Parathormone (PTH) is a component of the Mineral Metabolism (MM) system that has been shown recently to add prognostic value in pts. with stable coronary artery disease (SCAD) and average renal function. However, the influence of renal function on the prognostic role of PTH in pts. with SCAD has not been shown yet.
PurposeTo assess the influence of estimated glomerular filtration rate (eGFR) on the prognostic role of PTH and other MM markers in pts. with SCAD.
MethodsWe analyzed the prognostic value of MM markers (PTH, klotho, phosphate, calcidiol [25-hydroxyvitamin D], and fibroblast growth factor-23 [FGF-23]) in 964 pts. with SCAD and eGFR < 60 mL/min/1.73 m2 (LGFR) vs pts. with eGFR ≥ 60 mL/min/1.73 m2 (HGFR) included in five hospitals of Madrid. The main outcome was the combination of death with ischemic events (any acute coronary syndrome, ischemic stroke or transient ischemic attack). eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI).
ResultsAge was 60.0 (52.0–72.0) years, 76.2% of patients were men, and eGFR was 80.4 (65.3–93.1) ml/min/1,73 m2. Median follow-up was 5.39 (2.81–6.92) years. There were 790 pts. with HGFR and 174 with LGFR. In HGFR pts., predictors of ischemic events or death were plasma levels of calcidiol [HR = 0.023 (0.94−0.99) P = .023], FGF-23 [HR = 1.00 (1.00–1.003) P = .036], non-HDL cholesterol [HR = 1.01 (1.00−1.01) P = .026] and high sensitivity troponin I [HR = 5.12 (1.67–15.59) P = .004], along with age [HR = 1.03 (1.01−1.05) P = .01], treatment with statins [HR = 0.36 (0.19−0.68) P = .002], nitrates [HR = 1.13 (1.07−2.79) P = .027], dihydropyridines [HR = 1.71 (1.05−2.77) P = .032], verapamil [HR = 5.71 (1.35–24.1) P = .018], and proton-pump inhibitors [HR = 2.23 (1.36−3.68) P = .002]. In the LGFR subgroup, predictors of death or ischemic events were PTH plasma levels, [HR = 1.01 (1.00−1.01) P = .005], eGFR [HR = 0.96 (0.94−0.99) P = .004], age [HR = 1.06 (1.02−1.10) P = .003], caucasian race [HR = 0.04 (0.004−0.380) P = .005], and treatment with insulin [HR = 2.6 (1.20–5.63) P = .015].
ConclusionsIn pts. with SCAD, PTH is an independent predictor of poor outcomes only in those with eGFR < 60 mL/min/1.73 m2, while in pts. with eGFR ≥ 60 mL/min/1.73 m2 calcidiol and FGF-23 become the only components of MM that may predict prognosis. Then, renal function influences the predictive power of MM markers in pts. with SCAD.
La parathormona (PTH) es un componente del metabolismo mineral (MM) que ha demostrado aportar valor pronóstico en los pacientes con cardiopatía isquémica crónica (CIC) y función renal preservada. Sin embargo, la influencia de la función renal en el papel pronóstico de la PTH en los pacientes con CIC aún no se ha demostrado.
ObjetivoEvaluar la influencia del filtrado glomerular renal estimado (FGRe) sobre el papel pronóstico de la PTH y otros marcadores del MM en los pacientes con CIC.
MétodosAnalizamos el valor pronóstico de distintos componentes del MM (PTH, klotho, fósforo, calcidiol y factor de crecimiento de fibroblastos-23 [FGF-23]) en 964 pacientes con CIC incluidos en cinco hospitales de Madrid en función de si presentaban FGRe < 60 mL/min/1,73 m2 (LFGR) o FGRe ≥ 60 mL/min/1,73 m2 (HFGR). El objetivo primario fue la combinación de muerte con eventos isquémicos (cualquier síndrome coronario agudo, accidente cerebrovascular isquémico o accidente isquémico transitorio). El FGR se calculó mediante el método CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration equation).
ResultadosLa edad era de 60,0 (52,0–72,0) años y el 76,2% de los casos eran varones, con una mediana del FGRe de 80,4 (65,3–93,1) ml/min/1,73 m2. El seguimiento fue de 5,39 (2,81–6,92) años. 790 pacientes presentaron HGFR y 174 LGFR. En pacientes con HFGR, los predictores del endpoint combinado fueron los niveles plasmáticos de calcidiol [HR = 0,023 (0,94–0,99) P = ,023], FGF23 [HR = 1,00 (1,00–1,003) P = ,036], colesterol no HDL [ HR = 1,01 (1,00–1,01) P = ,026] y troponina de alta sensibilidad [HR = 5,12 (1,67–15,59) P = ,004], junto con la edad [HR = 1,03 (1,01–1,05) P = ,01], el tratamiento con estatinas [HR = 0,36 (0,19–0,68) P = ,002], nitratos [HR = 1,13 (1,07–2,79) P = ,027], dihidropiridinas [HR = 1,71 (1,05–2,77) P = ,032], verapamilo [HR = 5,71 (1,35–24,1) P = ,018] e inhibidores de la bomba de protones [HR = 2,23 (1,36–3,68) P = ,002]. En el subgrupo LFGR, los predictores de muerte o eventos isquémicos fueron los niveles plasmáticos de PTH, [HR por cada 10 unidades de PTH = 1,01 (1,02–1,11) P = ,008], el FGRe [HR = 0,96 (0,94–0,99) P = ,001], la edad [HR = 1,06 (1,02–1,10) P = ,003], la raza caucásica [HR = 0,04 (0,004–0,355) P = ,004], y el tratamiento con insulina [HR = 2,34 (1,11–4,95) P = ,026].
ConclusionesEn pts. con CIC, la PTH es un predictor independiente de mala evolución sólo en aquellos con FGRe < 60 mL/min/1,73 m2, mientras que en los pts. con FGRe ≥ 60 mL/min/1,73 m2, el calcidiol y el FGF-23 se convierten en los únicos componentes del MM que pueden predecir el pronóstico. Por lo tanto, la función renal influye en el poder predictivo de los componentes del MM en los pacientes con CIC.
Mineral metabolism (MM) comprises several components, such as calcidiol or vitamin D, fibroblast growth factor 23 (FGF23), parathormone (PTH), and phosphorus, which may be related to the incidence of cardiovascular disease. Vitamin D has attracted attention due to its association with the development of cardiovascular disease, as vitamin D deficiency is associated with an increased incidence of adverse cardiovascular events.1,2 FGF23 is a phosphaturic hormone that helps the diseased kidney eliminate phosphorus and reduces excessive vitamin D levels.3 High plasma levels of FGF23 have been associated with increased mortality, heart failure, and left ventricular hypertrophy.4,5 Similarly, increased plasma PTH levels are associated with hypertension, left ventricular hypertrophy and an increase in cardiovascular events.6–9 The soluble form of klotho, the co-receptor of FGF23, has recently been associated with cardiorenal protective effects and has even been attributed with anti-ageing properties.10,11 Despite this evidence, there are no studies that explore the influence of renal function on the prognostic role of all these components of MM in patients with chronic ischaemic heart disease (SCAD). Therefore, the aim of this study was to evaluate the influence of estimated renal glomerular filtration rate (eGFR) on the prognostic role of PTH and other MM markers in patients with SCAD.
Material and methodsPatientsThis study included 969 patients with SCAD, who had experienced an acute coronary syndrome 6–12 months earlier. These patients were part of the BACS & Biomarkers in Acute Coronary Syndrome & Biomarkers in Acute Myocardial Infarction (BAMI) studies, conducted in 5 hospitals in the Community of Madrid. The inclusion and exclusion criteria have been defined.12 Between July 2006 and June 2014, 2740 patients were discharged from the hospitals with a diagnosis of non-ST-elevation acute coronary syndrome (NSTE ACS) or acute ST-elevation myocardial infarction (STEMI). We excluded 1483 patients due to: age over 85 years (16.4%), presence of toxic disorders or habits limiting survival (29.8%), inability to perform cardiac revascularisation (9.6%), coexistence of other significant heart disease (5.7%), no follow up (11.9%), concomitant mental disorders (4.4%), clinical instability beyond the sixth day after the index event (10.9%), refusal to participate in the study (1.5%), and impossibility for the investigators to include the patient (9.8%). A total of 1257 was eventually included. The clinical variables were recorded on admission, and blood plasma was collected for analysis.
The patients had a second visit 6–12 months after hospital discharge, and clinical variables were again collected, and plasma collected for the second time. The present work is a substudy of the BACS & BAMI study, and reports data on clinical and analytical findings obtained at the time of this second plasma collection, relating them to data obtained during subsequent follow-up. Of the 1257 patients included in the acute phase, 284 did not attend the hospital for the second plasma collection and 4 were excluded because they developed cancer before this second visit. Therefore, 969 patients had plasma samples that were suitable for the present analysis. Plasma collection and baseline visits were performed between January 2007 and December 2014. The final visits were conducted in June 2016.
Ethics statementThe study protocol was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the human research committees of the institutions participating in this study: Fundación Jiménez Díaz, Hospital Fundación Alcorcón, Hospital de Fuenlabrada, Hospital Universitario Puerta de Hierro-Majadahonda, and Hospital Universitario de Móstoles. All the patients signed their informed consent.
Study designClinical variables were recorded, and venous blood samples were drawn after fasting for 12 h, and collected in an EDTA tube between 6 and 12 months after discharge from hospital for the acute ischaemic event. The blood samples were centrifuged at 2500 g/d for 10 min, and the plasma was stored at −80 °C. The patients received care in their hospital according to care protocols. At the end of follow-up, medical records were reviewed, and patient status was confirmed by telephone.
The population was divided according to whether or not they had an eGFR < 60 mL/min/1.73 m2 and we looked for variables associated with poor prognosis in each group.
The primary endpoint was the combination of acute ischaemic events (any acute coronary syndrome, stroke, and transient ischaemic attack) and all-cause mortality. NSTE ACS was defined as angina at rest lasting more than 20 min in the previous 24 h, or new-onset class III-IV angina, together with transient ST-segment depression or T-wave inversion on the electrocardiogram considered diagnostic by the treating cardiologist and/or troponin elevation. A diagnosis of STEMI required a picture compatible with angina of more than 20 min duration and ST elevation in 2 adjacent ECG leads with no response to nitroglycerin and troponin elevation. Stroke was defined as the rapid onset of a neurological deficit attributable to a focal vascular cause lasting more than 24 h or with evidence of new cerebral ischaemic lesions on imaging studies. A transient ischaemic attack was defined as a transient stroke with signs and symptoms that resolved in under 24 h without acute cerebral ischaemic lesions on imaging techniques. Events were ratified by at least two study investigators, together with a neurologist for the diagnosis of cerebrovascular events. Although all events were recorded for each case, patients were excluded from the Cox regression analysis after the first event. Thus, although the total number of events is also described, patients who had more than one event were counted only once for these analyses.
Biomarker and analytical studiesThe plasma tests were performed in the Nephrology Laboratory of the Hospital Gómez Ulla and the Biochemistry Laboratory of the Fundación Jiménez Díaz. The investigators who performed the laboratory studies were unaware of the clinical data. Plasma calcidiol levels were quantified by chemiluminescent immunoassay (CLIA) on the LIAISON® XL (LIAISON® 25OH-Vitamin D total Assay, DiaSorin, Saluggia, Italy), FGF23 levels were measured by an enzyme-linked immunosorbent assay that recognises epitopes within the carboxyl-terminal portion of FGF23 (Human FGF23, C-Term, Immutopics Inc, San Clemente, CA, USA), klotho levels were quantified by ELISA (Human soluble klotho alpha assay kit, Immuno-Biological Laboratories Co, Japan), intact PTH was analysed by a second generation automated chemiluminescence method (Elecsys® 2010 platform, Roche Diagnostics, Mannheim, Germany), phosphate was determined by an enzymatic method (Integra® 400 analyser, Roche Diagnostics, Mannheim, Germany), high-sensitivity troponin (hsTn) was assessed by direct chemiluminescence (ADVIA® Centaur; Siemens, Berlin, Germany), the amino-terminal portion of pro-BNP (NT-pro-BNP) was determined by immunoassay (VITROS®, Ortho Clinical Diagnostics, USA), and high-sensitivity C-reactive protein (CRP) was assessed by latex-enhanced immunoturbidimetry (ADVIA® 2400 Chemistry System, Siemens, Germany). Proprotein convertase subtilisin/kexin type 9 (PCSK9) was determined in duplicate by ELISA with specific anti-PCSK9 antibodies (ELLA® kit, R&D Systems). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI).
Statistical analysisThe quantitative data are shown as median (interquartile range) and qualitative variables as percentages. Normality of variables was assessed using the Kolmogorov-Smirnov or Shapiro-Wilk test depending on the sample size of each variable. To compare baseline values between the 2 groups according to whether or not they presented an eGFR < 60 mL/min/1.73 m2, Pearson's χ2 test or Fisher's exact test was used for qualitative variables. For quantitative variables, the Student's t-test or Mann-Whitney test was used, depending on whether or not the distribution was normal, respectively. The population was divided according to whether or not they had an eGFR < 60 mL/min/1.73 m2 and univariate Cox regression was performed to analyse the variables that were associated with the development of the different outcomes in each group. A multivariate regression analysis was then performed in both groups including those variables that reached a P < .20 in the univariate analyses. Analyses were performed with IBM® SPSS® Statistics for Windows, version 19.0. Armonk, NY: IBM Corp. and were considered significant at a P-value less than .05 (2-tailed).
ResultsPatientsOf the 969 patients included 5 were lost to follow-up, leaving 964 patients for the study. The age was 60.0 (52.0–72.0) years, 76.2% of cases were male and the median eGFR was 80.4 (65.3–93.1) ml/min/1.73 m2. Time since the previous acute coronary syndrome was 6.5 (6.2–7.6) months.
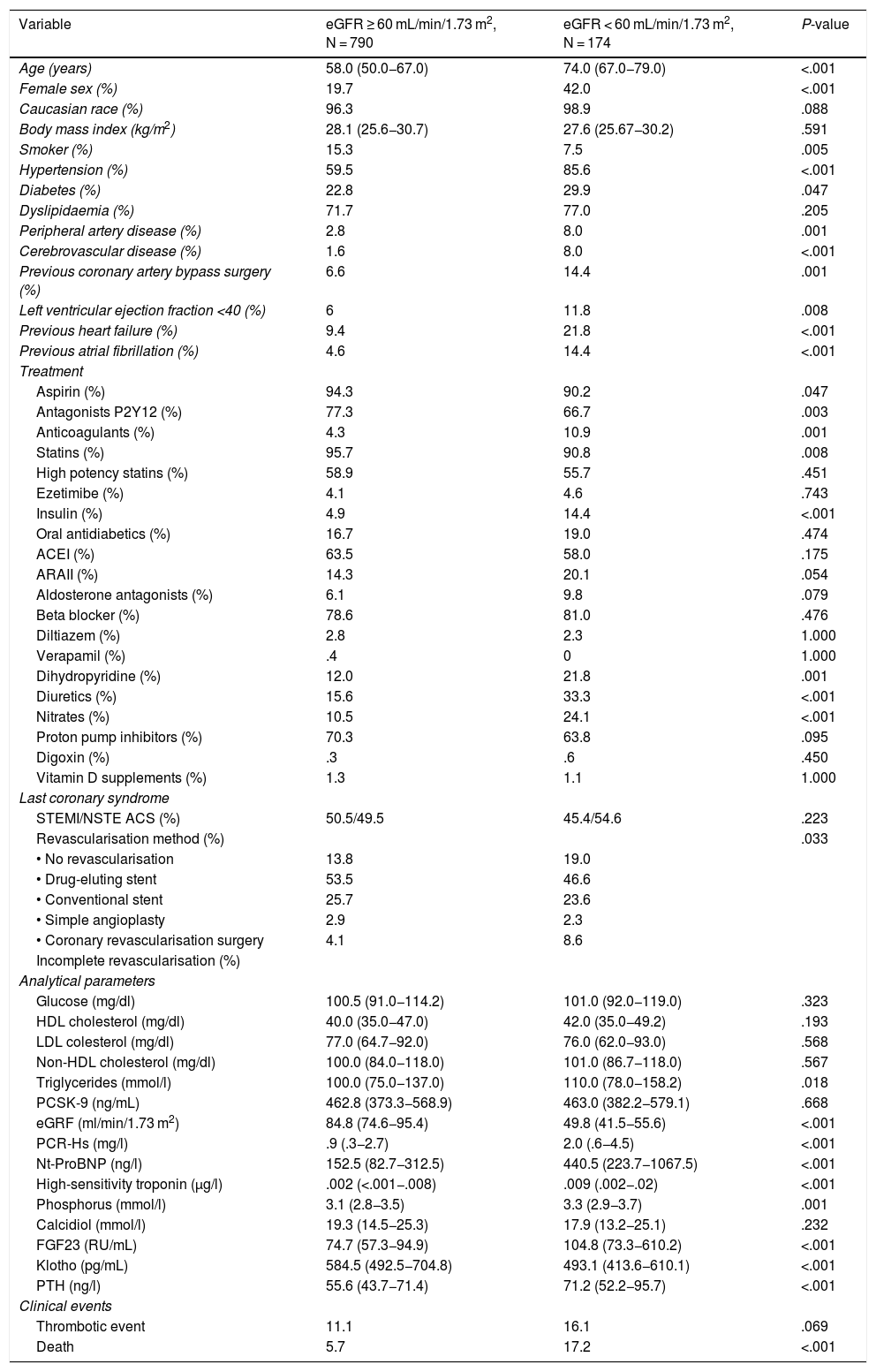
Table 1 details baseline population characteristics according to eGFR. The patients with an eGFR < 60 mL/min/1.73 m2 were older, a higher percentage were women, and they had a higher rate of comorbidities (hypertension, diabetes, peripheral arterial disease, ventricular dysfunction, heart failure, and atrial fibrillation). Analytically, they had higher levels of triglycerides, CRP, high-sensitivity troponin I, NT-ProBNP, phosphorus, FGF23 and PTH, with lower levels of klotho.
Baseline characteristics of the patients according to their renal function.
| Variable | eGFR ≥ 60 mL/min/1.73 m2, N = 790 | eGFR < 60 mL/min/1.73 m2, N = 174 | P-value |
|---|---|---|---|
| Age (years) | 58.0 (50.0−67.0) | 74.0 (67.0−79.0) | <.001 |
| Female sex (%) | 19.7 | 42.0 | <.001 |
| Caucasian race (%) | 96.3 | 98.9 | .088 |
| Body mass index (kg/m2) | 28.1 (25.6−30.7) | 27.6 (25.67−30.2) | .591 |
| Smoker (%) | 15.3 | 7.5 | .005 |
| Hypertension (%) | 59.5 | 85.6 | <.001 |
| Diabetes (%) | 22.8 | 29.9 | .047 |
| Dyslipidaemia (%) | 71.7 | 77.0 | .205 |
| Peripheral artery disease (%) | 2.8 | 8.0 | .001 |
| Cerebrovascular disease (%) | 1.6 | 8.0 | <.001 |
| Previous coronary artery bypass surgery (%) | 6.6 | 14.4 | .001 |
| Left ventricular ejection fraction <40 (%) | 6 | 11.8 | .008 |
| Previous heart failure (%) | 9.4 | 21.8 | <.001 |
| Previous atrial fibrillation (%) | 4.6 | 14.4 | <.001 |
| Treatment | |||
| Aspirin (%) | 94.3 | 90.2 | .047 |
| Antagonists P2Y12 (%) | 77.3 | 66.7 | .003 |
| Anticoagulants (%) | 4.3 | 10.9 | .001 |
| Statins (%) | 95.7 | 90.8 | .008 |
| High potency statins (%) | 58.9 | 55.7 | .451 |
| Ezetimibe (%) | 4.1 | 4.6 | .743 |
| Insulin (%) | 4.9 | 14.4 | <.001 |
| Oral antidiabetics (%) | 16.7 | 19.0 | .474 |
| ACEI (%) | 63.5 | 58.0 | .175 |
| ARAII (%) | 14.3 | 20.1 | .054 |
| Aldosterone antagonists (%) | 6.1 | 9.8 | .079 |
| Beta blocker (%) | 78.6 | 81.0 | .476 |
| Diltiazem (%) | 2.8 | 2.3 | 1.000 |
| Verapamil (%) | .4 | 0 | 1.000 |
| Dihydropyridine (%) | 12.0 | 21.8 | .001 |
| Diuretics (%) | 15.6 | 33.3 | <.001 |
| Nitrates (%) | 10.5 | 24.1 | <.001 |
| Proton pump inhibitors (%) | 70.3 | 63.8 | .095 |
| Digoxin (%) | .3 | .6 | .450 |
| Vitamin D supplements (%) | 1.3 | 1.1 | 1.000 |
| Last coronary syndrome | |||
| STEMI/NSTE ACS (%) | 50.5/49.5 | 45.4/54.6 | .223 |
| Revascularisation method (%) | .033 | ||
| • No revascularisation | 13.8 | 19.0 | |
| • Drug-eluting stent | 53.5 | 46.6 | |
| • Conventional stent | 25.7 | 23.6 | |
| • Simple angioplasty | 2.9 | 2.3 | |
| • Coronary revascularisation surgery | 4.1 | 8.6 | |
| Incomplete revascularisation (%) | |||
| Analytical parameters | |||
| Glucose (mg/dl) | 100.5 (91.0−114.2) | 101.0 (92.0−119.0) | .323 |
| HDL cholesterol (mg/dl) | 40.0 (35.0−47.0) | 42.0 (35.0−49.2) | .193 |
| LDL colesterol (mg/dl) | 77.0 (64.7−92.0) | 76.0 (62.0−93.0) | .568 |
| Non-HDL cholesterol (mg/dl) | 100.0 (84.0−118.0) | 101.0 (86.7−118.0) | .567 |
| Triglycerides (mmol/l) | 100.0 (75.0−137.0) | 110.0 (78.0−158.2) | .018 |
| PCSK-9 (ng/mL) | 462.8 (373.3−568.9) | 463.0 (382.2−579.1) | .668 |
| eGRF (ml/min/1.73 m2) | 84.8 (74.6−95.4) | 49.8 (41.5−55.6) | <.001 |
| PCR-Hs (mg/l) | .9 (.3−2.7) | 2.0 (.6−4.5) | <.001 |
| Nt-ProBNP (ng/l) | 152.5 (82.7−312.5) | 440.5 (223.7−1067.5) | <.001 |
| High-sensitivity troponin (μg/l) | .002 (<.001−.008) | .009 (.002−.02) | <.001 |
| Phosphorus (mmol/l) | 3.1 (2.8−3.5) | 3.3 (2.9−3.7) | .001 |
| Calcidiol (mmol/l) | 19.3 (14.5−25.3) | 17.9 (13.2−25.1) | .232 |
| FGF23 (RU/mL) | 74.7 (57.3−94.9) | 104.8 (73.3−610.2) | <.001 |
| Klotho (pg/mL) | 584.5 (492.5−704.8) | 493.1 (413.6−610.1) | <.001 |
| PTH (ng/l) | 55.6 (43.7−71.4) | 71.2 (52.2−95.7) | <.001 |
| Clinical events | |||
| Thrombotic event | 11.1 | 16.1 | .069 |
| Death | 5.7 | 17.2 | <.001 |
Clinical and analytical characteristics of patients according to glomerular filtration function.
ACEI: Angiotensin-converting enzyme inhibitor; ARA II: Angiotensin II receptor blocker; eGFR: Estimated renal glomerular filtration rate; FGF23: Fibroblast growth factor 23; HDL: High density lipoprotein; LDL: Low density lipoprotein; NSTE ACS: Non-ST elevation-acute coronary syndrome; NT-ProBNP: Amino-terminal portion of pro BNP; PCSK-9: Proprotein convertase subtilisin/kexin type 9; PTH: Parathyroid hormone; STEMI: ST-elevation myocardial infarction.
Over a median follow-up of 5.39 (2.81–6.92), 168 patients developed the primary endpoint with a total of 191 events. There were 116 patients who developed an ischaemic event and 75 died. Twenty-three patients died in addition to having an ischaemic event.
Of the 75 deaths observed during follow-up, 28 were of cardiovascular origin, 15 cancer, 9 infection, 3 renal failure, 3 advanced cognitive impairment, 2 pancreatitis, 2 gastrointestinal bleeding, 2 exacerbation of pulmonary disease, 3 other causes and 8 were of unknown origin.
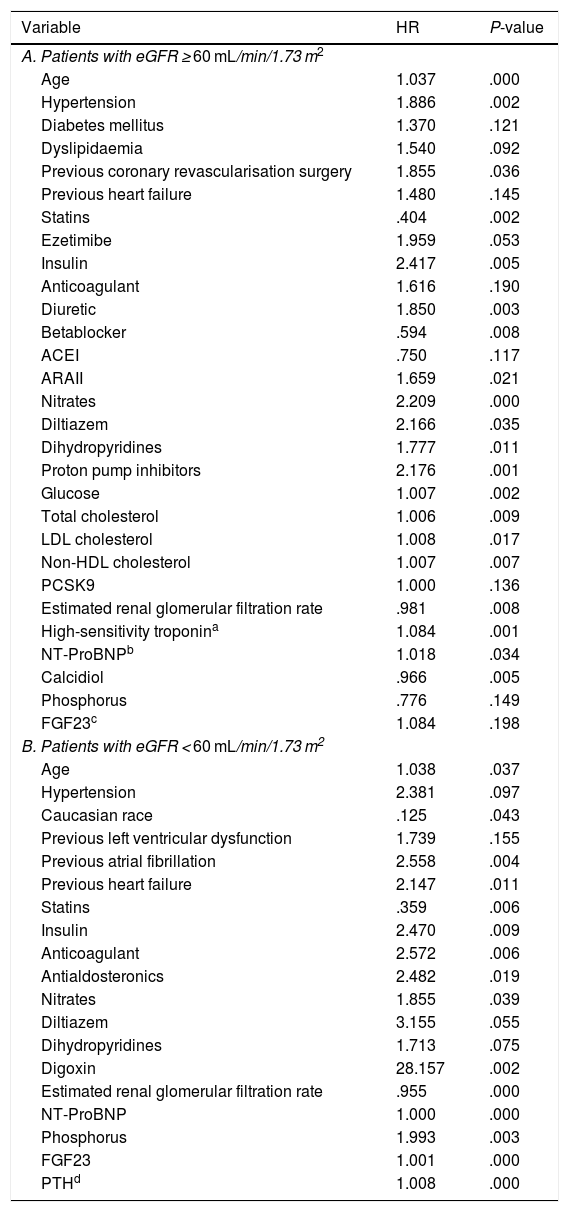
Prognostic value of the components of mineral metabolism according to estimated renal glomerular filtration rateIn the univariate Cox analysis in the group with an eGFR ≥ 60 mL/min/1.73 m2 the variables that were predictors of the development of the primary endpoint with a P < .2 were age, HT, DM, DL, previous surgical coronary revascularisation, previous HF, statin treatment, ezetimibe, insulin, anticoagulation, diuretic, BB, ACEI/ARAII, nitrates, calcium antagonists, proton pump inhibitors, and analytical parameters such as glucose, total cholesterol, LDL, non-HDL, PCSK9, eGFR, Tn, NT-ProBNP, calcidiol, phosphorus, and FGF23 (Table 2A).
Univariate Cox analysis with significance at P < .2 according to eGFR.
| Variable | HR | P-value |
|---|---|---|
| A. Patients with eGFR ≥ 60 mL/min/1.73 m2 | ||
| Age | 1.037 | .000 |
| Hypertension | 1.886 | .002 |
| Diabetes mellitus | 1.370 | .121 |
| Dyslipidaemia | 1.540 | .092 |
| Previous coronary revascularisation surgery | 1.855 | .036 |
| Previous heart failure | 1.480 | .145 |
| Statins | .404 | .002 |
| Ezetimibe | 1.959 | .053 |
| Insulin | 2.417 | .005 |
| Anticoagulant | 1.616 | .190 |
| Diuretic | 1.850 | .003 |
| Betablocker | .594 | .008 |
| ACEI | .750 | .117 |
| ARAII | 1.659 | .021 |
| Nitrates | 2.209 | .000 |
| Diltiazem | 2.166 | .035 |
| Dihydropyridines | 1.777 | .011 |
| Proton pump inhibitors | 2.176 | .001 |
| Glucose | 1.007 | .002 |
| Total cholesterol | 1.006 | .009 |
| LDL cholesterol | 1.008 | .017 |
| Non-HDL cholesterol | 1.007 | .007 |
| PCSK9 | 1.000 | .136 |
| Estimated renal glomerular filtration rate | .981 | .008 |
| High-sensitivity troponina | 1.084 | .001 |
| NT-ProBNPb | 1.018 | .034 |
| Calcidiol | .966 | .005 |
| Phosphorus | .776 | .149 |
| FGF23c | 1.084 | .198 |
| B. Patients with eGFR < 60 mL/min/1.73 m2 | ||
| Age | 1.038 | .037 |
| Hypertension | 2.381 | .097 |
| Caucasian race | .125 | .043 |
| Previous left ventricular dysfunction | 1.739 | .155 |
| Previous atrial fibrillation | 2.558 | .004 |
| Previous heart failure | 2.147 | .011 |
| Statins | .359 | .006 |
| Insulin | 2.470 | .009 |
| Anticoagulant | 2.572 | .006 |
| Antialdosteronics | 2.482 | .019 |
| Nitrates | 1.855 | .039 |
| Diltiazem | 3.155 | .055 |
| Dihydropyridines | 1.713 | .075 |
| Digoxin | 28.157 | .002 |
| Estimated renal glomerular filtration rate | .955 | .000 |
| NT-ProBNP | 1.000 | .000 |
| Phosphorus | 1.993 | .003 |
| FGF23 | 1.001 | .000 |
| PTHd | 1.008 | .000 |
Variables related to the combined endpoint (death or ischaemic event) with significance less than .2 in the univariate Cox study in the population divided according to glomerular filtration rate.
ACEI: Angiotensin-converting enzyme inhibitor; ARA II: Angiotensin II receptor blocker; eGFR: Estimated renal glomerular filtration rate; FGF23: Fibroblast growth factor-23; HDL: High density lipoprotein; HR: Hazard Ratio; LDL: Low density lipoprotein; NSTE ACS: Non-ST elevation-acute coronary syndrome; NT-ProBNP: Amino-terminal portion of pro BNP; PCSK-9: Proprotein convertase subtilisin/kexin type 9; PTH: Parathyroid hormone; STEMI: ST-elevation myocardial infarction.
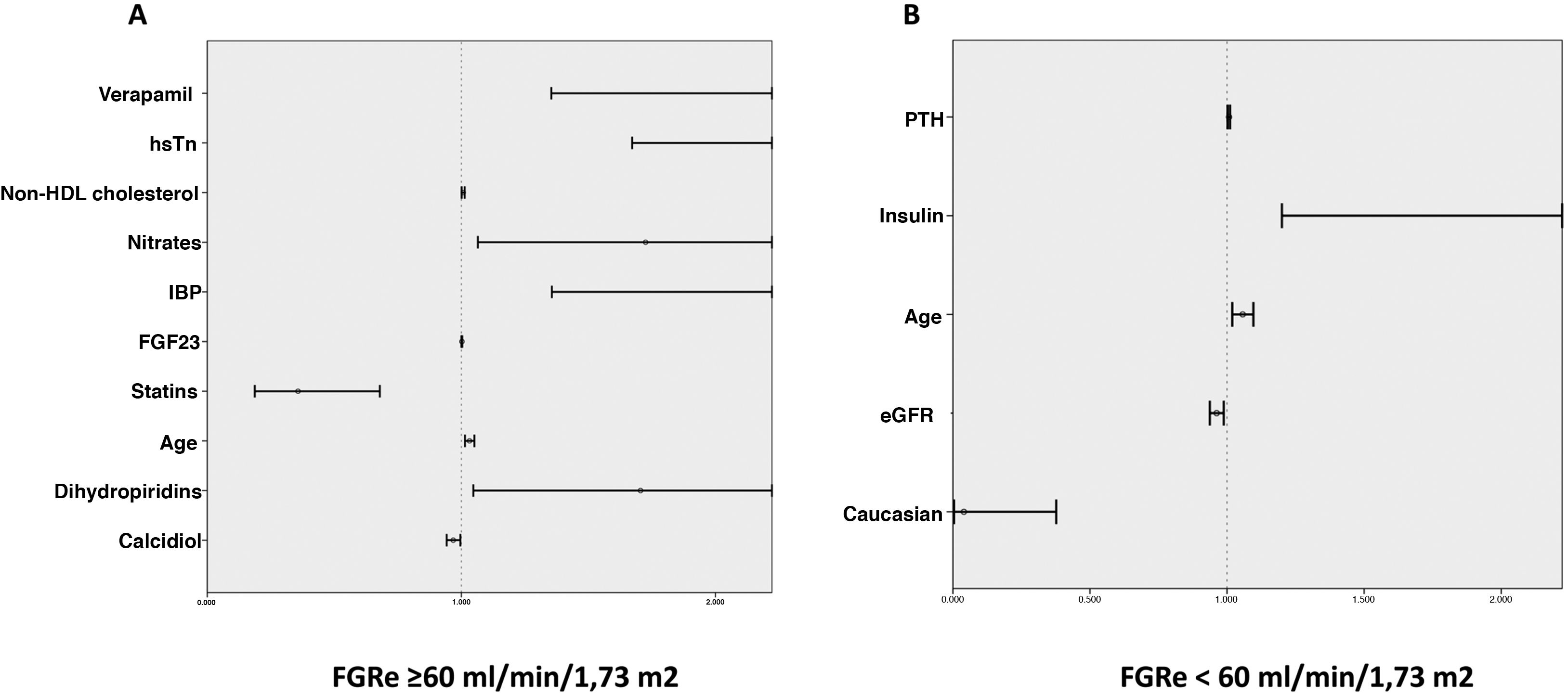
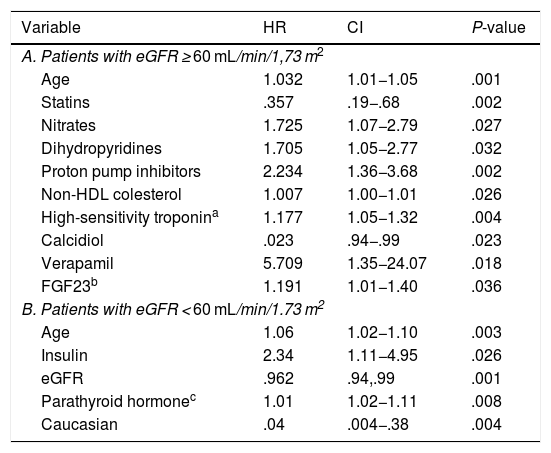
In the multivariate analysis, plasma calcidiol levels and statin treatment were inversely associated with the risk of developing the primary endpoint, whereas non-HDL cholesterol levels, high-sensitivity troponin I, age, and treatment with nitrates, dihydropyridines, verapamil and proton pump inhibitors were directly proportionally associated (Table 3A and Fig. 1A).
Multivariate Cox analysis for the search for predictors of adverse events according to eGFR.
| Variable | HR | CI | P-value |
|---|---|---|---|
| A. Patients with eGFR ≥ 60 mL/min/1,73 m2 | |||
| Age | 1.032 | 1.01−1.05 | .001 |
| Statins | .357 | .19−.68 | .002 |
| Nitrates | 1.725 | 1.07−2.79 | .027 |
| Dihydropyridines | 1.705 | 1.05−2.77 | .032 |
| Proton pump inhibitors | 2.234 | 1.36−3.68 | .002 |
| Non-HDL colesterol | 1.007 | 1.00−1.01 | .026 |
| High-sensitivity troponina | 1.177 | 1.05−1.32 | .004 |
| Calcidiol | .023 | .94−.99 | .023 |
| Verapamil | 5.709 | 1.35−24.07 | .018 |
| FGF23b | 1.191 | 1.01−1.40 | .036 |
| B. Patients with eGFR < 60 mL/min/1.73 m2 | |||
| Age | 1.06 | 1.02−1.10 | .003 |
| Insulin | 2.34 | 1.11−4.95 | .026 |
| eGFR | .962 | .94,.99 | .001 |
| Parathyroid hormonec | 1.01 | 1.02−1.11 | .008 |
| Caucasian | .04 | .004−.38 | .004 |
Variables related to the combined endpoint (death or ischaemic event) with significance less than .05 in the multivariate Cox study in the population divided according to glomerular filtration rate.
CI: Confidence Interval; eGFR: Estimated glomerular filtration rate; FGF23: Fibroblast growth factor 23; HDL: High density lipoprotein; HR: Hazard Ratio.
Predictors of a thrombotic event or death according to whether or not they have an eGFR < 60 mL/min/1.73 m2. Forest plot graph showing predictors of adverse events in patients: A) with an eFGR ≥ 60 mL/min/1.73 m2 and B) with an eFGR ≥ 60 mL/min/1.73 m2.
eGFR: estimated renal glomerular filtration rate; FGF23: fibroblast growth factor 23; HDL: high density lipoprotein; high-sensitivity troponin (hsTn): PPI: proton pump inhibitors; PTH: parathyroid hormone.
The variables associated with the development of the primary endpoint in the group with an eGFR < 60 mL/min/1.73 m2 were age, HT, Caucasian race, previous ventricular dysfunction, previous AF, previous HF, treatment with statins, insulin, anticoagulants, antialdosterone, nitrates, calcium antagonists, digoxin, and analytical parameters such as eGFR, NT-ProBNP, phosphorus, FGF23 and PTH (Table 2B).
In the multivariate analysis, plasma PTH levels, age, and insulin treatment were positively associated with the development of ischaemic events or death during follow-up, while Caucasian race and eGFR were inversely associated (Table 3B and Fig. 1B).
DiscussionMM abnormalities have traditionally been associated with chronic kidney disease. However, we have previously described that these abnormalities are also present in patients with coronary artery disease and normal renal function.13 Furthermore, these abnormalities are associated with an increased incidence of cardiovascular events.2,4,14
The main finding of the present study is that the components of MM that are prognosis predictors for patients with ischaemic heart disease differ according to the patients’ renal function. In patients with an eGFRe ≥ 60 mL/min/1.73 m2 the main predictors of adverse events are calcidiol and GFR23. In contrast, in those with an eGFR < 60 mL/min/1.73 m2 PTH is the main predictor of poor prognosis within the different components of MM.
We have recently shown in this same population that PTH is a predictor of cardiovascular events.15 However, this sub-analysis demonstrates that this result applies to patients with stage 3 or above advanced renal failure, but not to those with an eGFR of 60 mL/min/1.73 m2 or more. Therefore, the predictive ability of MM components may vary in different patient subgroups. On this basis, we previously described an interaction between the predictive value of MM components, as low calcidiol levels predicted cardiovascular events only in the presence of high FGF23 levels,14 but not in cases with low FGF23. Similar data have been observed for renal function, and pre-dialysis patients with low calcidiol and high FGF23 are more likely to require dialysis or double their plasma creatinine levels than those in whom only one of these markers is impaired or in whom both are at normal levels.16
These data could be relevant when designing and interpreting the results of clinical trials investigating possible cardiovascular benefits of vitamin D supplementation. In this regard, it is possible that positive effects may be concentrated in certain population subgroups determined by renal function and FGF23 levels. In this regard, several recent clinical trials have not demonstrated a beneficial effect of vitamin D supplementation,17–19 but we do not know whether subgroup analysis would have provided different results. Furthermore, it is important to bear in mind that the prognostic value of the various components of MM may vary, depending on the clinical targets being analysed. Thus, calcidiol levels are more involved in the genesis and development of atherosclerosis, and have been reported to be related to vascular calcification, severity of coronary heart disease, and incidence of acute myocardial infarction and cardiovascular death,2,6,15,20 while plasma PTH levels are more related to myocardial problems. In this regard, we described previously that PTH was associated with left ventricular hypertrophy in patients with SCAD.8
Finally, this study has some limitations. Firstly, urinary albumin levels were not measured, and therefore the early stages of CKD may have been underdiagnosed. However, excluding patients without clinical stability during the first days after the index event might create some selection bias, because patients with prolonged stays are likely to have a poorer prognosis. This is reflected by the relatively low prevalence of ventricular dysfunction in this population.
ConclusionsIn patients with SCAD, PTH is an independent predictor of poor outcome only in those with an eGFR < 60 mL/min/1.73 m2, whereas in patients with an eGFR ≥ 60 mL/min/1.73 m2, calcidiol and FGF23 become the only components of MM that can predict prognosis. Therefore, renal function influences the predictive power of MM components in SCAD patients.
Conflict of interestsJose Tuñón has given talks for Diasorin Spain.
This work has been funded by grants from the Spanish Society of Arteriosclerosis, Instituto de Salud Carlos III (ISCIII) [PI17/01495 and P20/00923] and the Spanish Ministry of Science and Innovation [RTC2019-006826-1].
Please cite this article as: Aceña Á, Pello-Lázaro AM, Martínez-Milla J, González-Lorenzo Ó, Tarín N, Cristóbal C, et al. Impacto de la función renal en el valor pronóstico del metabolismo mineral en pacientes con cardiopatía isquémica crónica. Clin Investig Arterioscler. 2022;34:1–9.