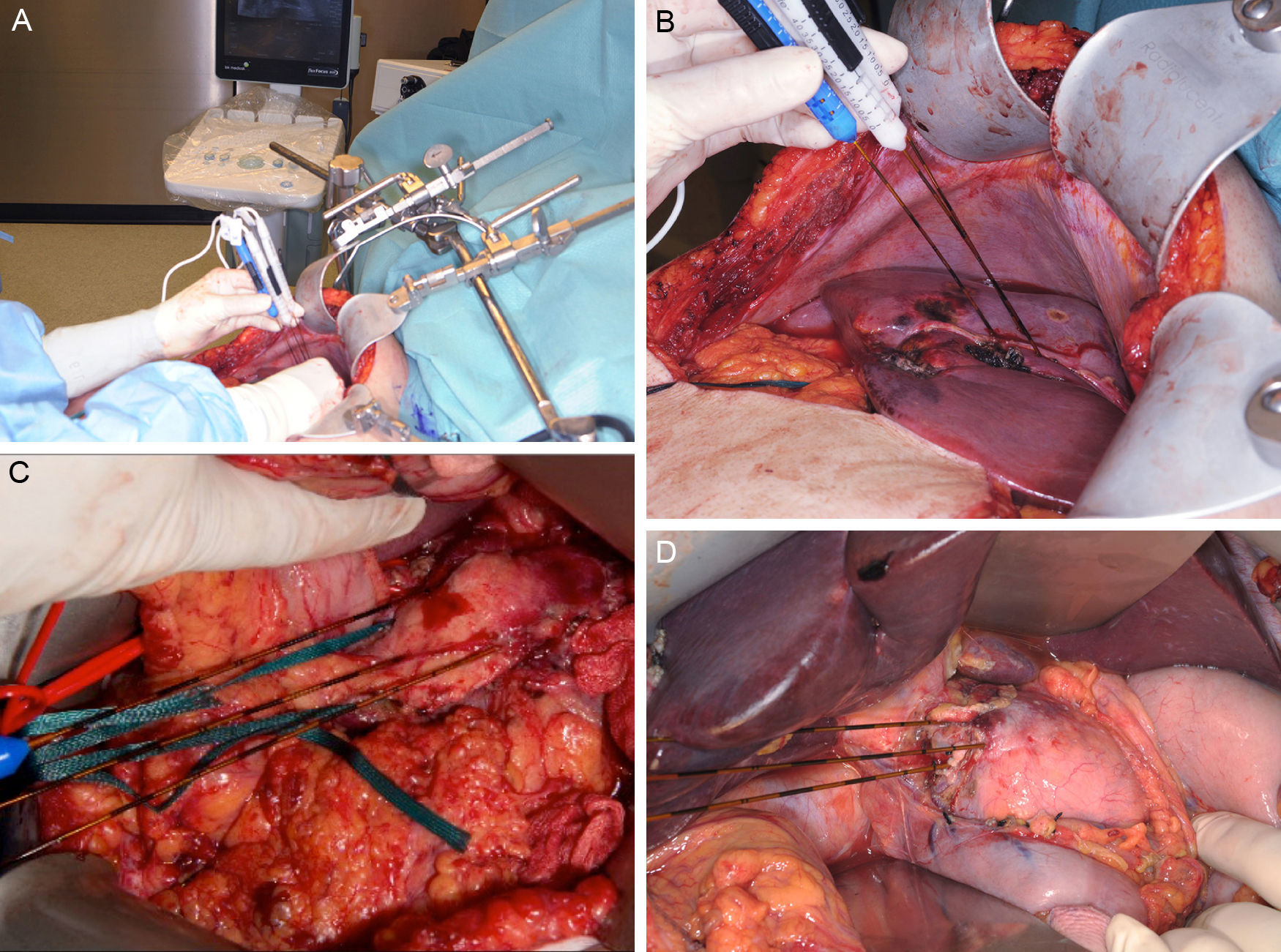
Clinical Device for Applying Irreversible ElectroporationCurrently, only one device has been approved by the FDA (510[k] Number: K080376) for the clinical use of IRE (Nanoknife® [AngioDynamics, Latham, NY, USA). The electrical pulses are applied by means of a varying number of bipolar or monopolar electrodes, depending on the lesions to treat. The device can generate a maximum energy of 70mA or 3000V. The application protocols are variable, but the majority of the studies execute 70–90 pulses with a duration of 70–100μs.7–10 The device admits up to a maximum of 6 electrodes of 19G, whose maximum distance should not surpass 2cm and whose alignment should be parallel to maintain the uniformity of the electrical field (Fig. 1). There is no standardized protocol for the number or placement of the electrodes, but the uniformity of the electrical field pays an essential role in the success of the ablation.11 Appelbaum et al.12 demonstrated that, by applying 4 electrodes in parallel, greater ablation volume is achieved with better oncological results, since in this manner the protocol can be personalized to each patient.
Images (A) and (B) show an example of the intraoperative placement of the electrodes for ablation of a hepatic lesion using the open approach. A closer look at the placement of the electrodes for the ablation of a lesion depending on its location in the neck/body of the pancreas (C) or the head (D). Images provided by Dr. ML de Oliveira.
A threshold for the electrical field has been reported at approximately 700V/cm, after which point the electroporation becomes irreversible.3 Nonetheless, it is important to note that this calculation is based on a theoretical mathematical model and that this threshold is probably not directly translatable to clinical practice since the extracellular matrix, blood vessels and other structures play a role in the homogeneity of the electrical field.13 Therefore, in general practice, an electrical field between 1000 and 1500V/cm is usually used.
For the application of the pulses, it is essential to synchronize them with the electrocardiogram in order to avoid arrhythmias. The electrical pulse should be applied exactly 50μs after the R wave to coincide with the absolute refractory period of the myocardium in the cardiac cycle.14 Likewise, it is necessary for patients to be under general anesthesia and under the effect of muscle relaxants, like rocuronium, to avoid muscle contractions triggered by the pulses. Most studies monitor patients with TOF-watch® type devices in order to ensure the maintenance of correct muscle relaxation, and defibrillator paddles are placed as a preventive measure.
Application of Irreversible Electroporation in Hepato-Bilio-Pancreatic TumorsHepatic AblationAblation techniques are currently an essential part of the treatment of hepatic tumors. In primary tumors such as hepatocellular carcinoma (HCC), which is currently in the sixth position in worldwide prevalence,15 as well as metastatic gastrointestinal tumors, ablation is a reasonable alternative for patients who are not candidates for surgical resection.
The first experimental studies were aimed at hepatic ablation in HCC, since radiofrequency ablation has been internationally established as the treatment of choice in early stages (BCLC 0 or A).15 In a murine HCC model, Guo et al. described for the first time that ablation by means of IRE demonstrates a significant decrease in tumor size and a greater percentage of necrosis induced by the treatment in ablated tumors.16 In later use in clinical practice, the studies by Cheung et al.7 and Bhutiani et al.,8 directed specifically at the treatment of HCC in a limited series of 11 and 55 patients, reported the feasibility of the technique and very few adverse effects. Even in patients with Child B cirrhosis, good oncological results and very few complications have been demonstrated.8,17
In the same manner, IRE can be used in the ablation of colorectal metastases that are not surgically resectable due to their central or perivascular location. To date, greater effectiveness of IRE has not been demonstrated according to tumor type, although it has been indicated that the histologic subtype could play a role in the success of the ablation.18 In most series, the number of patients is limited and samples are very heterogeneous, which limits the interpretation of the oncological results. Only Scheffer et al.19 prospectively included 10 patients with exclusive diagnosis of hepatic metastasis of colorectal carcinoma, in which the lesion was ablated and then resected for histological analysis. The results showed extensive areas of non-viable tumor in 80% of patients confirmed by a positive marker for apoptosis, caspase-3. However, the study did not provide data on long-term follow-up or the recurrence rate.
There is no limit established on the size of the lesion to be treated, but better oncologic results are described in lesions smaller than 3cm,20 and an increased risk of local recurrence has been described when the tumor volume exceeds 5cm.3,18 The efficacy and viability of IRE in perivascular ablation is one of its greatest advantages,10,21 and ablations have been reported with a mean proximity to the large blood vessels of 0.5cm, with no observed thrombosis or stenosis at follow-up.22 In a comprehensive study, Dollinger et al.17 specifically reported that the risk of post-IRE thrombosis is greater in ablations done near the portal vein compared to the suprahepatic veins or hepatic artery. However, out of a total of 172 ablations near large vessels, only 9.9% of patients experienced vascular alterations, which became permanent in certain cases. In this context, it is important to highlight the potential role of IRE in the downstaging of tumors prior to surgery, a use that has already been described in the pancreas.23 In the era of ALPPS or 2-stage hepatectomy, IRE is a more-than-reasonable alternative to treat lesions that infiltrate the suprahepatic veins and to be able to complete the surgery in a deferred manner. Our group has experience in the use of IRE for the downstaging of liver metastases of colorectal carcinoma in the context of the ALPPS procedure, and even for the local control of tumors as a step prior to liver transplantation.
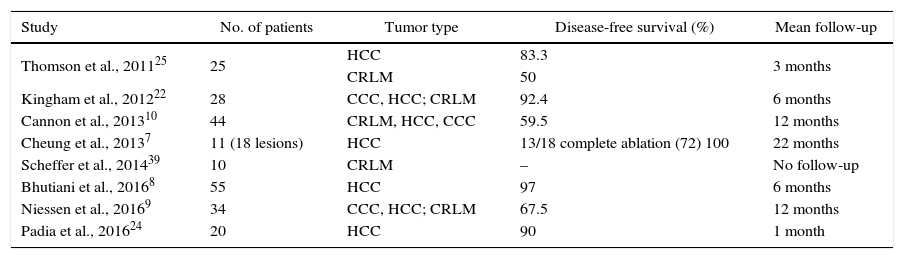
Oncologic outcomes vary greatly according to authors and series. Success rates range from 50% to 100%, depending on the diagnostic criteria applied and the length of follow-up (Table 1). Most authors have a maximum follow-up of 6 or 12 months post-ablation and in some cases the disease-free survival is not accurately reported since the objective is not so much to demonstrate the effectiveness of the treatment as it is to determine the tolerability of the treatment. It should also be taken into account that IRE is a technique that is in its early stages, so there are no established guidelines regarding the evaluation of the long-term radiological behavior of the lesion. In dynamic gadolinium-enhanced magnetic resonance studies, a hypointense area is usually observed in T1, corresponding with the treated area, surrounded by a halo of contrast uptake. This hypointense area decreases in diameter and the enhancement halo gradually disappears.24,25 However, the correlation of these radiological findings with the histology is challenging as no histological specimen is available to compare the results.
Summary of the Series of Patients Treated With Hepatic Ablation Using IRE.
| Study | No. of patients | Tumor type | Disease-free survival (%) | Mean follow-up |
|---|---|---|---|---|
| Thomson et al., 201125 | 25 | HCC | 83.3 | 3 months |
| CRLM | 50 | |||
| Kingham et al., 201222 | 28 | CCC, HCC; CRLM | 92.4 | 6 months |
| Cannon et al., 201310 | 44 | CRLM, HCC, CCC | 59.5 | 12 months |
| Cheung et al., 20137 | 11 (18 lesions) | HCC | 13/18 complete ablation (72) 100 | 22 months |
| Scheffer et al., 201439 | 10 | CRLM | – | No follow-up |
| Bhutiani et al., 20168 | 55 | HCC | 97 | 6 months |
| Niessen et al., 20169 | 34 | CCC, HCC; CRLM | 67.5 | 12 months |
| Padia et al., 201624 | 20 | HCC | 90 | 1 month |
CCC, cholangiocarcinoma; CRLM, colorectal liver metastases; HCC, hepatocellular carcinoma; IRE, irreversible electroporation.
Pancreatic adenocarcinoma is an entity with a grim prognosis, causing 266000 deaths/year worldwide.26 At the time of diagnosis, 80%–90% of patients present disseminated disease (stage IV) or regional lymphovascular infiltration (stage III), so the surgical results are not encouraging.27 Because of its complex anatomical location, conventional thermal ablation does not provide good palliation as it has a high rate of associated complications, such as hemorrhages, necrotizing pancreatitis or injury to the main biliary tract or the Wirsung duct.
IRE has demonstrated good results in the palliative ablation of stage III tumors of the neck/body as well as in the head in a long series of 200 patients, with a mean survival of 24.9 months.28 Adverse effects include gastrointestinal/hematological complications and infections, although their spectrum of severity varies. There are reports of 36% of complications derived directly from the procedure, in spite of which groups with great experience in pancreatic ablation report no pancreatic fistulas or clinically relevant pancreatitis,29,30 as has already been shown in experimental studies.31
In its clinical application, staging by exploratory laparoscopy with cytology is required prior to ablation, as well as good local tumor control after induction chemotherapy for 3–4 months and a tumor diameter less than or equal to 3.5cm.32 In patients with metallic stents, their withdrawal is necessary prior to the application of pulses, as the presence of metal increases the risk for thermal damage in the surrounding tissue.33 In most cases, an open approach is necessary for the correct localization, placement of electrodes and tumor treatment (Fig. 1).
In a comparison with patients treated with standard therapy, Martin32 describes a clear benefit in the local recurrence-free survival in patients treated with IRE (14 vs 6 months, p=.01), disease-free survival (15 vs 9 months, p=.02), and overall survival (20 vs 13 months, p=.03).
Clinical Safety of Irreversible ElectroporationSince the introduction of the technique in humans, clinical safety has been the big question in IRE. The most common adverse effects are cardiovascular events since the application of the electric field as pulses entails, as previously reported, the frequent appearance of arrhythmias and even bursts of ventricular fibrillation.34 Since the introduction of standardized electrocardiogram synchronization, the appearance of these arrhythmias has decreased substantially.
Rhythm alterations vary depending on the area to be treated. Our group has recently shown in a series of 43 patients that cardiovascular events are significantly related to the location of the electrodes. Thus, ablations in the celiac trunk region were a risk factor in the multivariate analysis for developing a cardiovascular event.35 These arrhythmias do not usually involve hemodynamic compromise for the patient and only require medical treatment.36 Furthermore, IRE can cause a transient increase in the patient's blood pressure. Some 77% of the patients in our series had a mean increase in blood pressure of 15mmHg, a fact that correlates with the results of other authors.36 In some cases, extreme elevation of systolic blood pressure to 200mmHg has been reported, but these were tumors of the upper pole of the kidney or ablations related with the adrenal glands.34
Fluid and electrolyte imbalances have also been reported, such as hyperkalemia34,35 or metabolic acidosis. Taking into account the mechanism of action, the hypothesis is that the formation of nanopores in the plasma membrane of cells leads to the massive release of intracellular potassium into the blood flow, similar to what occurs in tumor lysis syndrome. This alteration has also been confirmed at the experimental level, since there are studies that indicate that very large ablations of tissue lead to severe fluid and electrolyte imbalance.37
Apart from those already mentioned, there are complications directly derived from the placement of the electrodes, such as pneumothorax (3.9%), pleural effusion or hematomas (11.8%), which are usually related to percutaneous procedures.36 Pain has also been reported after the procedure, triggered by muscle hyperstimulation.38
Future PerspectivesIRE is certainly a promising technique and a feasible alternative in cases of unresectable tumors in both the liver and the pancreas. Its characteristic mechanism of action gives it an essential role to play in perivascular ablation. However, it is a new technique, and the related evidence is based on case series and limited patient cohorts that do not provide conclusions that could be generalized or standardized for clinical use. Multicenter randomized clinical trials with extensive follow-up periods are necessary to determine long-term oncological outcomes.
Currently, there are more than 10 registered randomized studies underway. Among them is the COLFIRE-II study (NCT02082782), whose preliminary results (COLDFIRE I) have already been published,39 with 29 recruited patients with liver metastases of colorectal carcinoma. The primary objective is to assess disease-free survival with PET-MRI during a one-year follow-up.
In addition, it is also interesting to highlight the NCT02787954 study comparing with RECIST criteria the time until progression of the HCC depending on the TACE applied technique, radioembolization with Itrio90, MWA or IRE.
Beyond a doubt, IRE is a technique with great potential, whose future clinical uses have still yet to be seen. Until now, however, it has demonstrated its capacity for the treatment of certain tumors, for which there is currently no better therapeutic alternative.40
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Sánchez-Velázquez P, Clavien P-A. El rol de la electroporación irreversible en la cirugía hepato-bilio-pancreática. Cir Esp. 2017;95:307–312.