The use of vacuum-assisted therapies for anastomotic leakage after rectal resection is a well-known minimally invasive technique. It showed excellent results in the literature, with successful curative rates over 90%.1
A novel use of endoluminal vacuum-assisted therapies for treatment of upper gastrointestinal (GI) tract defects has recently been proposed. Several studies have been published in the literature, reporting promising results on the use of these techniques after esophageal anastomotic leakages, gastric (mainly in bariatric surgery) and colorectal leaks and fistulas.2 We herein present, to our knowledge, the first case of endoluminal vacuum-assisted therapy for treatment of gastric fistula secondary to gastric ischemia after Appleby procedure for pancreatic ductal adenocarcinoma (PDAC).
A 67-year-old male with history of tobacco consumption, Hodgkin's lymphoma and lumbar spinal stenosis and a 6-month low back pain resistant to analgesic treatment. Finally, he was diagnosed with a pancreatic body tumor, which was considered locally advanced because of infiltration of less than 50% of the celiac trunk without involvement of the superior mesenteric artery (SMA). Splenic artery and vein were also involved. He received neoadjuvant chemotherapy (gemcitabine plus abraxane) for 9 cycles, followed by concomitant radiation therapy and oral capecitabine for 2 months.
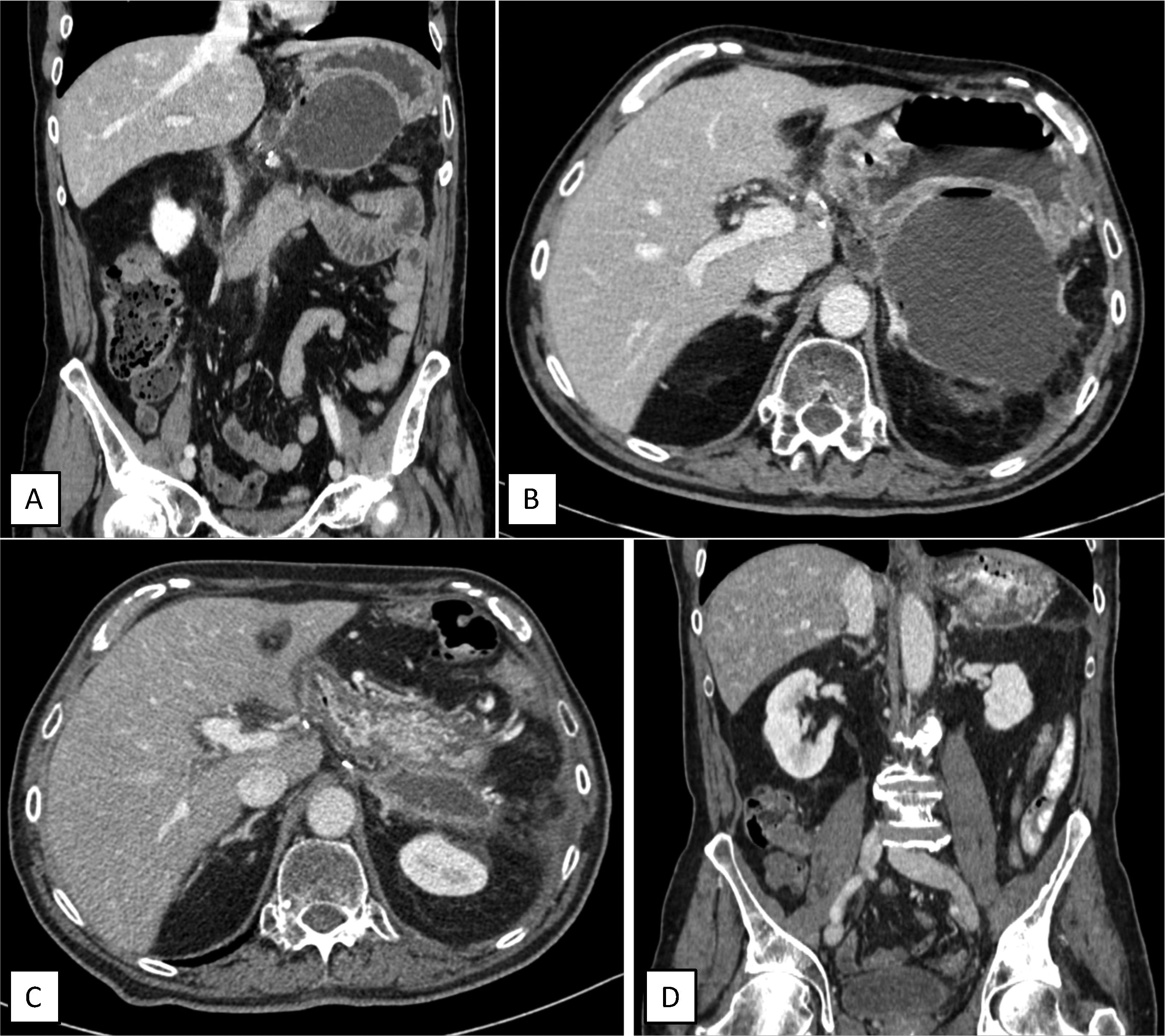
The case was presented in the Multidisciplinary Meeting after neoadjuvant therapy and because there was no disease progression, still invading the celiac trunk without involvement of neither gastroduodenal artery, nor SMA, nor portal vein, a curative resection was decided. A modified Appleby procedure (en-bloc distal pancreatectomy and celiac trunk resection with gastric preservation) was performed. The immediate post-operative period was uneventful, so the patient could be discharged 7 days later. The pathology report informed of infiltrating ductal adenocarcinoma of high-grade clear cells, stage ypT1cN0 (0 out of 33 lymph nodes). One month after surgery, in a scheduled post-operative CT scan, a 108×79mm retrogastric collection was diagnosed, alongside a marked thinning of a 1cm in diameter area in the lower face of the gastric fundus, in contact with the collection, suggestive of gastric rupture secondary to ischemia (Fig. 1A and 1B). Although the patient was completely asymptomatic, he was readmitted and a CT scan-guided puncture was performed, obtaining purulent liquid positive to multisensitive Escherichia coli. Pancreatic amylase levels in the collection were in normal range (<1000U/L). Antibiotic treatment with Amoxicillin/clavulanic acid was initiated, alongside nasogastric tube placement, fasting and parenteral nutrition.
Post-operative scheduled CT scan showing retrogastric collection (A: Coronal view. B: Cross-section showing posterior gastric wall thinning) and control CT scan after completing endoluminal vacuum-assisted therapy, showing decrease in collection size, without defects on the gastric wall (C: Cross-sectional view. D: Coronal view).
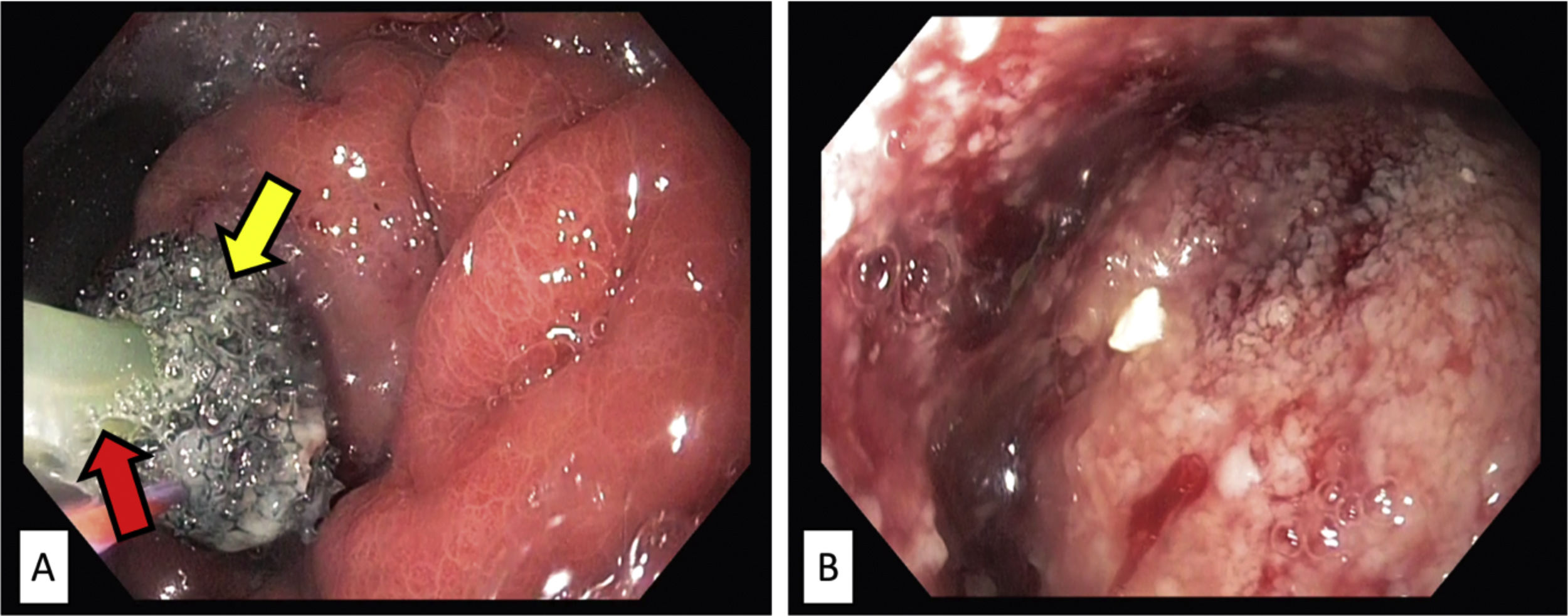
An esophagogastroduodenoscopy (EGD) was performed 6 days later, showing a 1cm wall defect in the posterior gastric wall, without signs of ischemia in the rest of the stomach. It was decided to treat the gastric defect with endoluminal vacuum-assisted therapy (Eso-SPONGE®, Braun). A sponge was placed inside the cavity through endoscopic direct view and afterwards connected to a negative pressure device (Fig. 2). Follow-up endoscopies were repeated every 48–72h, showing progressive improvement, with a decrease in cavity size, as well as in purulent content and formation of granulation tissue. In each control, the sponge was replaced by a smaller version according to the observed changes in the defect size. The duration of treatment was 11 days, including a total of 4 sponge replacements.
Endoscopic images showing endoluminal vacuum-assisted therapy (EsoSponge®, Braun) replacement. (A) The sponge covering the gastric defect (yellow arrow), in contact with the negative pressure therapy probe (red arrow). (B) Inside the cavity, in which granulation tissue and fibrin detritus are observed.
A control CT scan performed afterwards showed a 23×61×17mm collection, with no evidence of oral contrast leak (Fig. 1C and D). Food intake was restarted, and the patient was discharged without new complications after 5 months of follow-up and normal CT-scans.
Locally advanced pancreatic cancer involving the celiac axis has classically been considered unresectable. In selected patients, following neoadjuvant therapy, modified Appleby technique following neoadjuvant therapy has proven to be a safe procedure with favorable outcomes. Recent publications3 demonstrated a median survival of three years after successful arterial resection. However, gastric ischemia is one of the most feared complications of this technique, being responsible for a wide range of problems that go from gastric motility disorders to gastric perforation. In order to avoid that, it is recommended to preserve the left gastric artery (LGA), that was routinely included in en-bloc resection during modified Appleby procedures. In our case, the tumor involved the celiac trunk including the LGA, whose origin was not independent as an antecedent first branch, which only occurs in 68–72% of cases.4
Nowadays, minimally invasive and bloodless methods are arising for the treatment of upper GI defects. For example, in hemodynamically stable patients with benign esophageal perforations, endoscopic stenting may allay the potential morbidity of a surgical intervention. Comparing this technique to surgical repair, a recent systematic review reported a higher success rate (88% vs 83%) as well as a decrease in mortality rate (7.5% vs 17%).5 Nevertheless, stenting can present potentially serious complications such as migration, dysphagia, hemorrhage, stent fracture, airway compromise or allergic reactions.6
Endoluminal vacuum-assisted therapies have been recently proposed as a novel technique for treatment of upper GI tract defects. A drainage sponge is placed under direct endoscopic view either inside the cavity or into the upper GI lumen and afterwards it is connected to a continuous negative pressure system via a nasogastric tube. This system allows drainage of the perianastomotic leak abscess, as well as favors changes in perfusion and reduction of interstitial edema, stimulating granulation and re-epithelialization of surrounding tissues.7 In a recent study, complete restoration of the esophageal defect was achieved in 60 out of 77 patients (77.9%).7
In conclusion, in our specific case, the use of endoluminal vacuum-assisted therapy (Eso-SPONGE®, Braun) was a good option to treat gastric perforation secondary to ischemic gastropathy after modified Appleby procedure for pancreatic cancer in the reported case, allowing intraabdominal abscess drainage, granulation of the cavity walls and closure of the gastric wall defect. Moreover, this avoided the need for reoperation and also a longer hospital stay. In the future, randomized controlled trials will be required to confirm our proposed treatment strategy.