A group of experts convened by the Spanish Society of Arteriosclerosis (SEA) has been in charge of updating the SEA document on the indications of PCSK9 inhibitors (PCSK9i) in clinical practice that was published in 2016. This update is justified by the fact that the data from clinical trials carried out on a large scale with PCSK9i have shown that in addition to their high potency to lower atherogenic cholesterol, they reduce the risk of atherosclerotic cardiovascular disease, both in patients with stable disease, and with recent disease, and with a high degree of security. This update provides the recommendations and level of evidence for the prescription of iPCSK9 in patients with homozygous and heterozygous familial hypercholesterolemia, with atherosclerotic cardiovascular disease, and in primary prevention in patients with very high cardiovascular risk. These recommendations have been established taking into account the concentration of LDL-C, the clinical situation of the patient, the additional risk factors and the cost-effectiveness of their use.
Un grupo de expertos convocado por la Sociedad Española de Arteriosclerosis (SEA) se ha encargado de actualizar el documento de la SEA sobre las indicaciones de los inhibidores de PCSK9 (iPCSK9) en la práctica clínica publicadas en 2016. Esta actualización es necesaria porque en el periodo transcurrido hasta la actualidad se han publicado los resultados de los ensayos clínicos realizados a gran escala con iPCSK9 que demuestran que, además de su alta potencia para disminuir el colesterol aterogénico, disminuyen el riesgo de presentar episodios de enfermedad cardiovascular aterosclerótica en los pacientes con enfermedad tanto estable como reciente, y con un alto grado de seguridad. La presente actualización aporta las recomendaciones y el nivel de evidencia para la prescripción de los iPCSK9 en los pacientes con hipercolesterolemia familiar homo y heterocigota, con enfermedad cardiovascular aterosclerótica y en prevención primaria en los pacientes de muy alto riesgo cardiovascular. Dichas recomendaciones se han establecido teniendo en cuenta la concentración de c-LDL, la situación clínica del paciente, los condicionantes de riesgo adicionales y la relación coste-efectividad de su utilización.
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) have signified a substantial change in the clinical management of hypercholesterolaemia due to their high lipid-lowering efficacy and their preventive effects in ischaemic disease of atherothrombotic origin.1,2
PCSK9i have been studied in a wide range of clinical trials that have included populations with cardiovascular disease (CVD), heterozygous and homozygous familial hypercholesterolaemia (FH), mixed dyslipidaemia, and patients with statin intolerance. In all PCSK9i there has been a marked lowering effect of low-density lipoprotein cholesterol (LDL-C), even higher than 60%, which has allowed most patients to achieve their therapeutic goals. This cholesterol-lowering effect has been observed regardless of the intensity of the basic treatment.3 In 2015, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) authorised Praluent® and Repatha®, the registered names of alirocumab and evolocumab, respectively. These are the two monoclonal antibodies directed to inhibit PCSK9 which are currently available.
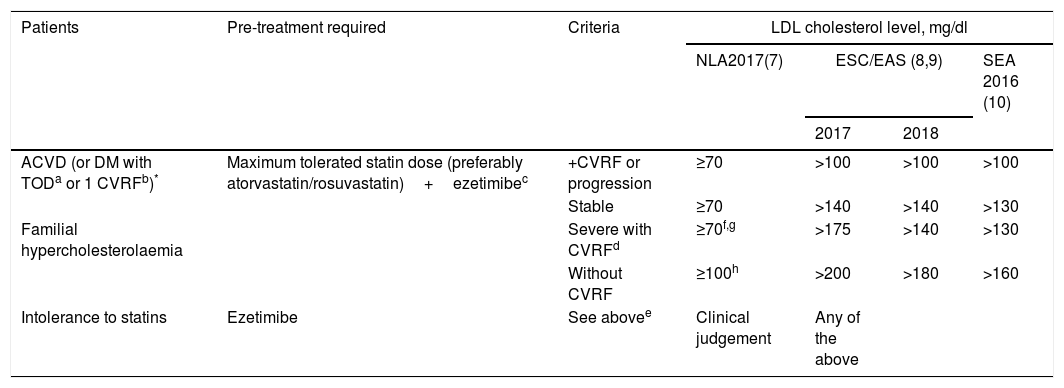
In its therapeutic positioning reports, the Spanish Agency of Medicines and Medical Devices (AEMPS) establishes that evolocumab and alirocumab will be funded in patients with heterozygous FH and in patients with established CVD (ischaemic heart disease, ischaemic cerebrovascular disease and peripheral arterial disease), in both cases with LDL-C concentrations >100mg/dl and undergoing treatment with the maximum tolerated dose of statin. Likewise, any of the patients in the previous groups that present contraindication or are intolerant to statins, and whose LDL-C is >100mg/dl, are included. In addition, evolocumab is funded in patients with homozygous FH. Given the transfers of the Spanish National Health System to the autonomous communities, some of these have added limitations to those indicated by the AEMPS. The most common are: to check the criteria in detailed clinical reports, especially in the classification of the statin intolerance, and to include the administration of ezetimibe before authorising the prescription of PCSK9i. After approval by the regulatory agencies, the main scientific societies involved in the treatment and control of dyslipidaemia established the first indications for the use of PCSK9i. Table 1 shows the recommendations of the Expert Panel of the National Lipid Association,4 the joint guideline of the European Society of Cardiology and the European Atherosclerosis Society,5,6 and that promoted by the Spanish Society of Arteriosclerosis [Sociedad Española de Arteriosclerosis] (SEA) in 2016.7
Current recommendations for the use of PCSK9i according to the main scientific societies of the United States of America, Europe and the Spanish Society of Arteriosclerosis.
| Patients | Pre-treatment required | Criteria | LDL cholesterol level, mg/dl | |||
|---|---|---|---|---|---|---|
| NLA2017(7) | ESC/EAS (8,9) | SEA 2016 (10) | ||||
| 2017 | 2018 | |||||
| ACVD (or DM with TODa or 1 CVRFb)* | Maximum tolerated statin dose (preferably atorvastatin/rosuvastatin)+ezetimibec | +CVRF or progression | ≥70 | >100 | >100 | >100 |
| Stable | ≥70 | >140 | >140 | >130 | ||
| Familial hypercholesterolaemia | Severe with CVRFd | ≥70f,g | >175 | >140 | >130 | |
| Without CVRF | ≥100h | >200 | >180 | >160 | ||
| Intolerance to statins | Ezetimibe | See abovee | Clinical judgement | Any of the above | ||
ACVD, atherosclerotic cardiovascular disease; CVRF, cardiovascular risk factor; DM, diabetes mellitus; ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; NLA, National Lipid Association; SEA, Spanish Society of Arteriosclerosis; TOD, target organ damage.
In the first assessments, there were no intervention studies with clinical final objectives and the recommendations were established based on theoretical assumptions about lipid-lowering efficacy and the expected impact on the cardiovascular prevention of these drugs.8,9 The results of the FOURIER trial with evolocumab,1 the Spire trial with bococizumab10 and the ODYSSEY OUTCOMES trial with alirocumab2 have provided robust scientific evidence on the role of PCSK9i in cardiovascular prevention, as reflected in the indications approved by the European Medicines Agency.11,12
Studies on cardiovascular prevention with PCSK9iThe FOURIER trial1 included patients with CVD and associated cardiovascular risk factors (CVRF), who maintained LDL-C >70mg/dl despite treatment with high-intensity statins in combination with ezetimibe or alone. The patients were randomised to receive evolocumab (140mg every two weeks or 420mg monthly) or placebo, with a mean follow-up of 2.2 years. A total of 9.8% of patients treated with evolocumab and 11.3% of those treated with placebo had an ischaemic recurrence (death due to CVD, acute myocardial infarction [AMI], stroke, hospitalisation due to angina or coronary revascularisation) (hazard ratio [HR] 0.85; 95% CI: 0.79–0.92; p<0.001). This preventive effect was observed regardless of the intensity of the baseline statin and the initial LDL-C value.13 It was also observed that the lower the LDL-C obtained with the treatment, the lower the incidence of CVD.14 The ODYSSEY OUTCOMES trial included patients who had presented an acute coronary syndrome in the 12 months prior to their inclusion and who maintained LDL-C at >70mg/dl, or cholesterol linked to non-high-density lipoproteins (non-HDL-C)>100mg/dl, or apolipoprotein B (>80mg/dl) despite treatment with the maximum tolerated dose of statins, combined with ezetimibe or alone. Patients were randomised to alirocumab or placebo and underwent follow-up for a mean of 2.8 years, titrating the doses of alirocumab (75 or 150mg/dl every two weeks) to reach a therapeutic target of LDL-C between 25 and 50mg/dl. A total of 9.5% of patients treated with alirocumab and 11.1% of those treated with placebo had an ischaemic recurrence (death due to CVD, AMI, stroke, or hospitalisation due to angina) (HR: 0.85; 95% CI: 0.78–0.93; p<0.001). The effect of alirocumab was more pronounced in the pre-specified subgroup with LDL-C ≥100mg/dl (HR: 0.76; 95% CI: 0.65–0.87). Also, treatment with alirocumab was associated with a lower incidence of death from any cause compared to placebo (3.5% vs. 4.1%; HR: 0.85; 95% CI: 0.73–0.98; nominal p-value=0.026).2,15 Both the FOURIER trial and the ODYSSEY OUTCOMES trial showed that a decrease in LDL-C below 70mg/dl, both at fixed and modulated doses to reach lipid targets, allowed a reduction in cardiovascular complications with a high degree of safety. The beneficial effects were as expected for the LDL-C decrease achieved and the duration of the studies. A third PCSK9i, bococizumab, showed a cardiovascular benefit consistent with the previous results, but its development was interrupted prematurely after attenuation of lipid-lowering efficacy due to the development of neutralising antibodies to the drug, because it was a “humanised” monoclonal antibody.10
Decrease in LDL-C and cardiovascular preventionIn recent decades, prior to the arrival of PCSK9i, a large number of randomised clinical trials had shown the effectiveness of lipid-lowering treatment with statins to reduce the rate of cardiovascular complications. This is summarised in the meta-analyses of the Cholesterol Treatment Trialists’ (CTT) Collaboration.16,17 In the two main meta-analyses of this group, it is consistently established that treatment with statins is associated with a reduction in cardiovascular morbidity of between 20 and 25% for each reduction of LDL-C by 1mmol/l (38.7mg/dl).16,17 Likewise, it is shown that intensive treatment with statins is associated with a similar effect, maintaining the aforementioned relationship of LDL-C reduction and the reduction of cardiovascular morbidity. Likewise, the IMPROVE-IT trial demonstrated, for the first time for a drug other than statins, that ezetimibe induced an additional risk reduction, consistent with that predicted by the CTT.18 The analyses of the relationship between cholesterol levels reached during treatment and the rate of cardiovascular complications in studies with statins, ezetimibe and PCSK9i show a progressively increasing benefit for LDL-C reductions of at least 30–40mg/dl, without having detected a threshold from which the beneficial effect disappears or there is an increase in side effects.13,19–21 Recent analyses suggest that 70% of patients with established CVD would achieve the target of LDL-C <70mg/dl with high-dose statins, and that figure would reach 86% with the addition of ezetimibe.22 However, for some authors it would be reasonable to consider directly the combination with PCSK9i in patients treated with statins whose LDL-C levels require additional decreases of more than 20–25%.23,24
Based on the results of the FOURIER and ODYSSEY OUTCOMES trials, it would seem reasonable to use PCSK9i to treat all patients with atherosclerotic CVD who do not reach the LDL-C target of <70mg/dl with the usual treatments. Nevertheless, it is necessary to select candidates with a greater probability of benefiting from this treatment to improve its therapeutic efficacy. Based on the foregoing, we present some updated recommendations on the use of PCSK9i, drafted by a group of SEA experts.
Familial hypercholesterolaemiaDecreasing LDL-C is a priority in FH. However, as in other populations, the intensity of the reduction must be established by taking into account the benefits of the intervention, its side effects and the costs of said reduction.25 Given that the side effects of PCSK9i are very rare, the benefits of the intervention can be deducted based on the reduction of LDL-C and baseline cardiovascular risk (CVR). The number of subjects to be treated to prevent an event, in other words, the net benefit of the intervention, is the parameter that we considered essential in order to establish the indication of these drugs.
This document addresses two aspects that, in the opinion of the panel, are key: (a) the definition of heterozygous FH (HeFH) in subjects with and without lipid-lowering treatment, and (b) the risk factors for developing a cardiovascular event in subjects with HeFH on treatment with statins.
Definition of heterozygous familial hypercholesterolaemia for the indication of PCSK9iThe most frequently recommended definition for the diagnosis of HeFH is the one proposed by the Dutch Lipid Clinic Network (DLCN) and adopted by the European Society of Atherosclerosis and the European Society of Cardiology.26 However, this definition depends very much on family clinical information, which is not always available, on subjective clinical criteria such as the presence of tendinous xanthomas, and information on the candidate genes causing HeFH. Since genetic testing is not available to many patients, it is discriminatory to use criteria that put patients without this information in a situation of inferiority.
We recommend the clinical diagnosis of HeFH for the indication of PCSK9i based on any of the following situations:
- •
Heterozygous familial hypercholesterolaemia with genetic confirmation.
- •
System scoring proposed by the DLCN ≥6 points.
- •
Primary hypercholesterolaemia with an LDL-C ≥220mg/dl (or LDL-C ≥130mg/dl if on high-intensity lipid-lowering treatment) and a history of hypercholesterolaemia in a first-degree relative.27,28
The prevalence of CVD in patients with HeFH is between three and eight times higher than in the general population.29 However, there are few studies to determine the incidence of CVD and the risk factors that modulate it in patients with HeFH on prolonged treatment with statins. The Montreal study is based on the association of clinical variables and the prevalence of the events.30 The SAFE-HEaRt study has shown in patients in our field the importance of the presence of classic risk factors in patients with HeFH when evaluating their risk and, from this, an equation for the calculation of risk in a patient with FH has been developed, although it is based on a limited number of cardiovascular events.31 Furthermore, data obtained from very extensive databases from electronic records allow the estimation of the risk of patients with FH phenotype, with the strength to base their calculations on a large number of patients (Ramos et al., 2019, remitted for publication).
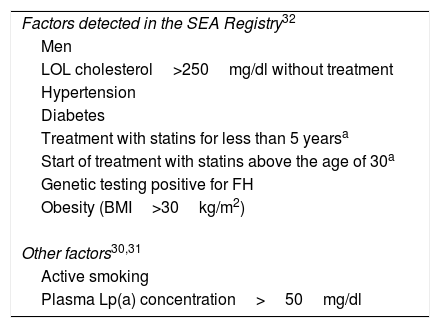
The SEA Registry of Dyslipidaemias, with more than 3000 patients with HeFH, is the source of information that we have applied preferentially in this document. This study prospectively analyses the onset of cardiovascular events in patients with HeFH on treatment with statins. The risk factors identified in the Registry are described in Table 2.32 Data from this and other studies show that patients with HeFH without established vascular disease and with at least four associated risk factors have a risk of future cardiovascular complications greater than 20%.
Risk factors for cardiovascular disease in subjects with familial hypercholesterolaemia on stable treatment with high-potency statins to be calculated for the indication of PCSK9i.
| Factors detected in the SEA Registry32 |
| Men |
| LOL cholesterol>250mg/dl without treatment |
| Hypertension |
| Diabetes |
| Treatment with statins for less than 5 yearsa |
| Start of treatment with statins above the age of 30a |
| Genetic testing positive for FH |
| Obesity (BMI>30kg/m2) |
| Other factors30,31 |
| Active smoking |
| Plasma Lp(a) concentration>50mg/dl |
Finally, the prevalence of diabetes is reduced in patients with FH.33 However, the coexistence of DM and FH causes the vascular risk to triple (an increase explained, at least in part, by the concurrence of other vascular risk factors).34
Homozygous familial hypercholesterolaemiaThe homozygous form of FH (HoFH) is a rare disorder, and its prevalence is estimated at 1/450,000 individuals. The LDL-C levels are extremely high and cardiovascular involvement is very premature. The reduction of LDL-C is an absolute priority, but current treatments are extremely ineffective, requiring extraordinary measures such as LDL apheresis. Evolocumab has demonstrated an ability to reduce LDL-C in patients with HoFH by around 20%.35 Although we do not have, nor will we have, studies on the impact of evolocumab in cardiovascular complications, its use is indicated in all patients with HoFH from the age of 14, except in those who have a double-null mutation of the LDL receptor.
Secondary prevention in patients with established cardiovascular disease. Risk stratificationPatients with established atheromatous vascular disease have a high risk of recurrence. For this reason, it is indicated that all of them receive intensive lipid-lowering therapy, usually with high-potency statins or therapeutic combinations, with the aim of reducing their LDL-C concentration by at least 50% and reaching a value lower than 70mg/dl.36 Despite this, many patients maintain LDL-C levels above those recommended and could benefit from treatment with PCSK9i, fundamentally those whose persistent vascular risk is extraordinarily high and whose LDL-C levels are far away from the therapeutic target.
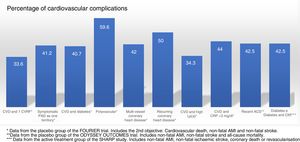
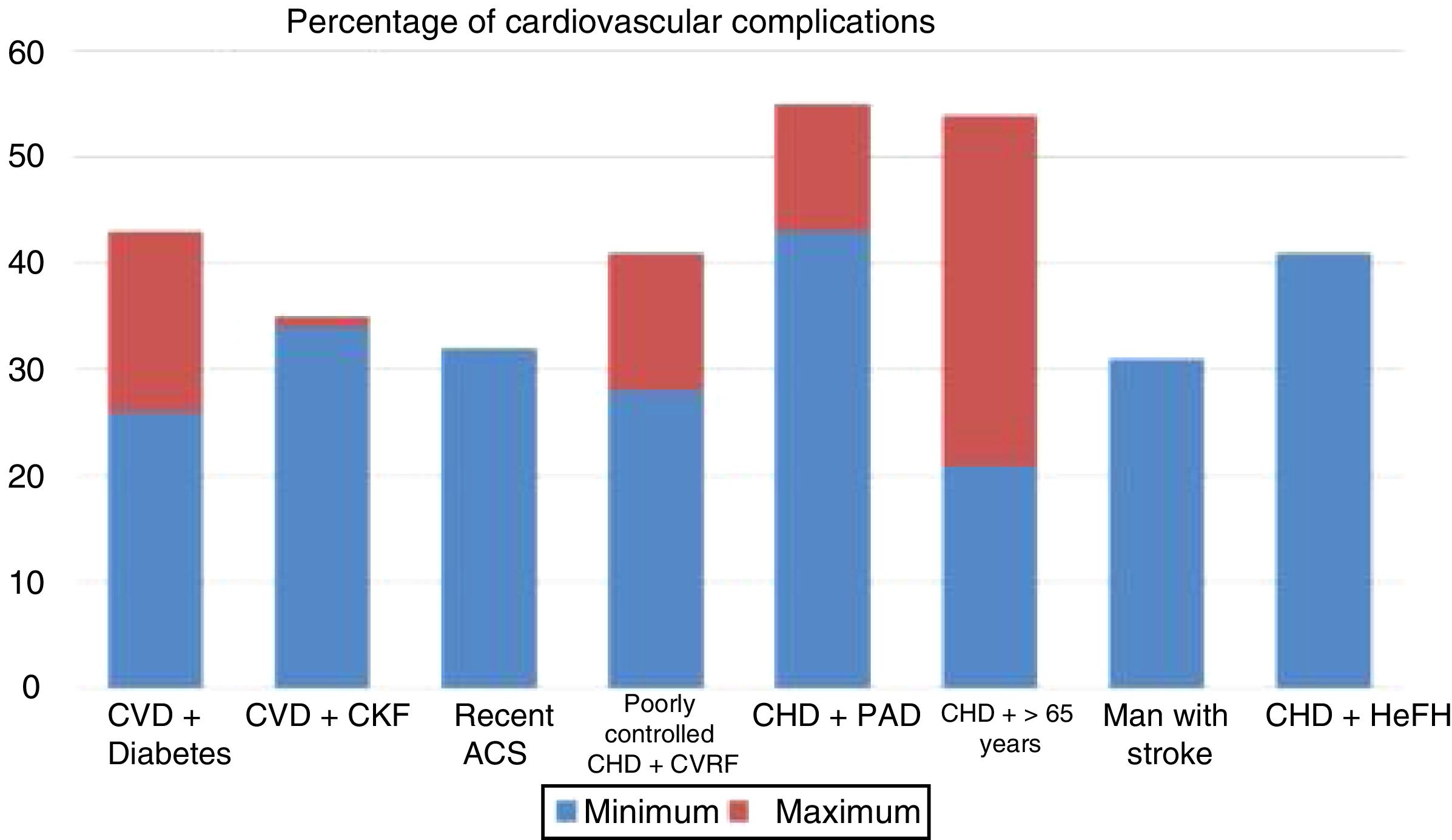
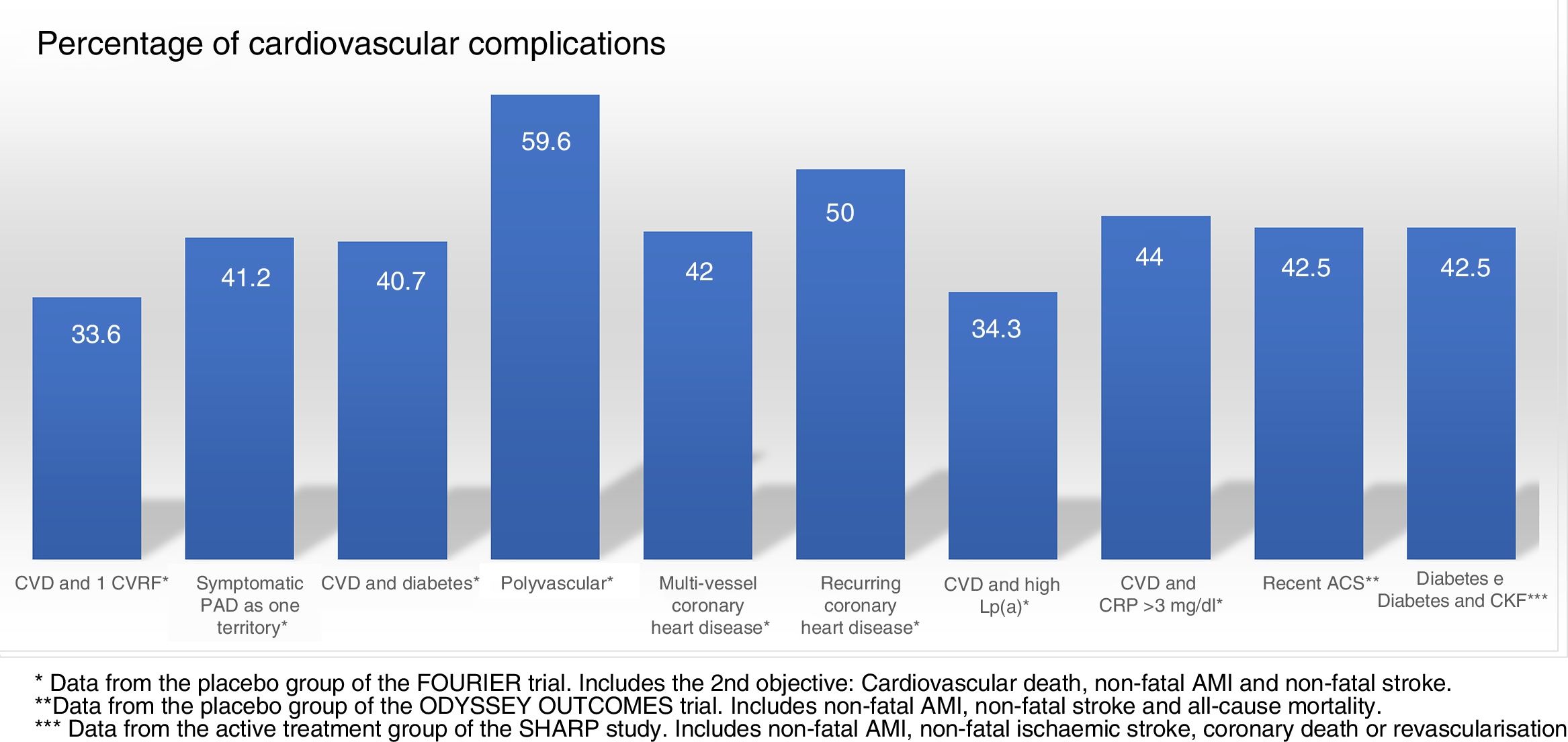
Randomised and controlled clinical trials indicate that, despite treatment with statins, some patient subgroups show an estimated rate of events of more than 30 and 40% at 10 years (Fig. 1).37 Similarly, some patient subgroups in the placebo group of the FOURIER and ODYSSEY OUTCOMES trials, despite treatment with high doses of statins and some cases with ezetimibe, maintained a high rate of cardiovascular complications, so they are still very high-risk patients (Fig. 2).
Rate of vascular complications (non-fatal acute myocardial infarction [AMI]+non-fatal stroke+cardiovascular death) extrapolated at 10 years of treatment with statins in different intervention studies. ACS: acute coronary syndrome; CHD: coronary heart disease; CKF: chronic kidney failure; CVD: cardiovascular disease; CVRF: cardiovascular risk factors; HeFH: heterozygous familial hypercholesterolaemia; PAD: peripheral arterial disease.
Source: Robinson et al.37.
Cardiovascular complications rate, extrapolated at 10 years in the placebo groups and subgroups of the FOURIER trial, in the placebo group of ODYSSEY OUTCOMES and in the active treatment group of the SHARP study. ACS: acute coronary syndrome; CKF: chronic kidney failure; CRP: C-reactive protein; CVD: cardiovascular disease; CVRF: cardiovascular risk factors; Lp(a): lipoprotein(a); PAD: peripheral arterial disease.
The risk of ischaemic episodes in patients with type 2 diabetes mellitus (DM2) is four to eight times higher than that found in the non-diabetic population. This high risk is maintained after adjusting for age, gender and other classic CVR factors. In turn, atherosclerotic lesions in DM2 are more extensive, distal and premature. Therefore, DM2 has been considered as a high or very high CVR38 situation, especially when associated with classic CVR factors, if there is target organ damage (albuminuria, retinal involvement or ventricular hypertrophy), if there is kidney failure, if atherosclerotic plaques are observed by any diagnostic method, and, in particular, if clinical CVD exists in any territory.
In the diabetic patient, a direct relationship between the decrease in LDL-C levels and the reduction of the risk of developing CVD has also been demonstrated.39,40
The FOURIER trial also shows an additional benefit in the absolute reduction of cardiovascular events in diabetic patients (2.7 vs. 1.6% at three years).41 However, this benefit was restricted to a reduced need for revascularisation, there being no differences in the combined variable of AMI, stroke or cardiovascular death (absolute risk reduction [ARR] 2%).41 Likewise, preliminary results of the ODYSSEY trial show that treatment with alirocumab in diabetic patients provides a greater benefit than in the overall population, given its higher baseline risk (ARR in diabetics 2.3% vs. 1.6% in the study as a whole), its use being justifiable with LDL-C levels less than 100mg/dl.42 However, this recommendation should be qualified with a more detailed analysis of this group of patients, since, when analysing the ODYSSEY subgroup of patients with an LDL-C <100mg/dl, no benefit was shown in cardiovascular prevention.2 Taken together, both studies, along with cumulative evidence of the risk of diabetics, support a more intensive treatment in diabetic patients, although direct evidence is limited.
Elevation of lipoprotein(a)The elevation of lipoprotein(a) [Lp(a)] is an independent vascular risk factor, both in the general population and in the population with dyslipidaemia, especially with FH,43 and in patients with established CVD.44 There are forms of premature ischaemic heart disease with familial aggregation in which the elevation of Lp(a) is the only identifiable risk factor. A concentration of Lp(a) >50mg/dl (>125nmol/l) is associated with a higher CVR.45 PCSK9i have been proven to reduce levels of Lp(a) significantly, by around 25–30%.46,47 It is unknown whether this reduction in Lp(a) contributes to reducing ischaemic events. However, the higher baseline risk of patients with elevated Lp(a) causes them to benefit from a further reduction in their LDL-C concentration. In the FOURIER trial, the absolute reduction in the risk of cardiovascular complications in patients with Lp(a) above the median (>37mg/dl) was 2.9% (NNT at three years of 40; extrapolated at five years, of 24).44 An Lp(a) concentration located in the 3rd and 4th quartiles of Lp(a) was associated with an increased risk of 17 and 22% compared to the lower quartile, after adjusting for other risk factors. A preliminary subanalysis of the ODYSSEY OUTCOMES trial has shown that the reducing effect of alirocumab on Lp(a) concentrations was associated with a decrease in cardiovascular events regardless of its action on LDL-C.48
Chronic kidney failureChronic kidney failure in advanced stages is a very high vascular risk condition. The prescription of statins in monotherapy or combined with ezetimibe in patients with chronic kidney disease in stages 3 and 4 has a preventive effect against CVD, while this has not been observed in patients already on dialysis.49
The FOURIER and ODYSSEY OUTCOMES studies excluded patients with estimated glomerular filtration rate <20ml/min/1.73m2 and <30ml/min/1.73m2, respectively. Therefore, patients with KDIGO stage 3 or lower kidney failure50 have no contraindications to receiving these drugs. The use of PCSK9i has been shown to be safe in mild forms of kidney failure. In addition, preliminary results from the FOURIER trial indicate that evolocumab is effective in cardiovascular prevention in patients with mild or moderate kidney failure (stages 3 or lower), resulting in a greater reduction in absolute risk than in patients without kidney failure.51 If cardiovascular complications are common in patients with kidney failure, they are even more so in diabetic patients with kidney failure. In the SHARP study,52 the rate of major cardiovascular complications (non-fatal AMI, non-fatal ischaemic stroke, coronary death or revascularisation) in the treatment group with simvastatin and ezetimibe was 11.3% (estimated to be 23.1% at 10 years). This rate in patients who also had DM2 increased to 18.3% (estimated to be 37.3% at 10 years). The effect of alirocumab is similar in patients with moderate kidney failure (stage IIIa), with no increase in side effects, but there are no data in relation to patients with advanced CKD.53
Indications for using PCSK9iPCSK9i have been shown to be effective in reducing cardiovascular morbidity and mortality in patients with atherosclerotic CVD and LDL-C values higher than 70mg/dl. However, given that they are expensive therapies that should be maintained in the long term, we believe it is useful to indicate such treatment to patients who are more likely to benefit, in order to limit non-effective treatments as much as possible and adjust their cost to health resources.
In this sense, cost-benefit studies are very dependent on the assumptions of the theoretical models on which they are based,54 which has led to controversial results.55–57 In addition, the cost of PCSK9i is subject to discounts or downward revisions. Therefore, current economic estimates have a transitory value. On the other hand, a reference indicator to consider the use of PCSK9i cost-effective for disability-adjusted life years has not been established in the Spanish national health system.
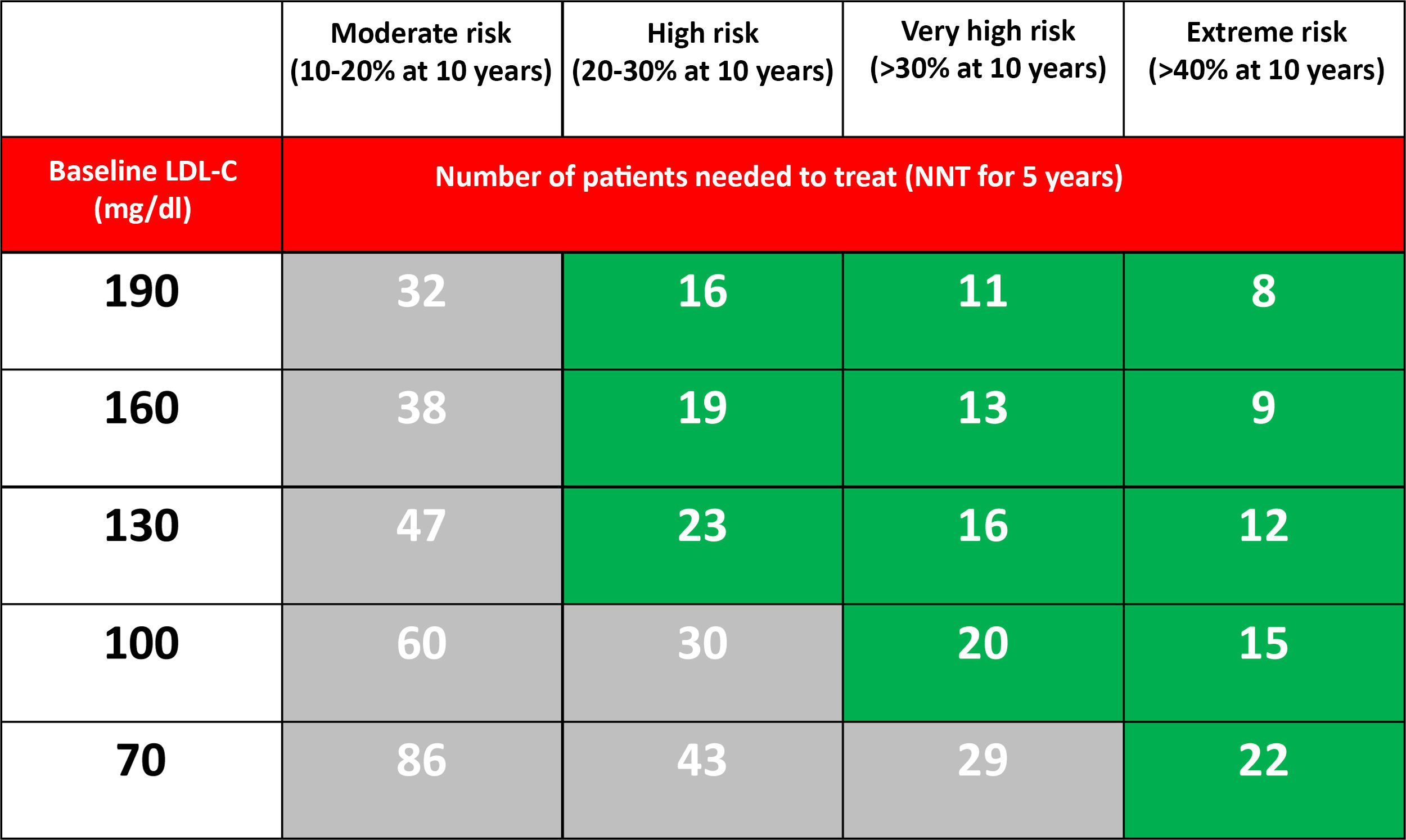
Several studies suggest that absolute risk reductions greater than 1% per year would be necessary (>10% at 10 years, NNT <20 at five years) to be able to justify treatment with PCSK9i.58–60 In this document, we used a five-year NNT <25 as a reference for these recommendations.
Our recommendations on the use of PCSK9i aim to obtain the maximum benefit by treating the smallest number of patients. This means selecting the populations where the NNT to prevent an event is lower. In other words, choosing the patients whose absolute risk reduction is maximum with the treatment. This is synonymous with treating patients with higher concentrations of LDL-C and with a higher baseline risk, especially if both circumstances are combined.
From a theoretical point of view, if we accept that PCSK9i at the maximum dose reduces LDL-C by 60%, the NNT predicted at five years to prevent a major cardiovascular event depending on the CVR and the level of LDL-C in optimised lipid-lowering treatment is reflected in Table 3.
Number of patients needed to treat (NNT) with PCSK9i for 5 years (assuming an additional LDL-C reduction of 60%) to prevent a major cardiovascular event (non-fatal AMI, non-fatal stroke or cardiovascular death) in patients already treated with statins, based on the estimated 10-year risk and the LDL-C level.37
Populations with a >30% risk of events despite optimised lipid-lowering therapy, in this case through the use of statins, have been extracted from the active treatment group in clinical trials with these drugs37 and are summarised in Fig. 1. In these same studies, it has been observed that there are groups of patients with a risk extrapolated to 10 years of >40%. Similarly, data from the ODYSSEY OUTCOMES trial and from subgroups of the FOURIER trial1,61–63 validated these findings, indicating that there are subgroups of patients with a >40% risk of cardiovascular complications, extrapolated at 10 years (Fig. 2).
Regarding the treatment prior to the indication of PCSK9i, the FOURIER and ODYSSEY OUTCOMES trials included patients on treatment with high-dose statins, in various percentages, and with a small proportion on combined treatment with ezetimibe. The SEA recommends that patients who are candidates for treatment with PCSK9i be previously treated with optimised lipid-lowering therapy,64 except in the case of patients with intolerance to statins. The difference in efficacy of additional reduction of LDL-C over the baseline value of a statin at the maximum dose or in combination with ezetimibe ranges from 50 to 60%.65 For this document, optimised lipid-lowering treatment is defined as that which theoretically produces at least a 50% reduction of LDL-C and with an adherence of 80%. The number of patients to be treated with PCSK9i, if they follow an optimised treatment regimen, is reduced by a third.66
Methodology used to define the recommendations for use of PCSK9iFor the recommendations, we have prioritised the results of clinical trials with PCSK9i with the objectives of prevention of cardiovascular events as a direct reference. From these trials, we have stratified the benefits based on subgroup analyses and approximated the potential benefits for different levels of starting LDL-C.
In the absence of clinical trials demonstrating the real benefit of treatment, we have developed recommendations based on the risk estimates and the expected benefit of LDL-C reduction with the use of PCSK9i.
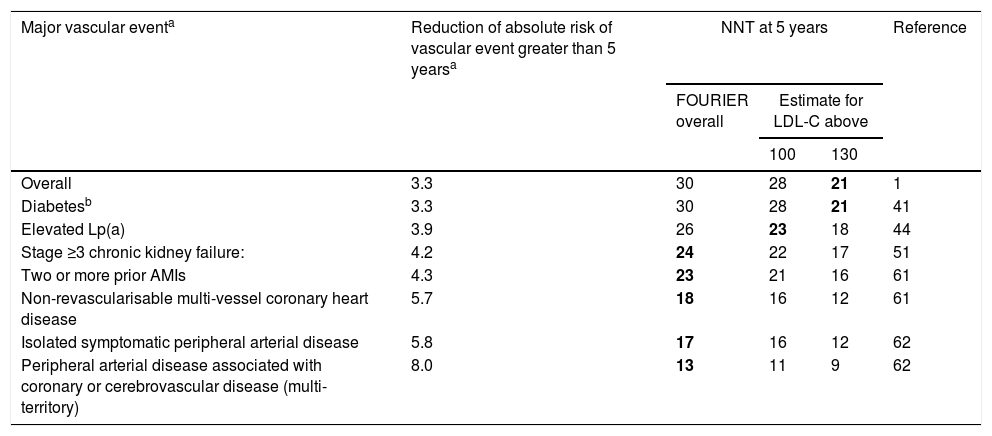
Table 4 offers the real reduction of the absolute risk of major vascular events and the estimated NNT at five years for the main subgroups of the FOURIER trial.
Real reduction of the absolute risk of major vascular events and the estimated number of patients needed to treat (NNT) at 5 years for the main subgroups of the FOURIER trial.
| Major vascular eventa | Reduction of absolute risk of vascular event greater than 5 yearsa | NNT at 5 years | Reference | ||
|---|---|---|---|---|---|
| FOURIER overall | Estimate for LDL-C above | ||||
| 100 | 130 | ||||
| Overall | 3.3 | 30 | 28 | 21 | 1 |
| Diabetesb | 3.3 | 30 | 28 | 21 | 41 |
| Elevated Lp(a) | 3.9 | 26 | 23 | 18 | 44 |
| Stage ≥3 chronic kidney failure: | 4.2 | 24 | 22 | 17 | 51 |
| Two or more prior AMIs | 4.3 | 23 | 21 | 16 | 61 |
| Non-revascularisable multi-vessel coronary heart disease | 5.7 | 18 | 16 | 12 | 61 |
| Isolated symptomatic peripheral arterial disease | 5.8 | 17 | 16 | 12 | 62 |
| Peripheral arterial disease associated with coronary or cerebrovascular disease (multi-territory) | 8.0 | 13 | 11 | 9 | 62 |
LDL-C: low-density lipoprotein cholesterol; NNT: number needed to treat (number of patients needed to treat to prevent a vascular event).
Major vascular event defined as the key secondary variable: AMI, stroke or cardiovascular death. The benefit estimates for different concentrations of LDL-C have been made from the different LDL-C reductions, assuming a reduction of ≈55% and that the clinical benefit is proportional to the absolute reduction of LDL-C. The combinations of clinical groups with LDL-C levels starting with those for which the NNT at 5 years is lower than 25 are highlighted in bold.
To modulate the possible benefit of treatment according to baseline LDL-C levels, the potential benefit has been estimated assuming that the clinical benefit is linearly related to the absolute reduction of LDL-C when the results of the study do not allow a direct estimation of an NNT <25 at five years. Thus, the combination of beneficial effects in high-risk subgroups related to different levels of starting LDL-C offers an estimate of patients who achieve a higher ARR and who would therefore be a priority for treatment with PCSK9i.
In some cases, as in the case of poor control of other risk factors or a recurrent disease despite maintaining a controlled LDL-C level, we offer a weak recommendation based on potential benefits, in the absence of direct evidence or a specific analysis of subgroups.
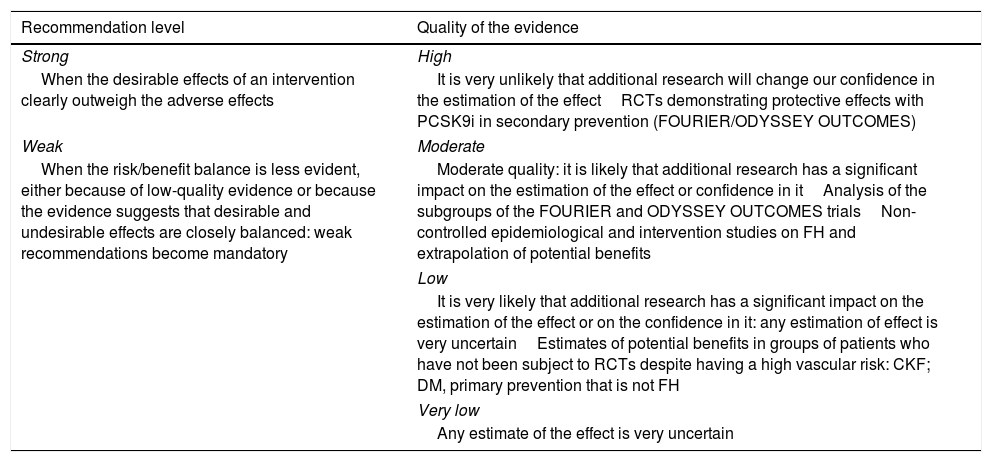
To qualify the recommendations, we have used the GRADE consensus as a reference.54,67 The recommendations are identified as strong if it is considered unlikely that new studies will significantly change the effect of the treatment with PCSK9i. Weak recommendations are those in which the risk/benefit balance is considered less established and can be modified significantly with new studies (Table 5). We have considered the quality of the evidence to be high in the direct evaluations of the FOURIER and ODYSSEY clinical trials, moderate for the subgroup analyses of these trials, and low in the case of potential risk estimates not evaluated directly in the studies. For FH, we have considered the evidence as moderate in the epidemiological studies or in large cohorts with these patients.
‘GRADE’ classification of the recommendations.
| Recommendation level | Quality of the evidence |
|---|---|
| Strong | High |
| When the desirable effects of an intervention clearly outweigh the adverse effects | It is very unlikely that additional research will change our confidence in the estimation of the effectRCTs demonstrating protective effects with PCSK9i in secondary prevention (FOURIER/ODYSSEY OUTCOMES) |
| Weak | Moderate |
| When the risk/benefit balance is less evident, either because of low-quality evidence or because the evidence suggests that desirable and undesirable effects are closely balanced: weak recommendations become mandatory | Moderate quality: it is likely that additional research has a significant impact on the estimation of the effect or confidence in itAnalysis of the subgroups of the FOURIER and ODYSSEY OUTCOMES trialsNon-controlled epidemiological and intervention studies on FH and extrapolation of potential benefits |
| Low | |
| It is very likely that additional research has a significant impact on the estimation of the effect or on the confidence in it: any estimation of effect is very uncertainEstimates of potential benefits in groups of patients who have not been subject to RCTs despite having a high vascular risk: CKF; DM, primary prevention that is not FH | |
| Very low | |
| Any estimate of the effect is very uncertain | |
CKF: chronic kidney failure; DM: diabetes mellitus; FH: familiar hypercholesterolaemia; RCTs: randomised clinical trials.
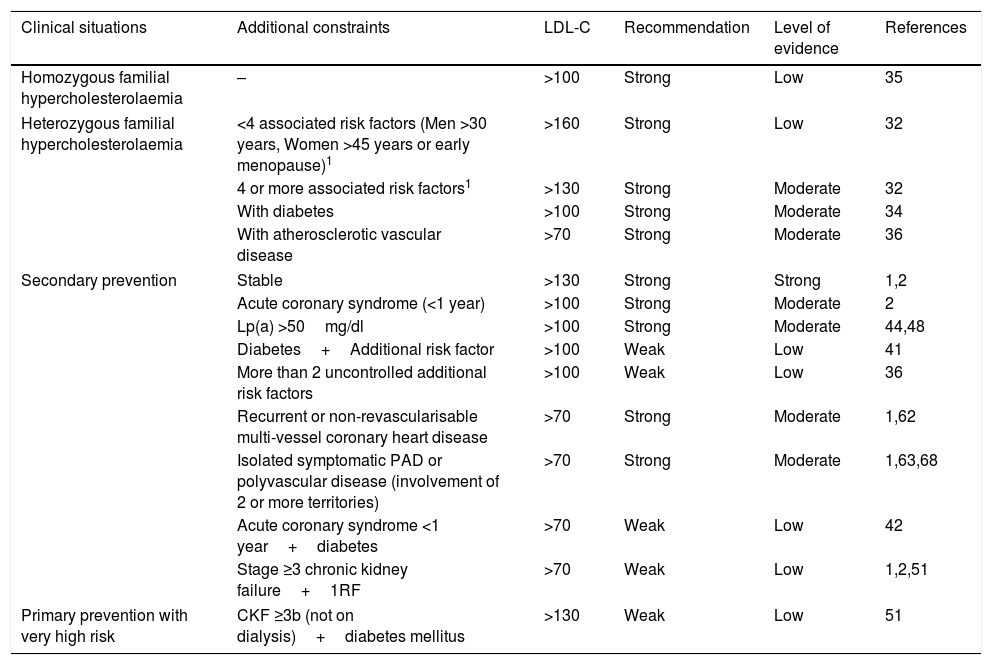
The indications proposed by the SEA for the use of PCSK9i, taking into account a NNT at five years of <25, are summarised in Table 6.
Recommendations and level of evidence for the prescription of PCSK9i in relation to the patient's clinical situation and LDL-C levels.
| Clinical situations | Additional constraints | LDL-C | Recommendation | Level of evidence | References |
|---|---|---|---|---|---|
| Homozygous familial hypercholesterolaemia | – | >100 | Strong | Low | 35 |
| Heterozygous familial hypercholesterolaemia | <4 associated risk factors (Men >30 years, Women >45 years or early menopause)1 | >160 | Strong | Low | 32 |
| 4 or more associated risk factors1 | >130 | Strong | Moderate | 32 | |
| With diabetes | >100 | Strong | Moderate | 34 | |
| With atherosclerotic vascular disease | >70 | Strong | Moderate | 36 | |
| Secondary prevention | Stable | >130 | Strong | Strong | 1,2 |
| Acute coronary syndrome (<1 year) | >100 | Strong | Moderate | 2 | |
| Lp(a) >50mg/dl | >100 | Strong | Moderate | 44,48 | |
| Diabetes+Additional risk factor | >100 | Weak | Low | 41 | |
| More than 2 uncontrolled additional risk factors | >100 | Weak | Low | 36 | |
| Recurrent or non-revascularisable multi-vessel coronary heart disease | >70 | Strong | Moderate | 1,62 | |
| Isolated symptomatic PAD or polyvascular disease (involvement of 2 or more territories) | >70 | Strong | Moderate | 1,63,68 | |
| Acute coronary syndrome <1 year+diabetes | >70 | Weak | Low | 42 | |
| Stage ≥3 chronic kidney failure+1RF | >70 | Weak | Low | 1,2,51 | |
| Primary prevention with very high risk | CKF ≥3b (not on dialysis)+diabetes mellitus | >130 | Weak | Low | 51 |
CKF: chronic kidney failure; PAD: peripheral arterial disease.
In summary, PCSK9i are a new group of drugs with an intense lipid-lowering action that has resulted in a reduction of cardiovascular complications in secondary prevention, which has been demonstrated in controlled clinical trials. However, its high cost and the absence of long-term studies recommend directing the use of these drugs to patients who can obtain a greater benefit, namely high-risk vascular patients who maintain high LDL-C levels despite a high intensity lipid-lowering treatment. These recommendations are intended as a reference when identifying patients for whom there is a greater potential benefit from treatment with PCSK9i.
Conflicts of interestJuan Francisco Ascaso has received fees for conferences or participation in the scientific committees of AstraZeneca, MSD, Lilly, Novartis, Recordati, Esteve, Ferrer, Novo Nordisk, Danone, Praxis, Amgen, Sanofi and Mylan.
Fernando Civeira has received fees for lectures, training or consultancy from the laboratories of Amgen, Ferrer, Merck and Sanofi.
Carlos Guijarro has received fees for lectures, training or consultancy from the laboratories of Amgen, Ferrer, MSD, Rubió and Sanofi.
Jose López Miranda has received fees for conferences or participation in the scientific committees of AstraZeneca, Esteve, Ferrer, Amgen and Sanofi.
Luis Masana has received fees for conferences and participation as a consultant for Amgen, Sanofi, MSD, Mylan and Daiichi.
José María Mostaza has received fees for conferences or participation in the scientific committees of Ferrer, Pfizer, Amgen and Sanofi.
Juan Pedro-Botet has received fees for conferences or participation in the scientific committees of Amgen, AstraZeneca, Esteve, Ferrer, MSD, Mylan, ROVI, Sanofi and Servier.
Xavier Pintó has received fees for conferences or participation in the scientific committees of Esteve, Ferrer, Rubió, Amgen, Sanofi and Mylan.
Pedro Valdivielso has received fees for consultancy or participation in scientific committees and conferences of Amgen, Sanofi, MSD, Ferrer and Almirall. He has received research grants from Ferrer.
Please cite this article as: Ascaso JF, Civeira F, Guijarro C, López Miranda J, Masana L, Mostaza JM, Pedro-Botet J, Pintó X, Valdivielso P. Indications of PCSK9 inhibitors in clinical practice. Recommendations of the Spanish Sociey of Arteriosclerosis (SEA) 2019. Clín Investig Arterioscler. 2019;31:128–139.






![Rate of vascular complications (non-fatal acute myocardial infarction [AMI]+non-fatal stroke+cardiovascular death) extrapolated at 10 years of treatment with statins in different intervention studies. ACS: acute coronary syndrome; CHD: coronary heart disease; CKF: chronic kidney failure; CVD: cardiovascular disease; CVRF: cardiovascular risk factors; HeFH: heterozygous familial hypercholesterolaemia; PAD: peripheral arterial disease. Source: Robinson et al.37. Rate of vascular complications (non-fatal acute myocardial infarction [AMI]+non-fatal stroke+cardiovascular death) extrapolated at 10 years of treatment with statins in different intervention studies. ACS: acute coronary syndrome; CHD: coronary heart disease; CKF: chronic kidney failure; CVD: cardiovascular disease; CVRF: cardiovascular risk factors; HeFH: heterozygous familial hypercholesterolaemia; PAD: peripheral arterial disease. Source: Robinson et al.37.](https://static.elsevier.es/multimedia/25299123/0000003100000003/v3_201908220859/S2529912319300269/v3_201908220859/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)