Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causal agent of coronavirus disease 2019 (COVID-19). Diabetes is one of the most frequent comorbidities in people with COVID-19 with a prevalence that varies between 7 and 30%. Diabetics infected with SARS-CoV-2 have a higher rate of hospital admission, severe pneumonia, and higher mortality compared to non-diabetic subjects. Chronic hyperglycemia can compromise innate and humoral immunity. Furthermore, diabetes is associated with a low-grade chronic inflammatory state that favours the development of an exaggerated inflammatory response and therefore the appearance of acute respiratory distress syndrome. Recent evidence has shown that SARS-CoV-2 is also capable of causing direct damage to the pancreas that could worsen hyperglycemia and even induce the onset of diabetes in previously non-diabetic subjects. Therapeutic strategies should be aimed at facilitating patient access to the healthcare system. Control of blood glucose and comorbidities must be individualised in order to reduce the incidence of complications and decrease the burden on health systems. In this article we will review the pathophysiological mechanisms that explain the bidirectional relationship between COVID-19 and diabetes mellitus, its implication in the prognosis and management of hyperglycemia in this group of patients.
El coronavirus 2 del síndrome respiratorio agudo grave (SARS-CoV-2) es el agente causal de la enfermedad por coronavirus 2019 (COVID-19). La diabetes es una de las comorbilidades más frecuentes en personas con COVID-19, con una prevalencia que varía según los estudios entre el 7 y el 30%. Los diabéticos infectados con SARS-CoV-2 tienen una tasa más alta de admisión hospitalaria, neumonía severa y mayor mortalidad en comparación con sujetos no diabéticos. La hiperglucemia crónica puede comprometer la inmunidad innata y la inmunidad humoral. Además, la diabetes se asocia con un estado inflamatorio crónico de bajo grado que favorece el desarrollo de una respuesta inflamatoria exagerada y, por tanto, la aparición del síndrome de distrés respiratorio agudo. Evidencia reciente ha demostrado que el SARS-CoV-2 también es capaz de producir un daño directo al páncreas, que podría empeorar la hiperglucemia e incluso inducir la aparición de diabetes en sujetos previamente no diabéticos. Las estrategias terapéuticas deben dirigirse a facilitar el acceso de los pacientes al sistema sanitario. El control de la glucemia y de las comorbilidades debe ser individualizado a fin de reducir la incidencia de complicaciones y disminuir la carga en los sistemas de salud. En este artículo revisaremos los mecanismos fisiopatológicos que explican la relación bidireccional entre COVID-19 y diabetes mellitus, su implicación en el pronóstico y el manejo de la hiperglucemia en este grupo de pacientes.
In December 2019 an acute respiratory disease outbreak started in China, characterised by fever, a dry cough and difficulty in breathing. One month later this was identified as a new coronavirus which was termed coronavirus 2 of the severe, acute respiratory syndrome (SARS-CoV-2), the causal agent of the disease from coronavirus 2019 (COVID-19).1
In general, people with diabetes are at greater risk of developing complications when they present with COVID-19.2,3 In Italy over two thirds of deaths associated with COVID-19 were observed in diabetic patients.4 This relationship between diabetes and mortality also appeared in previous epidemics caused by other coronavirus, such as that of the SARS in 2002 and the Middle East respiratory syndrome (MERS) in 2012.5
The development of diabetes in patients infected with SARS-CoV-2 has also been described, and it is therefore possible that SARS-CoV-2 may lead to changes in the metabolism of glucose which may lead to the appearance of diabetes mellitus.6 In this article we will review the pathophysiological mechanisms which explain the bidirectional relationship between COVID-19 and diabetes mellitus.
Diabetes mellitus as a risk factor for COVID-19Diabetics infected with SARS-CoV-2 have a higher ratio of hospital admission, severe pneumonia and greater mortality compared with non diabetic subjects infected with SARS-CoV-2.2,3 In fact, diabetes is a factor of bad prognosis in COVID-19, since a recent meta-analysis showed that diabetes increases by 2.3 times the risk of severity, and 2.5 times the risk of the COVID-197-associated death.
Acute respiratory distress syndrome (ARDS) is the main cause of death by COVID-19 and occurs as a consequence of an exaggerated inflammatory response leading to the release of pro-inflammatory cytokines such as interleukins (IL) and tumoral necrosis factor-alpha.3 Toll-like receptors [TLR]) are a family of proteins which act as sensors and help the immune system to discriminate between its own and foreign elements. SARS-CoV-1 and presumably SARS-CoV-2 interact with TLR in the host cell membrane and increase the expression of the primary response gene of myeloid (88 (MyD88) differentiation. This, in turn, activates the nuclear factor kappa beta, finally provoking an inflammatory cascade which increases lung damage.8
For its part, chronic hyperglycaemia may compromise innate immunity and humoral immunity. Diabetes is also associated with a chronic low grade inflammatory status which affects the regulation of glucose and the parietal sensitivity to insulin.9 In diabetic patients infected with SARS-CoV-2 an increase in IL-6 and reactive C protein (RCP) levels were in evidence, resulting in the actual pro-inflammatory status of the diabetes to promote the torment of cytokines and the systemic inflammatory response accompanying ARDS in patients with COVID-19.10
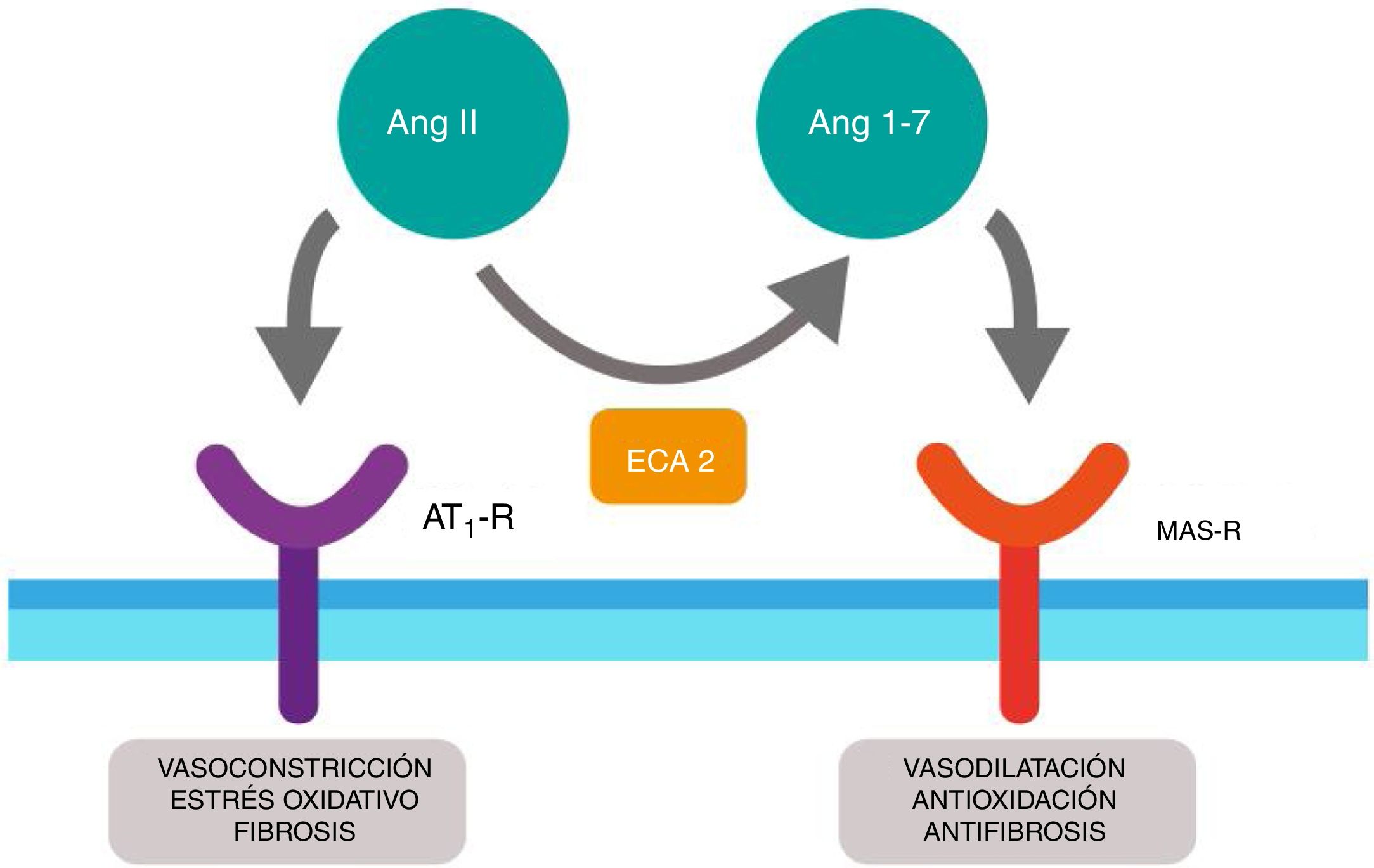
SARS-CoV-2 as a diabetogenic agentRenin-angiotensin systemThe renin-angiotensin system (RAS) is an elegant cascade of vasoactive peptides which orchestrate key processes in human physiology. The angiotensinogen produced in the liver is concealed by the action of the renin in a decapeptide called angiotensin (Ang) I and this in turn is converted by the angiotensin converting enzyme (ACE) into an octapeptide called Ang II which when acting on the Ang type I receptor (AT1-R), produces vasoconstrictor and oxidative effects and in the lungs induces contraction of the bronchial smooth muscle, a proliferation of fibroblasts, apoptosis of alveolar epithelial cells and increases vascular permeability.11,12
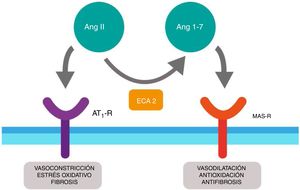
Furthermore, ACE2 is able to hydrolyze the Ang I and generate Ang (1–9); however, its catalytic activity is 400 times higher on Ang II and carries with it the formation of Ang (1–7) with vasodilation properties through the Mas (MAS-R)12 receptor. Thus, the RAS functions as a dual endocrine system whereby the vasoconstrictor/proliferative actions and the vasodilation/anti-proliferative actions are regulated by a balance between ACE and ACE2 (Fig. 1).
Duality of the renin-angiotensin (Ang.) system. When the angiotensin ii (Ang II) acts on the type 1 receptor of Ang (AT1-R) it causes vasoconstrictor and oxidative effects, and induces fibrosis. The angiotensin converting enzyme 2 (ACE2) converts the Ang II into Ang (1–7) with vasodilatory, antioxidant and antifibrosis properties through the Mas receptor (MAS-R).
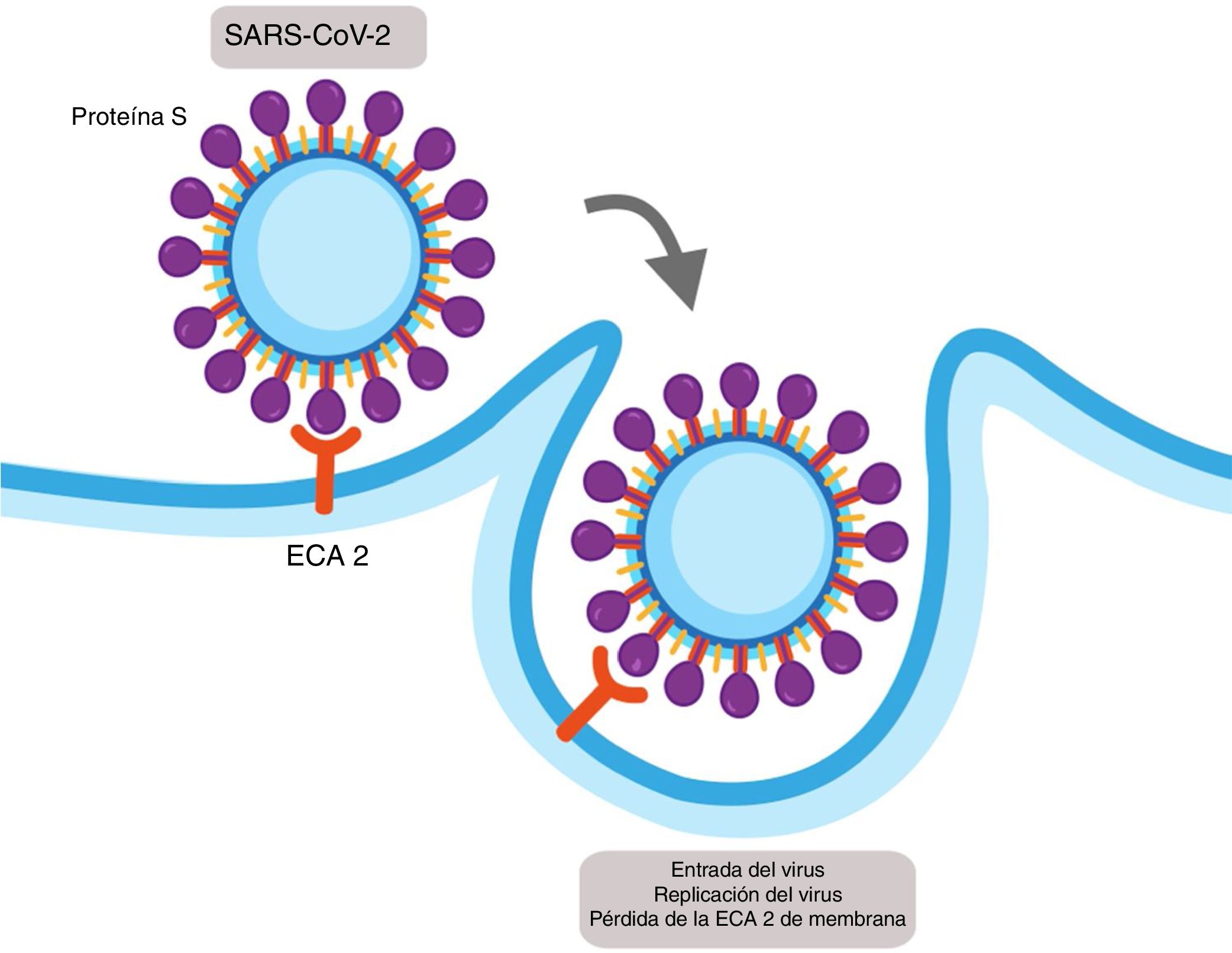
Viral infections depend on entry of the virus into the cell and the use of the cellular host mechanisms to replicate multiple copies which go on to infect more cells. Coronavirus SARS-CoV-1 and SARS-CoV-2 enter the host cells using the ACE2 as a functional receptor. ACE2 is expressed in alveolar epithelial cells type 1 and type 2 and has 2 fractions: one soluble and one bound to the membrane.13
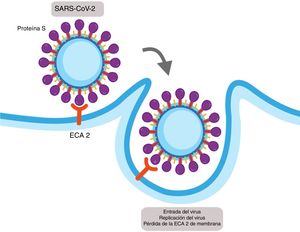
SARS-CoV-1 and SARS-CoV-2 express a protein in their structure called protein S, which contains a region of union that binds with high affinity to the extracellular domain of ACE2 leading to the fusion of the membrane and the internalisation of the virus by endocytosis (Fig. 2).14 The internalisation of the ACE2 by SARS-CoV-2 results in a loss of the ACE2 in the surface area of the cell and therefore avoids degradation of the Ang II in Ang (1–7), which could contribute to the lung damage and fibrosis associated with COVID-19.15
Mechanism of cellular infection of SARS-CoV-2. Structurally SARS-CoV-2 expresses a protein called protein S which binds with high affinity to the extracellular domain of the angiotensin converter 2 (ACE2) provoking the fusion of the membrane and the internalisation on the virus by endocytosis. This results in a loss of the ACE2 on the cell surface and also the entry of the virus allows for its replication.
Many viruses, such as Coxsackie B, enterovirus, rubeola, cytomegalovirus, Epstein–Barr virus and the varicela-zoster virus, have been implicated in the development of diabetes type 1.16 In fact, there is serological evidence of infection and isolation of the virus in the pancreas in patients with recent onset diabetes, and it is therefore possible that some viruses may act as diabetogenic agents.16
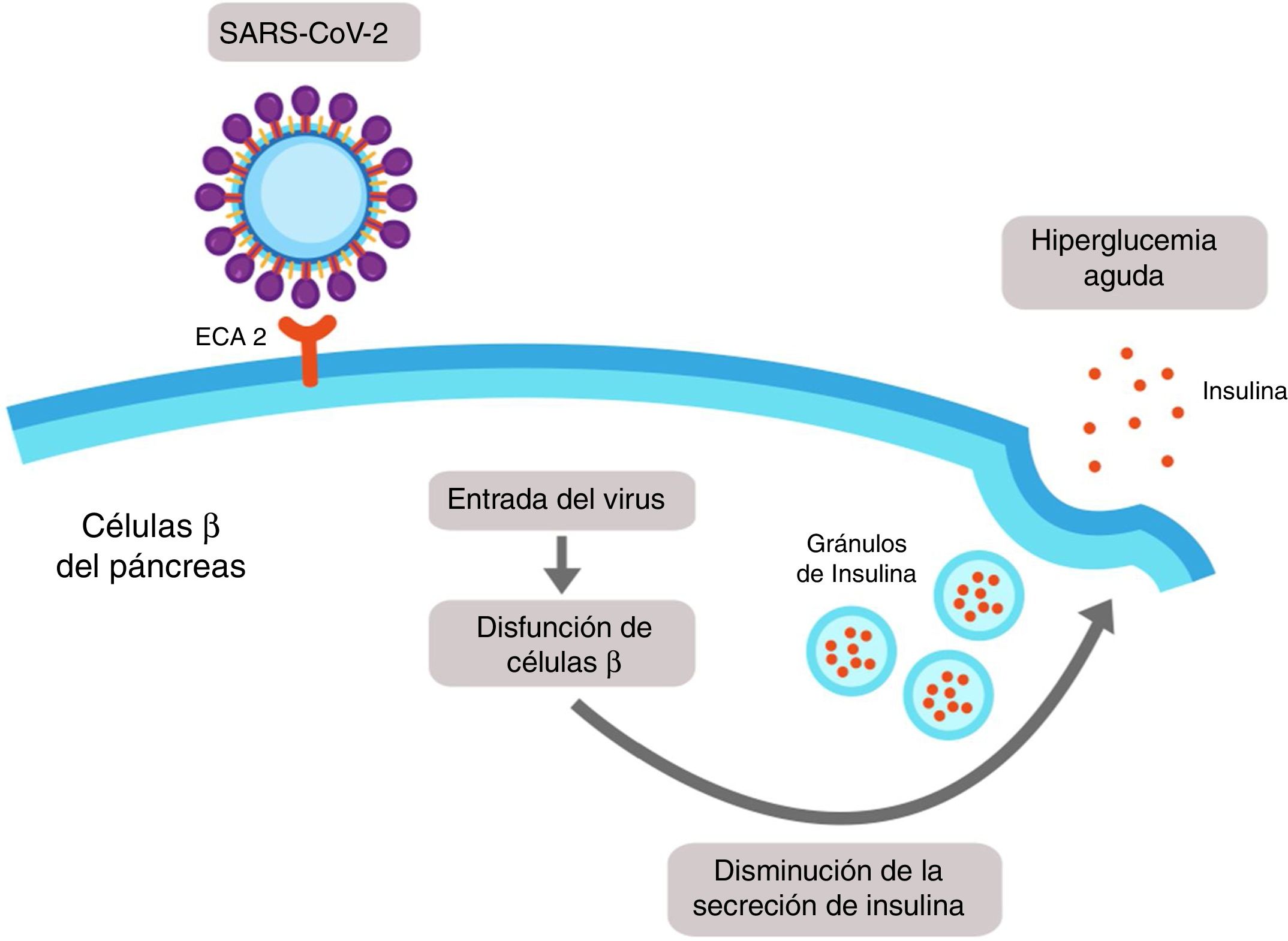
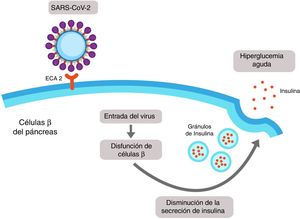
It has recently been demonstrated that expression of ACE2 in the pancreas (mainly in islet cells) is even higher than in the lungs, and it is therefore possible that SARS-CoV-2 may bind to this receptor and enter the β cells of the pancreas, producing cellule dysfunction with acute hyperglycaemia (Fig. 3).17
SARS-CoV-2-induced pancreatic damage. The islet cells of the pancreas express the angiotensin-converting enzyme 2 (ACE2) in its membrane. SARS-CoV-2 binds to the extracellular domain of the ACE2 and enters into the β cell of the pancreas, where it induces cellular dysfunction which could lead to a reduction in the secretion of insulin and finally to hyperglycaemia.
It is of note that only 1–2% of patients with mild infection by COVID-19 present with pancreatic lesions, whilst 17% of patients with severe cases present with pancreatic lesions, and this may emphasis the systemic inflammatory response and thereby accelerated the appearance of ARDS.17
Therapeutic considerations in the management of diabetic patients with COVID-19The impact of oral hypoglycaemics in COVID-19Appropriate control of hyperglycaemia has been shown to lead to a lower rate of adverse events in patients with diabetes mellitus and COVID-19.18 Metformin is the first line drug in the management of diabetes type 2 and improves sensitivity to insulin through the activation of the AMP dependent protein (AMPK) in the liver.19 It has been suggested that metformin could be useful in COVID-19 because activation of AMPK leads to the phosphorylation of ACE2 and therefore generates functional changes which reduce the binding of the SARS-CoV-2 to the receptor.20 In contrast, the agonists of the peptide receptor similar to glucagon type 1 and the inhibitors of the co-transporter of type 2 sodium glucose may induce an over expression of ACE2, and they therefore may be inadequate in dietetic patients infected with SARS-CoV-2. However, they have a proven benefit in the prevention of cardiovascular and renal disease, and should therefore not be ruled out.21 Recently, it has been described that, based on its immunomodulator effect, the inhibitor of dipeptidyl peptidase 4 may reduce the severity of infection by SARS-CoV-29 and the thiazolidinediones are able to reduce the production of pro-inflammatory cytokines, such as that of IL-6, which may improve the prognosis of diabetic patients infected with SARS-CoV-2.22
Factors impacting metabolic control during the COVID-19 pandemicDiabetic patients are more susceptible to developing psychological stress, anxiety and depression.23 In diabetics stress is associated with a poorer metabolic control, which includes a higher level of glucosylated haemoglobin, a higher body mass index and raised blood pressure.23
The current scenario of the pandemic, even in non infected subjects, may promote the deterioration of metabolic control through the difficulties of access to the healthcare system, the lack of physical activity and the increase in stress associated with lockdown. Therapeutic tragedies should be aimed at facilitating access to the healthcare system through telemedicine so as to advise patients on how to adapt treatment, or on any other medical situation to guide patients and carers in the control of diabetes, so as to prevent hospitalisation (Table 1).24 Telemedicine in this context, apart from distanced medical care, would allow for strengthening the education of the diabetic patient, increasing medical education and exchanging information between specialists and even promoting clinical research with other healthcare centres.
Outpatient management of patients with diabetes mellitus and COVID-19.
| Measures | |
|---|---|
| Prevention of the infection | Intensify prevention measures (social distancing, mask, hand hygiene) |
| Healthy lifestyle | Healthy diet, physical exercise, not smoking |
| General measures for improving the control of diabetes | Weight control, adequate hydration, more frequent monitoring of glycaemia, inventory of monitoring and drug material, family and psycho-emotional support |
| Treatment of hyperglycaemia | Improving the HbA1c, glycaemia, revaluing pharmacological treatment with their physician, avoiding hypoglycaemias |
| Treatment of comorbidities | Blood pressure, cholesterol and triglyceride control. Heart care, kidney function feet, eyes |
| Healthcare support | Appropriate and permanent contact with their doctors, using telemedicine or virtual medicine, consulting serious and credible sources (WHO, PAHO, ADA, EASD, ALAD, VSEM, etc.). Hospitals only if necessary |
ADA: American Association of Diabetes; ALAD: Latin American Diabetes Association; COVID-19: coronavirus disease 2019; EASD: European Association for the Study of Diabetes; HbA1c: glycosated haemoglobin A1c; PAHO: Pan-American Health Organisation; VSEM: Venezuelan Society of Endocrinology and Metabolism; WHO: World Health Organisation.
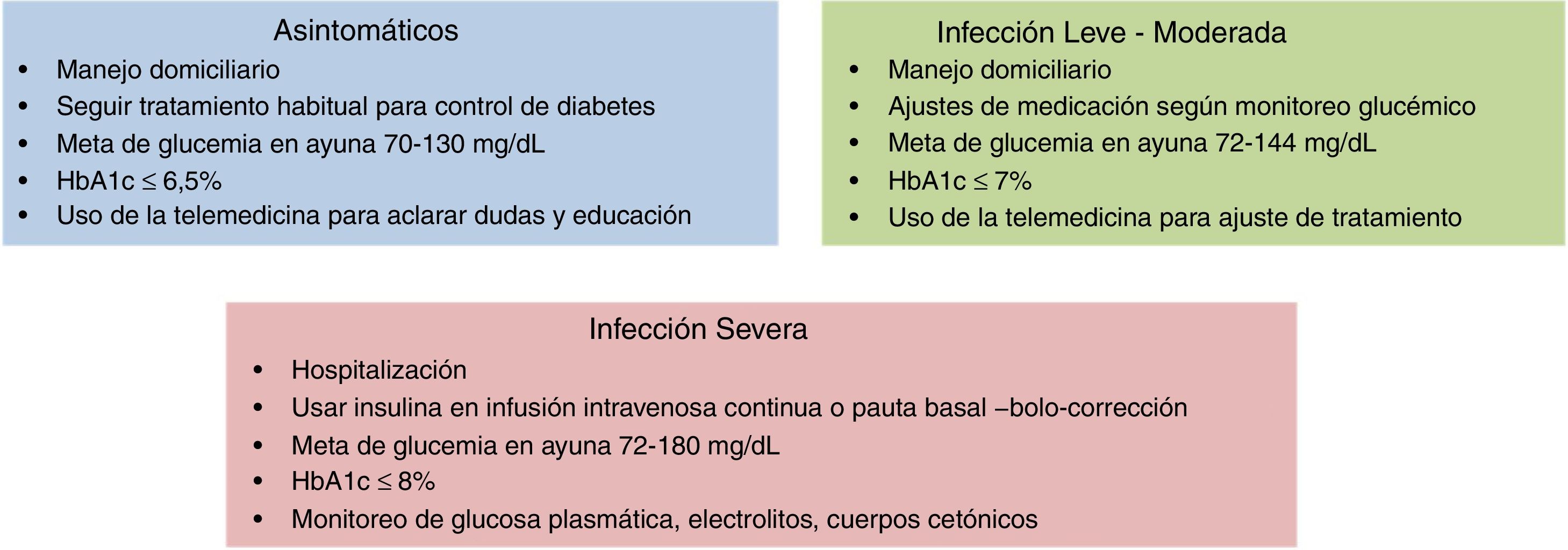
Treatment of diabetics infected with SARS-CoV-2 is basically the same as usual, but it is important to make certain considerations. If the person is asymptomatic and maintains a good glycaemic control, no changes to their medication should be made. If the diabetic contracts COVID-19 and it develops into a mild infection, with no complications, a simple adjustment to medication according to the directives of glycaemic monitoring could be sufficient. Where the evolution of the condition is severe, and breathing difficulty ensues or they are referred for hospitalisation, treatment must be reassessed, taking into account several special considerations for each drug (Table 2). Severe cases must be treated with insulin. The most effective and safest insulin administration guidelines are continuous intravenous administration in critical patients and the administration of insulin in baseline-bolus-correction factor guideline, adapted to type of nutrition in non critical patients.25
Special considerations of the drugs for diabetes mellitus in COVID-19.
| Drug | Considerations in COVID-19 |
|---|---|
| Metformin | Risk of lactic acidosis especially in renal patients, hepatic patients or if dehydration takes place. Avoid in severely ill patients. |
| ISGLT2 | Increased risk of dehydration, deterioration of renal function and ketoacidosis. Suspend in severely ill patient. |
| GLP-1 ARs | Potential gag reflex. Monitor hydration. |
| IDPP-4 | In general, quite safe |
| Sulfonylureas | High risk of hypoglycaemias. Moderate risk |
| Insulin | Drug of choice in diabetes type 1 and decompensated type 2. Drug of choice in severe diabetics or with COVID-19 complications. Need for very high doses in some cases. |
COVID-19: coronavirus disease 2019; GLP-1 ARs: glucagon-like peptide-1 receptor agonists; IDPP-4: Dipeptidyl-4 inhibitors; ISGLT2: sodium-glucose cotransporter 2 inhibitors.
Controversy exists regarding the glycaemic control goal in diabetics with COVID-19, and it should therefore be individualised. In diabetic patients with mild to moderate infection from COVID-19 who are not hospitalised, a goal of 72–144mg/dl has been proposed and in hospitalised patients that of 72–180mg/dl26 (Fig. 4). However, specific treatment of COVID-19 is similar in diabetics and non diabetics. Given the higher frequency of severe progression in hyperglycaemic conditions, a more intensive approach in patients with diabetes is accepted.
High blood pressure managementAppropriate management of all comorbidities presented is important. In particular, the management of blood pressure is key in diabetics with COVID-19.18 Controversy arises regarding the use of ACE inhibitors and angiotensin receptor blockers (ATB) in patients with COVID-19, since these drugs may increase the expression of the ACE2 and, therefore facilitate the virus entering into the cells.13,15 Despite this, several scientific societies and the European Medicines Agency have highlighted that there is insufficient evidence to justify the omission of these drugs in patients with COVID-19. Furthermore, recent studies have proven drug safety and even a potential benefit with the use of them.27,28
Anticoagulation in the diabetic with COVID-19Inflammation markers (RCP, erythrocyte sedimentation rate, IL-1, IL-6, ferritin) and hypercoagulability markers (D-dimer) are usually higher in patients with diabetes mellitus. This rationally supports the use of anti-inflammatory drugs and cytokine blockers, many of them experimental, with compassionate purposes in diabetic patients.
Diabetic patients have a propensity to develop thrombosis and in the context of infection by SARS-CoV-2 have a higher risk of thromboembolic events, which could justify treatment with anticoagulants.29 In diabetic patients hospitalised due to COVID-19 the use of prophylactic doses of low molecular weight heparin are suggested in the absence of contraindications (active bleeding or a platelet count of ˂25×109/l), with an adjustment of dose for patients with frank elevation of D-dimer and those who present with criteria of severity. The studies derived from COVID-19 use 40–60mg/day of enoxaparin for at least 7 days. The use of low molecular weight heparin reduces the generation of thrombin, has anti-inflammatory properties and reduces the appearance of a venous thromboembolic event.30
ConclusionsThere is a bidirectional relationship between COVID-19 and diabetes mellitus. On the one hand, people with diabetes have a higher risk of developing complications when they present with COVID-19 and, on the other, SARS-CoV-2could act as a diabetogenic agent on binding to the ACE2 in beta cells of the pancreas, causing acute dysfunction and changes to glucose. Up until now, no clear data has existed on the impact of this pandemic on chronic complications associated with diabetes; however, it is essential to optimise the metabolic management of patients in order to improve prognosis and reduce the healthcare system burden.
Please cite this article as: Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 y diabetes mellitus: una relación bidireccional. Clin Investig Arterioscler. 2021;33:151–157.