One of the objectives of the Spanish Society of Arteriosclerosis is to contribute to the knowledge, prevention and treatment of vascular diseases, which are the leading cause of death in Spain and entail a high degree of disability and health expenditure. Atherosclerosis is a multifactorial disease and its prevention requires a global approach that takes into account the associated risk factors. This document summarises the current evidence and includes recommendations for patients with established vascular disease or at high vascular risk: it reviews the symptoms and signs to evaluate, the laboratory and imaging procedures to request routinely or in special situations, and includes the estimation of vascular risk, diagnostic criteria for entities that are vascular risk factors, and general and specific recommendations for their treatment. Finally, it presents aspects that are not usually referenced in the literature, such as the organisation of a vascular risk consultation.
La Sociedad Española de Arteriosclerosis (SEA) tiene entre sus objetivos contribuir al conocimiento, prevención y tratamiento de las enfermedades vasculares, que son la primera causa de muerte en España y conllevan un elevado grado de discapacidad y gasto sanitario. La arteriosclerosis es una enfermedad multifactorial y su prevención exige un abordaje global que contemple los factores de riesgo asociados. Este documento resume la evidencia actual e incluye recomendaciones a seguir ante el paciente con enfermedad vascular establecida o con un elevado riesgo vascular (RV): se revisan los síntomas y signos a evaluar, los procedimientos de laboratorio e imagen a solicitar rutinariamente o en situaciones especiales, e incluye la estimación del RV, criterios diagnósticos de las entidades que son factores de riesgo vascular (FRV), y plantea recomendaciones generales y específicas para su tratamiento. Por último, se presentan aspectos poco referenciados en la literatura, como son, por ejemplo, la organización de una consulta de RV.
Medicine is an ever-evolving science. In recent years we have witnessed continuous advances in the diagnosis and treatment of atherosclerotic vascular disease (AVD) and its risk factors, and therefore, the therapeutic guidelines need constant updating.
One of the objectives of the Spanish Society of Arteriosclerosis (SEA) is to contribute to better knowledge and control of vascular risk factors (VRF) in our country, especially dyslipidaemia, through its network of lipids units. Hence the SEA’s decision to develop standards for the global control of VRFs, a way of summarising the scientific evidence and national and international recommendations on the main VRFs. As already indicated in the first version of these standards, when they were conceived the intention was for them to be regularly revised and updated, therefore, changes have been introduced in all sections of this third edition. These include, among others, the removal of the diagnosis of combined familial hyperlipaemia, the incorporation of new evidence of the beneficial effect of olive oil in vascular prevention (CORonary Diet Intervention with Olive Oil and cardiovascular PREVention [CordioPrev] study), the importance of following a healthy diet not only for the individual but also for the planet, the role of functional foods, the incorporation of treatment plans for new drugs approved in our country, such as bempedoic acid, icosapent ethyl (IPE), or the polypill, the indication for semaglutide to treat obesity, the incorporation of the recent 2023 European Society of Hypertension (ESH) guidelines, and the advances in the screening and prevention of atrial fibrillation (AF). The aim of this document is to serve all clinicians who in one way or another care for patients with vascular risk (VR), both in primary and hospital care, in primary or secondary prevention, and, in general, all members of the societies that make up the Spanish Interdisciplinary Committee for Vascular Prevention (CEIPV). This document is also addressed to professionals in training, not exclusively those in the health professions, and in particular to basic researchers interested in the atherosclerotic process.
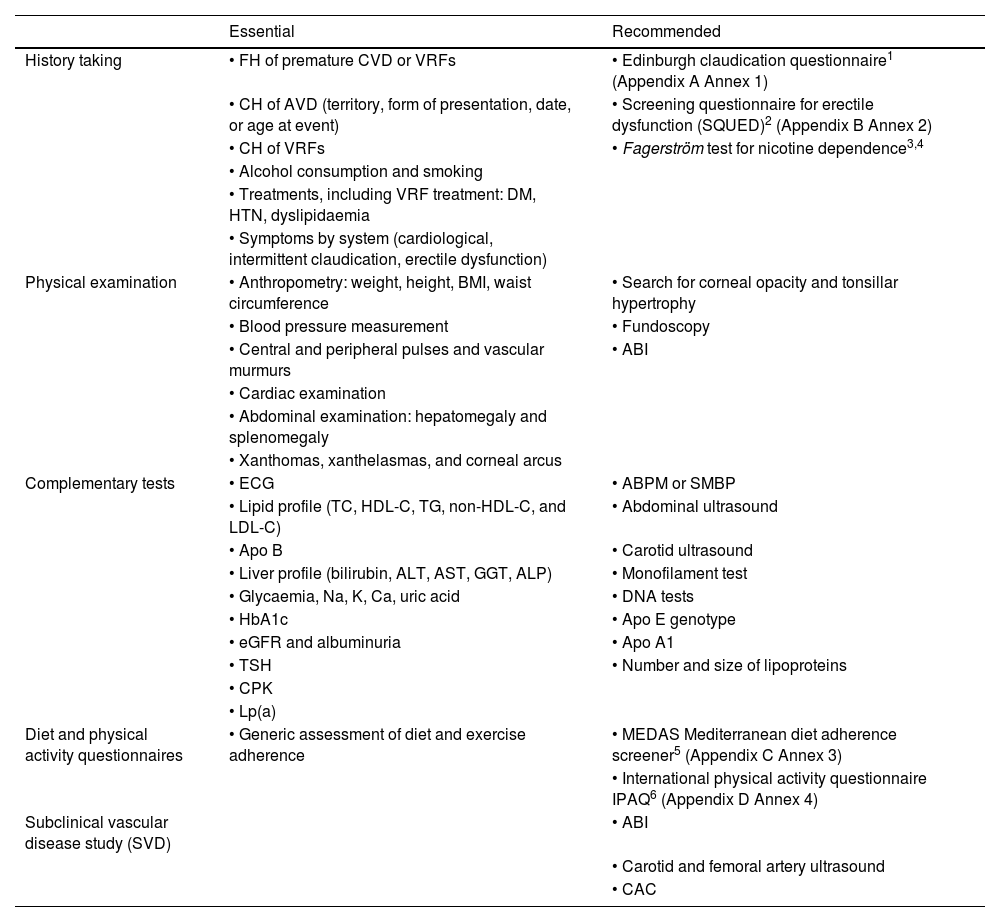
History taking, examination, and complementary tests in the consultation roomA conventional medical history and well-ordered recording of the patient's symptoms and signs are the standard procedure for establishing a clinical diagnosis. Table 1 summarises the elements that should be included in a VR consultation.
History taking, examination, and complementary tests to estimate VR.
| Essential | Recommended | |
|---|---|---|
| History taking | • FH of premature CVD or VRFs | • Edinburgh claudication questionnaire1 (Appendix A Annex 1) |
| • CH of AVD (territory, form of presentation, date, or age at event) | • Screening questionnaire for erectile dysfunction (SQUED)2 (Appendix B Annex 2) | |
| • CH of VRFs | • Fagerström test for nicotine dependence3,4 | |
| • Alcohol consumption and smoking | ||
| • Treatments, including VRF treatment: DM, HTN, dyslipidaemia | ||
| • Symptoms by system (cardiological, intermittent claudication, erectile dysfunction) | ||
| Physical examination | • Anthropometry: weight, height, BMI, waist circumference | • Search for corneal opacity and tonsillar hypertrophy |
| • Blood pressure measurement | • Fundoscopy | |
| • Central and peripheral pulses and vascular murmurs | • ABI | |
| • Cardiac examination | ||
| • Abdominal examination: hepatomegaly and splenomegaly | ||
| • Xanthomas, xanthelasmas, and corneal arcus | ||
| Complementary tests | • ECG | • ABPM or SMBP |
| • Lipid profile (TC, HDL-C, TG, non-HDL-C, and LDL-C) | • Abdominal ultrasound | |
| • Apo B | • Carotid ultrasound | |
| • Liver profile (bilirubin, ALT, AST, GGT, ALP) | • Monofilament test | |
| • Glycaemia, Na, K, Ca, uric acid | • DNA tests | |
| • HbA1c | • Apo E genotype | |
| • eGFR and albuminuria | • Apo A1 | |
| • TSH | • Number and size of lipoproteins | |
| • CPK | ||
| • Lp(a) | ||
| Diet and physical activity questionnaires | • Generic assessment of diet and exercise adherence | • MEDAS Mediterranean diet adherence screener5 (Appendix C Annex 3) |
| • International physical activity questionnaire IPAQ6 (Appendix D Annex 4) | ||
| Subclinical vascular disease study (SVD) | • ABI | |
| • Carotid and femoral artery ultrasound | ||
| • CAC |
ABI: ankle-brachial index; ABPM: ambulatory blood pressure monitoring; ALP: alkaline phosphatase; ALT: alanine aminotransferase; Apo A1: apolipoprotein A1; Apo B: apolipoprotein B; Apo E: apolipoprotein E; AST: aspartate aminotransferase; AVD: atherosclerotic vascular disease; BMI: body mass index; Ca: calcium; CAC: coronary artery calcium; CH: clinical history; DM: diabetes mellitus; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; FH: family history; GGT: gamma-glutamyl transferase; HbA1c: glycated haemoglobin; HDL-C: high-density lipoprotein cholesterol; HTN: hypertension; IPAQ: International Physical Activity Questionnaire; K: potassium; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein (a); MEDAS: Mediterranean Diet Adherence Screener; Na: sodium; non-HDL-C: non-HDL cholesterol; SMBP: self-measured blood pressure monitoring; SQUED: Screening Questionnaire for Erectile Dysfunction; TC: total cholesterol; TG: triglycerides; TSH: thyroid-stimulating hormone; VFR: vascular risk factors.
Knowledge of first-degree family history (FH) is necessary, both in terms of prevalent diseases related to AVD and CVR, especially in cases of suspected familial hypercholesterolaemia (FH) or premature AVD. FH is of greatest value when it occurs in first-degree relatives (father, mother, children, or siblings) and early in life, below the age of 55 years in men and below the age of 65 years in women.
Clinical historyIn addition to the conventional clinical history (CH) (allergies, surgical interventions, etc.), a history of AVD and the various major VRFs (diabetes mellitus [DM], hypertension [HTN], dyslipidaemia, smoking, and obesity) should be specifically interrogated. If any are present, the age of onset and current or previous treatments, regardless of their indication, should be noted. In the case of lipid-lowering therapy, the type of treatment, its intensity, and the months or years of treatment (or date of initiation) should be indicated. Adverse reactions or drug intolerance and existing or potential pregnancy should also be known. The potency and chronology of VRFs (number of cigarettes per day and years of smoking, peak levels of low-density lipoprotein cholesterol [LDL-C], glycosylated haemoglobin [HbA1c], systolic blood pressure [SBP], and weight or body mass index [BMI]) should be quantified. The presence of systemic disease with low-grade inflammatory burden, such as psoriasis, human immunodeficiency virus (HIV) disease, rheumatoid arthritis, or systemic lupus erythematosus, chronic obstructive pulmonary disease (COPD), and cancer, should also be recorded, as they themselves or their treatment increase VR. In women, a history of hypertension or gestational DM, polycystic ovary syndrome (PCOS), date of onset of menopause, and hormonal therapies should also be recorded.
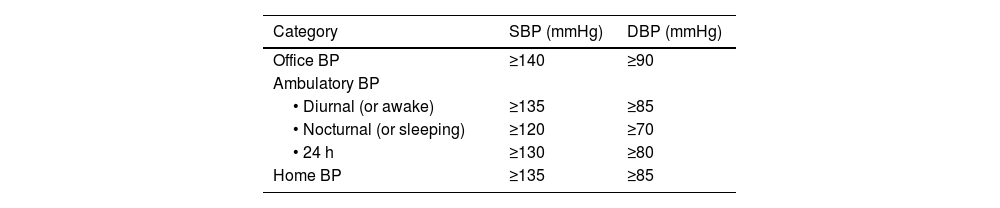
Taking a current history and by systemThe reason for consultation should be investigated, which in VR patients is usually lack of control of one or more VRFs. Symptoms associated with ischaemic events in the three main vascular territories, which may have gone unnoticed or undiagnosed (transient neurological deficits, exertional chest pain, palpitations, dyspnoea, or intermittent claudication), cardinal symptoms of DM, headache or dizziness associated with elevated blood pressure (BP), and symptoms related to secondary HTN conditions should be investigated (Table 2). If the patient has been instructed, ambulatory blood pressure monitoring readings should be noted (ABPM).
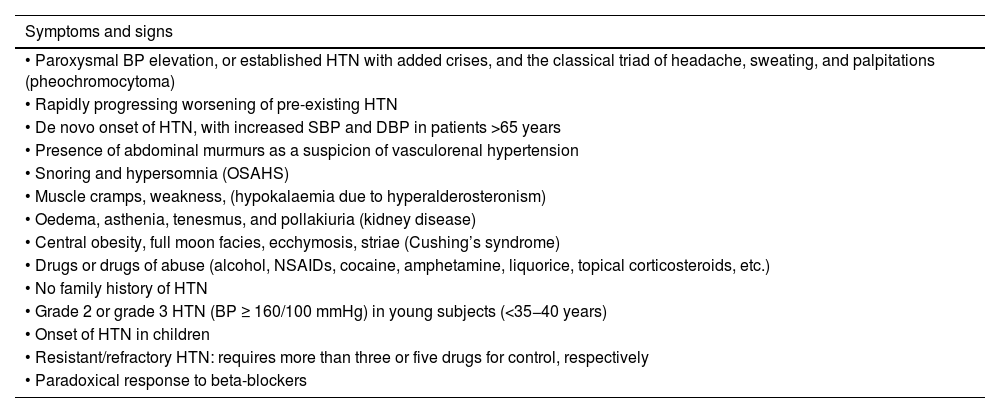
Symptoms and signs suggestive of secondary hypertension.
| Symptoms and signs |
|---|
| • Paroxysmal BP elevation, or established HTN with added crises, and the classical triad of headache, sweating, and palpitations (pheochromocytoma) |
| • Rapidly progressing worsening of pre-existing HTN |
| • De novo onset of HTN, with increased SBP and DBP in patients >65 years |
| • Presence of abdominal murmurs as a suspicion of vasculorenal hypertension |
| • Snoring and hypersomnia (OSAHS) |
| • Muscle cramps, weakness, (hypokalaemia due to hyperalderosteronism) |
| • Oedema, asthenia, tenesmus, and pollakiuria (kidney disease) |
| • Central obesity, full moon facies, ecchymosis, striae (Cushing’s syndrome) |
| • Drugs or drugs of abuse (alcohol, NSAIDs, cocaine, amphetamine, liquorice, topical corticosteroids, etc.) |
| • No family history of HTN |
| • Grade 2 or grade 3 HTN (BP ≥ 160/100 mmHg) in young subjects (<35−40 years) |
| • Onset of HTN in children |
| • Resistant/refractory HTN: requires more than three or five drugs for control, respectively |
| • Paradoxical response to beta-blockers |
Weight, height, abdominal circumference, and BMI should be recorded and calculated. BP should be measured according to the recommendations in Table 3, both in the consultation room and at home.9 Basic cardiocirculatory examination is mandatory, especially the presence of murmurs and the presence and symmetry of arterial pulses; interpretation of the findings will depend on the context: an absence of pedal pulses may indicate peripheral arterial disease (PAD) in an elderly patient with claudication, while asymmetry of pulses in a young hypertensive patient may indicate coarctation of the aorta. Hepatomegaly and/or splenomegaly should be noted. Xanthomas, their morphology, and location, are a primary diagnostic factor in many cases.
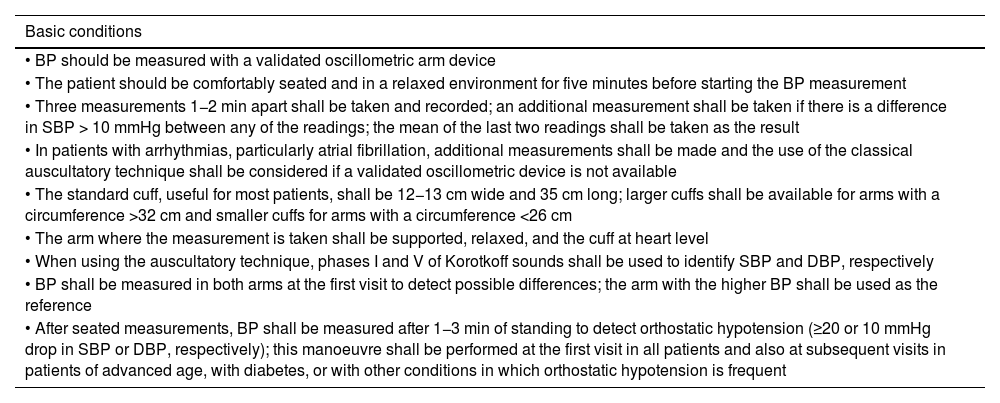
Basic conditions for adequate BP measurement in the consultation room.
| Basic conditions |
|---|
| • BP should be measured with a validated oscillometric arm device |
| • The patient should be comfortably seated and in a relaxed environment for five minutes before starting the BP measurement |
| • Three measurements 1−2 min apart shall be taken and recorded; an additional measurement shall be taken if there is a difference in SBP > 10 mmHg between any of the readings; the mean of the last two readings shall be taken as the result |
| • In patients with arrhythmias, particularly atrial fibrillation, additional measurements shall be made and the use of the classical auscultatory technique shall be considered if a validated oscillometric device is not available |
| • The standard cuff, useful for most patients, shall be 12−13 cm wide and 35 cm long; larger cuffs shall be available for arms with a circumference >32 cm and smaller cuffs for arms with a circumference <26 cm |
| • The arm where the measurement is taken shall be supported, relaxed, and the cuff at heart level |
| • When using the auscultatory technique, phases I and V of Korotkoff sounds shall be used to identify SBP and DBP, respectively |
| • BP shall be measured in both arms at the first visit to detect possible differences; the arm with the higher BP shall be used as the reference |
| • After seated measurements, BP shall be measured after 1−3 min of standing to detect orthostatic hypotension (≥20 or 10 mmHg drop in SBP or DBP, respectively); this manoeuvre shall be performed at the first visit in all patients and also at subsequent visits in patients of advanced age, with diabetes, or with other conditions in which orthostatic hypotension is frequent |
BP: blood pressure; DBP: diastolic blood pressure; SBP: systolic blood pressure.
As an indicative example, tendinous xanthomas suggest FH, tuberoeruptive xanthomas indicate chylomicronaemia, and palmar striated xanthomas are characteristic of dysbetalipoproteinaemia. The presence of stony xanthomas attached to bony surfaces is suggestive of cerebro-tendinous xanthomatosis (Fig. 1).10
Complementary testsEvaluation of VR and diagnosis of dyslipidaemias require a blood test. Optimal conditions for blood collection, processing, and evaluation have been published by consensus by the European Atherosclerosis Societies (EAS) and European Federation of Clinical Chemistry and Laboratory Medicine11 and are shown in Appendix E Annex 5.
According to the consensus document drawn up by 15 Spanish scientific societies,12 a basic lipid profile is required: total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C (estimated by the Friedewald, Samson or Martin Hopkins formula, or by direct method) and calculation of non-HDL-cholesterol (non-HDL-C), which is a measure of atherogenic cholesterol not influenced by TG concentration. The latest European guidelines on cardiovascular prevention (2021) include it in VR calculation.13
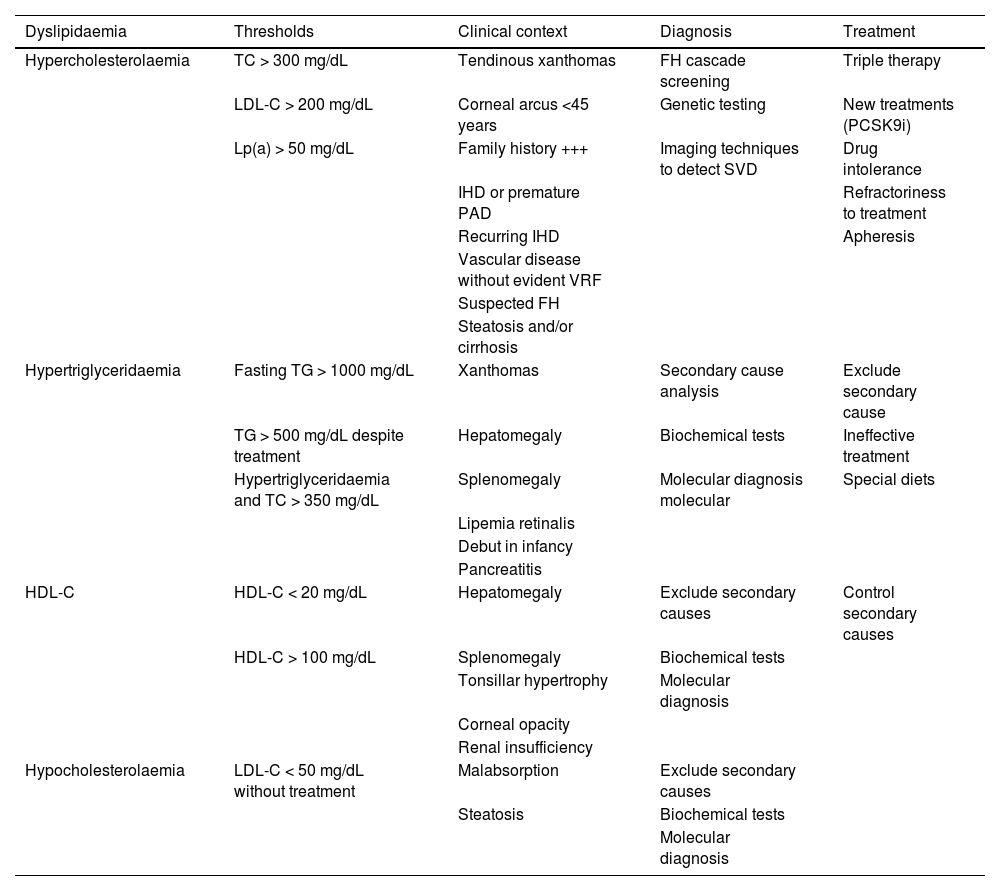
Apolipoprotein B (Apo B) testing can contribute to screening for dysbetalipoproteinaemia.14 It also indicates the total number of atherogenic lipoproteins and is an excellent marker of events. European cardiology guidelines recommend its testing, especially in patients with DM, visceral obesity, metabolic syndrome (MS), or in the case of low levels of LDL-C, when LDL-C testing is less reliable. Lipoprotein(a) (Lp[a]) concentration should be measured at least once in a lifetime and ideally at the first visit.12 In patients with significant elevation of Lp(a), if there is insufficient pharmacological response, LDL-C estimation could be corrected by the formula: LDL-C corrected by Lp(a) (mg/dL) = LDL-C (mg/dL) - [Lp(a) (mg/dL) × .3].12 This correction is highly dependent on the Lp(a) isoform and should only be used as an estimate.15 Elevated Lp(a) plays an important role in the increased VR shown by some patients with FH, and in subjects with premature or recurrent ischaemic disease, despite good control of the other VRFs.16 Patients with very high Lp(a) (>180 mg/dL) have an VR equivalent to those with heterozygous familial hypercholesterolaemia (HeFH).
At the first visit, a conventional haemogram and biochemical tests including glycaemic profile (fasting blood glucose, HbA1c), renal and liver function, as well as creatine phosphokinase (CPK), sodium (Na), potassium (K), calcium (Ca), uric acid, and thyroid stimulating hormone (TSH) levels should be requested. In urine, preferably an early morning sample, an albumin-to-creatinine ratio test should be ordered. Urine protein measurement is necessary to rule out nephrotic syndrome. Since the risk of hepatotoxicity from treatments is exceptional,17 routine transaminase monitoring during statin therapy is not recommended, except when there is a dose increase (European Atherosclerosis Society/European Society Cardiology [EAS/ESC] 2019).18 A resting electrocardiogram (ECG) provides valuable information in patients being assessed for HTN and may show signs compatible with myocardial ischaemia or necrosis, left ventricular enlargement, or rhythm disturbances such as AF.
History taking, examination, and complementary tests in the consultation room: recommendedHistory taking: Edinburgh questionnaire and erectile dysfunction questionnaireA specific history should be taken on smoking, including the Fagerström study in smokers (see below, in the section “Smoker patients”). If intermittent claudication is suspected, the Edinburgh questionnaire, validated in Spain (Appendix A Annex 1), helps support the clinical diagnosis of PAD.1 The questionnaire for the assessment of erectile dysfunction (SQUED) is shown in Appendix B Annex 2.
Physical examinationCorneal opacity (lecithin cholesterol acyltransferase [LCAT] deficiency, or tonsillar hypertrophy [Tangier's disease]) should be specifically looked for in patients with very low HDL-C. Fundoscopy provides valuable information in the examination of the patient with DM, in primary chylomicronaemias (lipidaemia retinalis), and in target organ damage (TOD) of HTN, essential in grade 3 HTN (SBP ≥ 180 mmHg and/or diastolic BP (DBP) ≥ 110 mmHg).
Additional complementary testsThe SEA deem it advisable to measure lipoparticle size and concentration in the presence of:
- •
Suspected mismatch between lipid concentration and particle number, a common situation in DM, obesity, and MS.
- •
Premature or recurrent AVD, with no underlying VRF.
- •
Rare or complex lipid disorders, such as extreme HDL-C concentrations.
- •
Clinical situations where classical analytical techniques cannot be applied, such as very low LDL-C concentrations.19
Lipoprotein ultracentrifugation could be of interest in confirming dysbetalipoproteinaemia (very low-density lipoprotein cholesterol [VLDL-C]/TG ratio in mg/dL > .3)20 and to determine the composition of plasma lipoproteins, but given its high cost and complexity, its use is rather limited. Apolipoprotein A1 (Apo A1) testing is recommended in the study of hypercholesterolaemia in childhood. An Apo B/Apo A1 ratio higher than .82 has shown increased sensitivity and specificity in detecting carriers of a genetic variant associated with FH.21
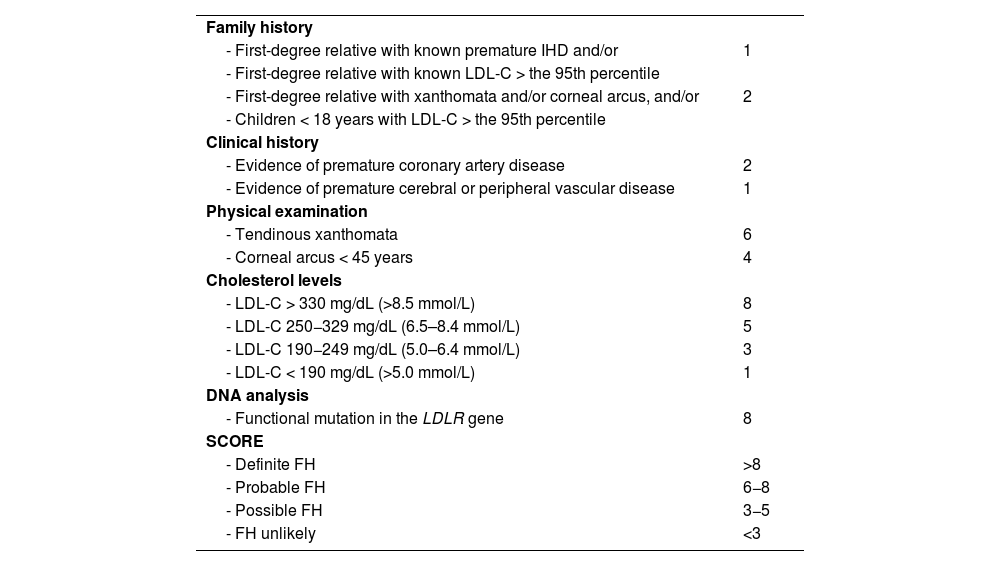
If FH is suspected, the clinical and biochemical scale of the Dutch Lipid Clinic Network Diagnostic criteria for Familial Hypercholesterolaemia (Appendix F Annex 6)22 should be used and confirmed with the genetic diagnosis. Current mass sequencing procedures and the commercialisation of gene panels for hypercholesterolaemia facilitate its diagnosis and the differentiation between heterozygous, compound heterozygous, double heterozygous, and homozygous forms (the latter three could be grouped as “biallelic”). There may be clinical and analytical overlap between all these forms),23 or other diseases with which it may share phenotype (lysosomal acid lipase deficiency or beta-sitosterolaemia).24 Apolipoprotein E (Apo E) genotyping should be requested when dysbetalipoproteinaemia is suspected. Genetic testing should be requested only when monogenic dyslipidaemia is suspected.25 Current genetic studies assessing the risk of severe polygenic hypercholesterolaemia have little impact on the diagnosis and management of patients with primary dyslipidaemias and should not be routinely ordered for clinical purposes.26 Of the additional tests, 24-h ambulatory BP monitoring (ABPM) should be requested in all patients with HTN or with suspected HTN, and is especially indicated when office and ambulatory BP measurements do not agree, when there is high variability in measurements, when nocturnal HTN is suspected (e.g. sleep apnoea) and in cases of resistant HTN.13 SMBP over five to seven days can replace ABPM, especially during follow-up, if there is good agreement between the two.
Screening for subclinical vascular diseaseThe tests discussed in this section are performed with the sole intention of re-stratifying an individual’s VR, as they concern a patient with no established AVD, or symptoms that would raise suspicion of AVD. They refer to a period in the natural history of the atherosclerotic process during which, in the absence of symptoms and signs, there are demonstrable vascular structural abnormalities. By definition, confirmation can only be by specific diagnostic tests. Both VRFs and atheromatous disease are systemic in nature, and therefore finding vascular involvement in one territory also provides information on the state of the disease in other territories. Exploratory techniques should be non-invasive and are proposed to obtain complementary information in estimating VR, to redefine lipid targets, and to guide therapeutic decisions.27 Some of these tests have also been proposed for routine screening; the most common for diagnosing subclinical AVD are set out below.
Ankle-brachial indexThe ankle-brachial index (ABI) is the ratio of ankle-to-arm systolic pressures for each lower limb. A value of less than .9 indicates greater than 50% stenosis between the aorta and distal leg arteries, with high specificity (90%) and acceptable sensitivity (79%)28; it helps identify significant PAD that may be silent or have poorly defined symptoms. Values ≥1.4 usually indicate the presence of arterial calcification, which is also associated with an increased risk of vascular complications, especially frequent in patients with DM. Because it is so simple, ABI can be performed routinely in the assessment of the patient's vascular status, provided that a handheld Doppler is available, and it takes 15 min to perform.
ABI measurement is not justified in low-risk patients because it is not very cost-effective,29 however, it is most cost-effective when performed in subjects with the two main VRFs associated with PAD, such as DM and smoking. In Spanish series, up to 25% of patients with type 2 DM (T2DM) without claudication have an ABI < .9.30 In those with long-standing DM and high probability of microangiopathy (which can be identified with monofilament testing), ABI has low sensitivity for detecting cases of PAD due to the high frequency of arterial calcification that masks its measurement.
Carotid and femoral ultrasonographyAlthough quantification of carotid intima-media thickness (IMT) by ultrasound has been widely used to assess the evolution of the atherosclerotic process and even the benefit of treating hypercholesterolaemia, it is not currently recommended for restratifying VR. Unlike the existence of a carotid plaque.27
A carotid plaque is considered to be a focal thickening of more than 50% of the surrounding vessel wall, or an IMT greater than 1.5 mm that protrudes into the adjacent lumen.31 Not only is its presence assessed but also its amount, size, irregularity, and echodensity, characteristics that are also associated with the risk of vascular complications in the cerebral and coronary territories.
The presence of femoral plaques detected by nuclear magnetic resonance predicts adverse outcomes of lower limb PAD.32 The evidence of the value of their detection by ultrasound to improve VR stratification is being assessed in several ongoing observational studies.33
Coronary calciumComputed tomography (CT) of the chest quantifies coronary artery calcium (CAC), which is expressed in Agatston units.34 The main indication would be for an individual >40 years old, asymptomatic, and at intermediate risk.35 The presence of CAC indicates an advanced stage of coronary atherosclerosis and is a better predictor of the risk of ischaemic events than the presence of carotid or femoral plaques.36,37 When there is no calcification (Agatston = 0), the probability of coronary obstructive lesion is almost nil; while the risk of vascular complications is greater the higher the degree of calcification.38 There is a relative consensus among scientific societies in considering CAC > 100 Agatston units a risk modifier, suggesting, in these circumstances, that treatment with high-intensity statins should be started, with or without low-dose acetylsalicylic acid.35 CAC > 400 Agatston units is associated with a high likelihood of haemodynamically significant lesions and may justify requesting cardiac catheterisation.
CT-guided angiography shows subclinical stenotic coronary artery disease, with the added advantage of reporting plaque volume, lipid-necrotic core volume, and maximum diameter of the stenosis.39 The information it provides is independent of that provided by CAC score, which continues to identify patients at higher risk even in the absence of stenosis.40
Arterial wall stiffnessAortic stiffness, measured as carotid-femoral pulse wave velocity (PWV), is an independent risk marker, but is currently not recommended as a routine procedure due to technical difficulties and reduced reproducibility.13,41
Diet and physical activity questionnaires: recommended scalesBeyond inquiring about general dietary data, such as whether the diet is rich in carbohydrates or saturated fats, or eating disorders, diet can be assessed with a simple 14-question Mediterranean Diet Adherence Screener (MEDAS) (Appendix C Annex 3), which has been validated in the Prevention with Mediterranean Diet (PREDIMED) trial and is associated with the presence of VRFs and VR.42 Alcohol consumption should also be quantified, by noting the number (volume in mL) of beers, wine, and/or spirits per week, or by quantifying the grams of alcohol per week, estimating alcohol content in degrees of 6, 12, and 40 proof, respectively, using the formula (volume in mL × alcohol content × .8)/100. Physical activity can be assessed semi-quantitatively during work (1 = not working or sedentary; 2 = walks regularly during work; 3 = walks regularly and lifts weights, and 4 = very physically active) and during leisure time (1 = no exercise; 2 = walks at least four hours in the week; 3 = walks > four hours in the week; and 4 = vigorous training).43 Finally, it is also possible to simply quantify physical activity using the International Physical Activity Questionnaire (IPAQ), also validated 6 and available online44 (Appendix D Annex 4).
Indication for special testsSome biomarkers have been extensively investigated as predictors of VR (homocysteine, lipoprotein-associated phospholipase A2, thrombogenic and fibrinolytic factors) and have not been incorporated into clinical routine because they do not provide additional information relevant to VR. Taken together, these biomarkers have no clinical justification as they do not increase the predictive capacity for events compared to the European Systematic Coronary Risk Evaluation (SCORE).13 Its testing in epidemiological studies makes it possible to detect patients who may have a residual risk irrespective of lipid parameters, although it has the disadvantage of high intra-individual variability, making it difficult to use in clinical practice.45
If there are suggestive symptoms or signs, or there is suspicion of disease, the relevant complementary tests should be requested, such as stress test in the case of chest pain, or imaging tests in the case of suspicion of secondary HTN, hormone tests, etc.
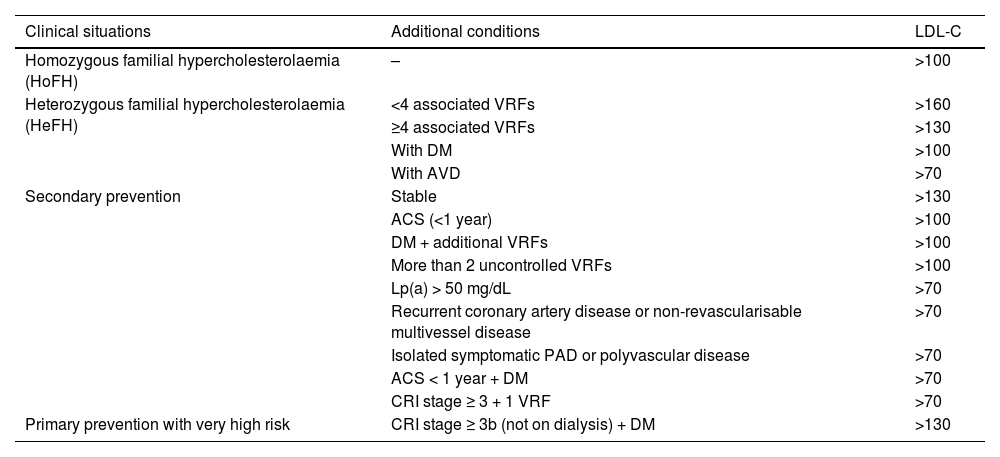
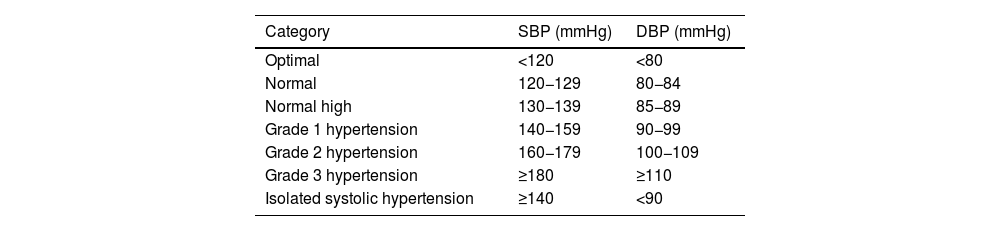
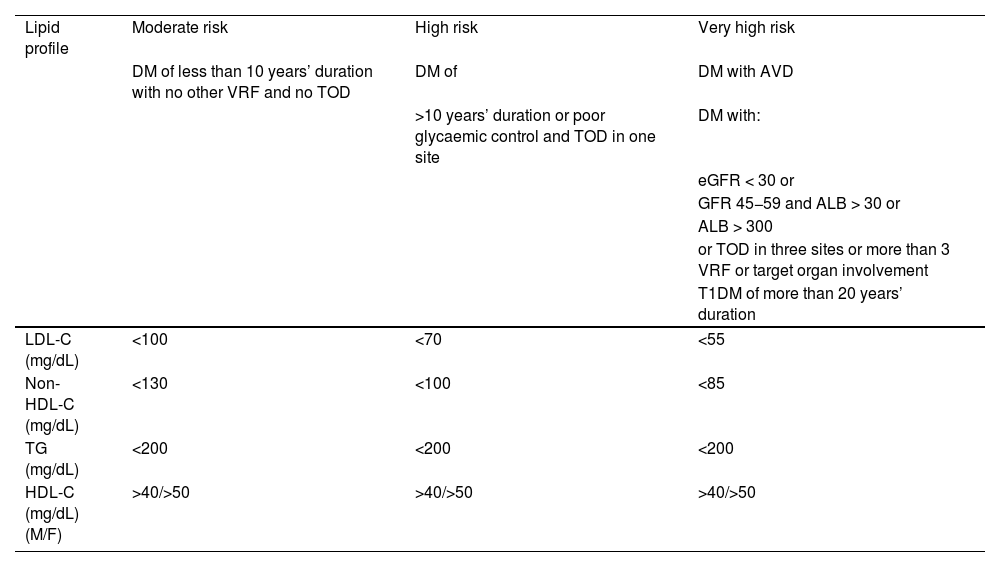
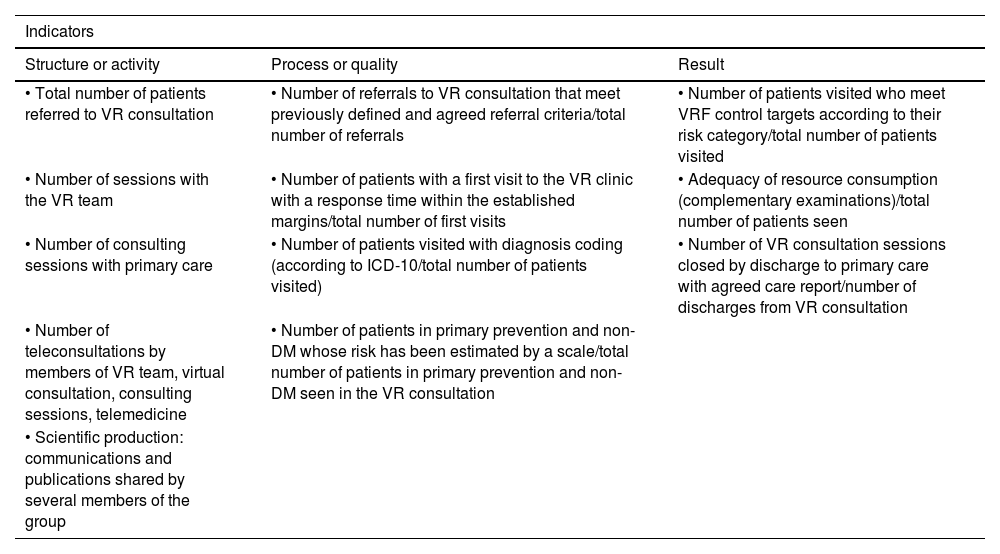
Diagnosis of vascular riskCollection of diagnoses in the medical record: diagnostic criteriaAll patients seen in a VR clinic should have a list of standardised diagnoses in their medical records, including those listed in Table 4. In addition, all diagnoses derived from any diseases they may have, both cardiovascular and non-cardiovascular, should be added.8,46–54
Diagnostic criteria.
| Diagnosis | Definition | ||
|---|---|---|---|
| Hypercholesterolaemia | • In secondary prevention (coronary, cerebrovascular or peripheral arterial disease) or if there are obstructive carotid or coronary plaques: LDL-C > 55 mg/dL or non-HDL-C >85 mg/dL | ||
| There is no optimal TC or LDL-C level, since the lower the concentration, the better. Elevated cholesterol would be considered levels at which lipid-lowering treatment is recommended, which depend on the baseline risk of each person (Table 10) | • In T2DM, with TOD, SVD, or with 3 or more VRFs: LDL-C >55 mg/dL or non-HDL-C 85 mg/dL | ||
| • In T2DM, without TOD, without SVD, and with fewer than 3 VRFs: LDL-C > 70 mg/dL or non-HDL-C > 100 mg/dL | |||
| • In patients with grade 3 CKD, without TOD or SVD: LDL-C >70 mg/dL or non-HDL-C >100 mg/dL | |||
| • In patients with grade 3 CKD, with TOD or SVD: LDL-C >55 mg/dL or non-HDL-C >85 mg/dL | |||
| • In patients with grade 4 or 5 CKD: LDL-C >55 mg/dL or non-HDL-C >85 mg/dL | |||
| • In patients without cardiovascular disease, DM, or CKD LDL-C >116 mg/dL according to whether the risk is low or moderate according to SCORE2 | |||
| Hypertriglyceridaemia | • desirable TG < 150 mg/dL | ||
| • Hypertriglyceridaemia: | |||
| ◦ Mild: 150–499 mg/dL | |||
| ◦ Moderate: 500–1.000 mg/dL | |||
| ◦ Severe: >1000 mg/dL | |||
| Mixed hyperlipidaemia | Elevated concentrations of both LDL-C or non-HDL-C and TG | ||
| Familial hypercholesterolaemia | According to the tables of the Dutch Lipid Clinic Network Diagnostic criteria for Familial Hypercholesterolemia (Appendix F Annex 6) | ||
| Atherogenic dyslipidaemia | Hypertriglyceridaemia (TG > 150 mg/dL) and low HDL-C (<40 mg/dL in males and <45 mg/dL in females). Increased number small, dense LDL particles | ||
| Abeta/hypobetaliproteinaemia | Apo B lower than the 10th percentile, according to age, race, and sex | ||
| Hypoalphalipoproteinaemia | HDL-C lower than the 10th percentile, according to age, race, and sex | ||
| Hyperlipoproteinaemia (a) | Lp(a) ≥50 mg/dL or ≥ 120 nmol/L | ||
| HTN (office blood pressure measurement) | • Optimal BP: SBP < 120 and DBP < 80 mmHg | ||
| • Normal: SBP 120−129 and DBP 80−84 mmHg | |||
| • Normal-high SBP 130−139 or DBP 85−89 mmHg | |||
| • Grade I HTN: SBP 140−159 and/or DBP 90−99 mmHg | |||
| • Grade II HTN: SBP 160−179 and/or DBP 100−109 mmHg | |||
| • Grade III HTN: SBP ≥ 180 and/or DBP ≥ 110 mmHg | |||
| • Isolated systolic HTN: SBP ≥ 140 and DBP < 90 mmHg | |||
| The diagnosis is established after checking BP values in at least two measurements at two or more visits several weeks apart. | |||
| When SBP and DBP fall into different categories, the higher category applies. | |||
| Isolated systolic HTN is classified into grades (1, 2, or 3) according to systolic BP value | |||
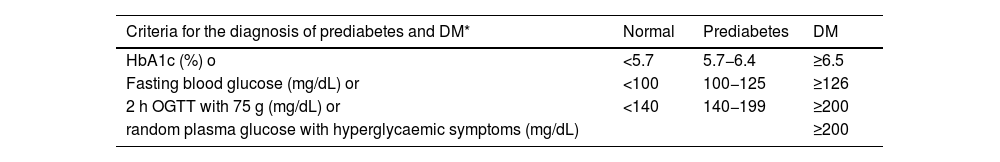
| DM | • Fasting blood glucose of at least 8 h ≥ 126 mg/dL (7.0 mmol/L)*, or | ||
| • Blood glucose 2 h after an OGTT of 75 g ≥ 200 mg/dL (11.1 mmol/L)*, o | |||
| • HbA1c ≥6.5% (48 mmol/mol), or | |||
| • Patient with classic symptoms of hyperglycaemia with a blood glucose value ≥200 mg/dL, regardless of fasting status (11.1 mmol/L)* | |||
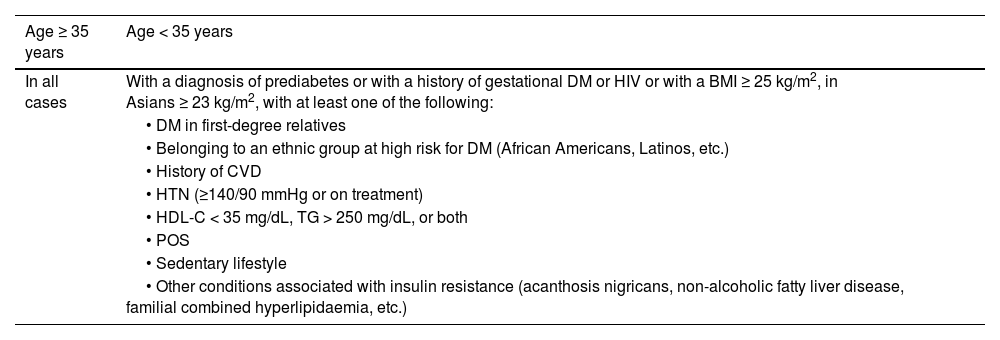
| Prediabetes | Presence of: | ||
| • Impaired fasting blood glucose: fasting blood glucose between 100 mg/dL (5.6 mmol/L) and 125 mg/dL (6.9 mmol/L), or | |||
| • Glucose intolerance: blood glucose 2 h after 75 g OGTT between 140 mg/dL (7.8 mmol/L) and 199 mg/dL (11.0 mmol/L), or | |||
| • HbA1c between 5.7% and 6.4% (39−47 mmol/mol) | |||
| Obesity | BMI ≥ 30.0 kg/m2 | ||
| • Grade I obesity: 30.0–34.9 kg/m2 | |||
| • Grade II obesity: 35.0–39.9 kg/m2 | |||
| • Grade III obesity: ≥40 kg/m2 | |||
| Overweight | BMI ≥ 25.0 kg/m2 y <30.0 kg/m2 | ||
| • Grade I: 25.1-27.5 kg/m2 | |||
| • Grade II: 27.6–30.0 kg/m2 | |||
| Normal weight | BMI 18.50-24.9 kg/m2 | ||
| Underweight | BMI <18.5 kg/m2 | ||
| • Extremely thin <16.0 kg/m2 | |||
| • Moderately thin 16.0-16.9 kg/m2 | |||
| • Mildly thin 17.0-18.4 kg/m2 | |||
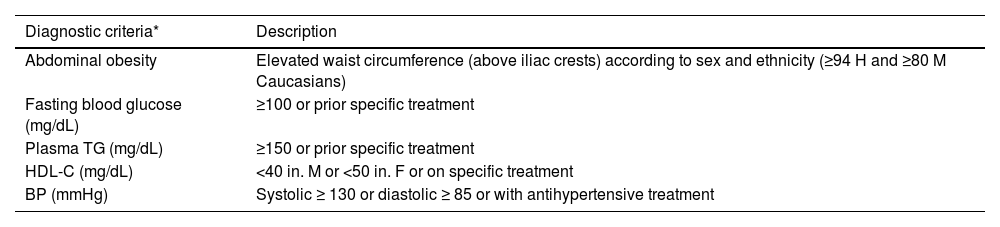
| Metabolic syndrome | Three of the following five criteria are required: | ||
| • High abdominal girth (≥94 cm in males and ≥80 cm in females of European origin) | |||
| • TG ≥ 150 mg/dL (1.7 mmol/L) or on treatment with TG-lowering drugs | |||
| • HDL-C <40 mg/dL (1.0 mmol/L) in males or <50 mg/dL (1.3 mmol/L) in females or on treatment with drugs to increase HDL-C | |||
| • BP ≥ 130/85 mmHg or on treatment with BP-lowering drugs | |||
| • Fasting blood glucose ≥100 mg/dL or on treatment with anti-diabetic drugs | |||
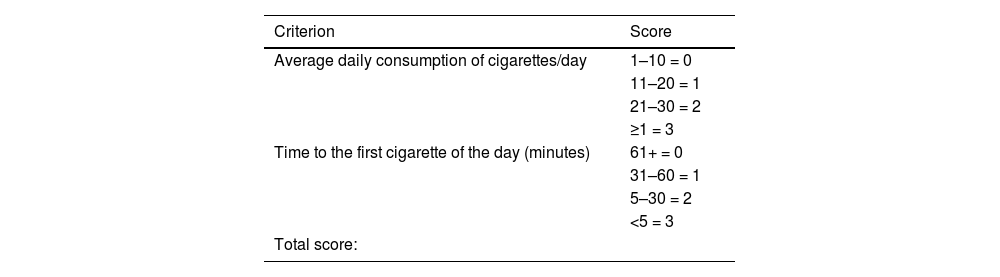
| Current smoker52 | A person who has smoked at least one cigarette in the last six months. This group includes: | ||
| • Daily smoker: a person who has smoked at least one cigarette a day, in the last six months | |||
| • Occasional smoker: a person who has smoked less than one cigarette per day | |||
| Quantification of tobacco consumption (pack/year rate): (N° cigarettes smoked per day × N° years of smoking)/20) | |||
| Ex smoker | A person who has been a former smoker and has been completely abstinent for at least the last six months | ||
| Never smoked | A person who has never smoked or has smoked fewer than 100 cigarettes in their lifetime | ||
| Passive smoker | A person who does not smoke, but regularly breathes second-hand or environmental tobacco smoke or second-hand smoke | ||
| Target organ damage | • Arterial stiffness: pulse pressure (in the elderly) ≥60 mmHg or PWV > 10 m/s | ||
| • Left ventricular hypertrophy: | |||
| ◦ on ECG (Sokolow–Lyon index >3.5 mV; RaVL >1.1 mV; Cornell voltage-duration product >2440 mV*ms), or | |||
| ◦ echocardiographic (left ventricular mass >115 g/m2 in males or >95 g/m2 in females per body surface area) | |||
| • Albuminuria/creatinine ratio >30 mg/g or albuminuria >30 mg/24 h | |||
| SVD | Presence of: | ||
| • ABI < .9 (for some authors a value >1.4 is also pathological), or | |||
| • At least one plaque in epicardial, carotid, or femoral coronary artery, or | |||
| • CAC quantification: Agatston ≥100 units | |||
| GFR (mL/min/1.73 m2) in CKD | Grade | GFR | Definition |
| G1 | ≥90 | Normal | |
| G2 | 60−89 | Slight decrease in GFR | |
| G3a | 45−59 | Mild-moderate decrease in GFR | |
| G3b | 30−44 | Moderate decrease in GFR | |
| G4 | 15−29 | Severe decrease in GFR | |
| G5 | <15 | Renal failure (pre-dialysis) | |
| Albuminuria categories (albuminuria/creatinine ratio in mg/g) in CKD | A1 | <30 | Normal |
| A2 | 30−300 | Moderately elevated | |
| A3 | >300 | Very elevated | |
ABI: ankle-brachial index; Apo B: apolipoprotein B; AVD: atherosclerotic vascular disease; BMI: body mass index; BP: blood pressure; CAC: coronary artery calcium; CKD: chronic kidney disease; DBP: diastolic blood pressure; DM: diabetes mellitus; ECG: electrocardiogram; GFR: glomerular filtration rate; HbA1c: glycated haemoglobin; HDL: high-density lipoprotein; HDL-C: high-density lipoprotein cholesterol; HTN: hypertension; IMT: intima-media thickness; LDL: low-density lipoprotein; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein (a); non-HDL-C: non-HDL-cholesterol: OGTT: oral glucose tolerance test; PWV: pulse wave velocity; RaVL: voltage of R wave in lead; SBP: systolic blood pressure; SCORE2: Systematic Coronary Risk Estimation 2; SVD: subclinical vascular disease; T2DM: type 2 diabetes mellitus; TC: total cholesterol; TG: triglycerides; TOD: target organ damage; VRFs: vascular risk factors.
Note: Definition adapted from the European Society of HTN and the European Society of Cardiology.
One of the first steps to be taken when assessing patients without AVD with VRFs is to calculate VR, because there are certain decisions that will be made in one direction or another depending on the level or value of VR, such as when to initiate lipid-lowering therapy and its therapeutic target.
(Absolute) risk is the probability of a given vascular event occurring in a defined period of time based on the VRFs of a patient belonging to a given population group. Therefore, there is no universal system to calculate VR. The European guidelines on cardiovascular prevention13 and those on dyslipdaemia control,27 to which the SEA adheres through the CEIPV, recommend the SCORE2 system55 to assess VR in its version for low-risk countries, in a situation of primary prevention, i.e., for individuals who have not yet suffered a vascular event.
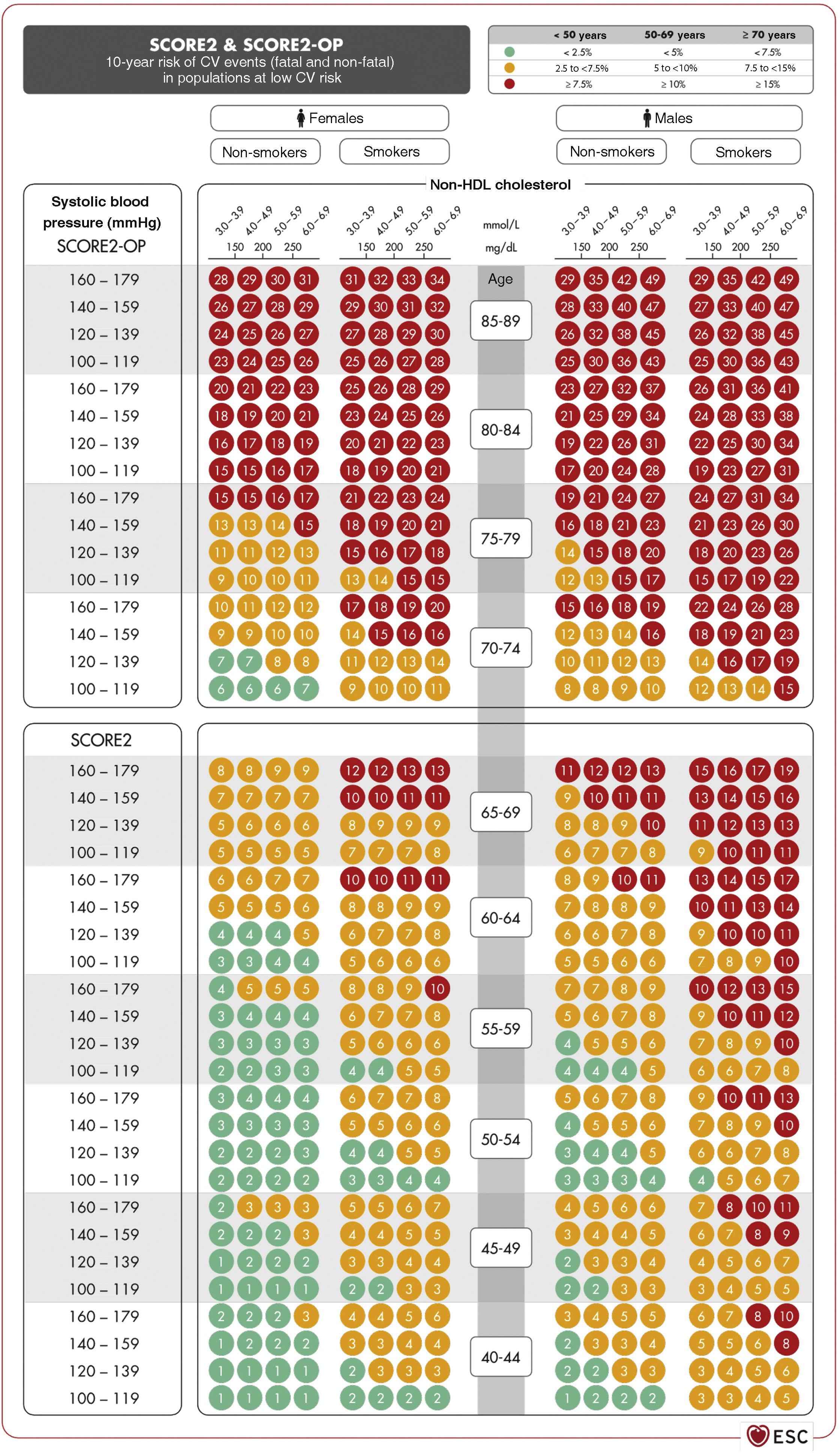
Vascular risk estimation: systematic coronary risk evaluation 2 (SCORE2) and systematic coronary risk estimation 2-older persons (SCORE2-OP) projectsSCORE256,57 captures the risk of both a vascular complication and vascular death in the next 10 years. To obtain the tables of the SCORE2 system, an analysis was performed of 45 cohorts from 43 countries, including 677,684 individuals and 30,121 CV events. The variables predicting the risk of fatal and non-fatal complications are sex, age, smoking (dichotomous), SBP, and the lipid parameter included in this new index is non-HDL-C. DM is not included as it is a priori considered a high-risk condition. However, specific risk tables for patients with DM have recently been published.58 The risk equation is modulated by the incidence of vascular events in each country and therefore the final indices are distributed in four zones: low risk (which includes Spain), moderate, high, and very high; showing a clear east-west gradient. The values are applicable up to 70 years of age, and specific tables have been developed separately for older persons up to 89 years of age (Systematic Coronary Risk Estimation 2-Older Persons [SCORE2-OP])57 based on other cohorts, but with the same mathematical analysis as SCORE2 (Fig. 2).13
SCORE2 and SCORE2-OP tables for low cardiovascular risk countries.13
CV: cardiovascular; HDL: high-density lipoprotein; SCORE2: Systematic Coronary Risk Estimation 2; SCORE2-OP: Systematic Coronary Risk Estimation 2-Older Persons.
Source: Visseren F.L.J., et al., 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC), European Heart Journal 2021; 42 (34): 3227–3337 doi:10.1093/eurheartj/ehab484. Translated and reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology. OUP and European Society of Cardiology accept no responsibility or liability for the accuracy of the translation. The licensee is solely responsible for the translation in this publication/reprint.13
Based on these new SCORE2 and SCORE2-OP indices, the 10-year VR is distributed into three categories in three age bands:
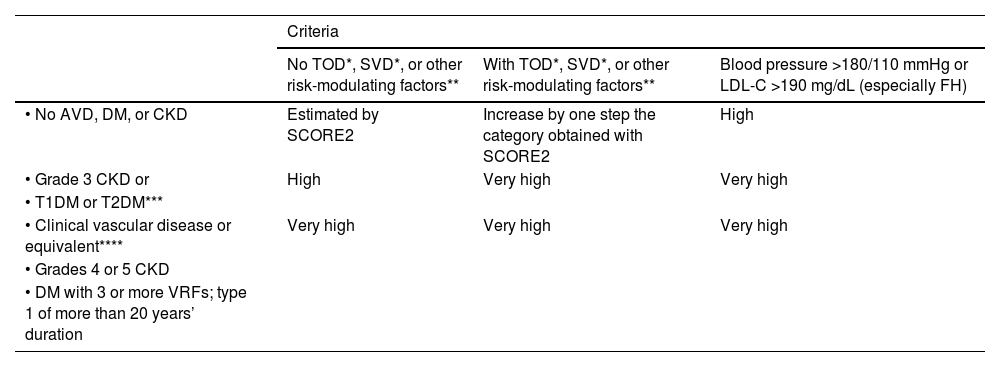
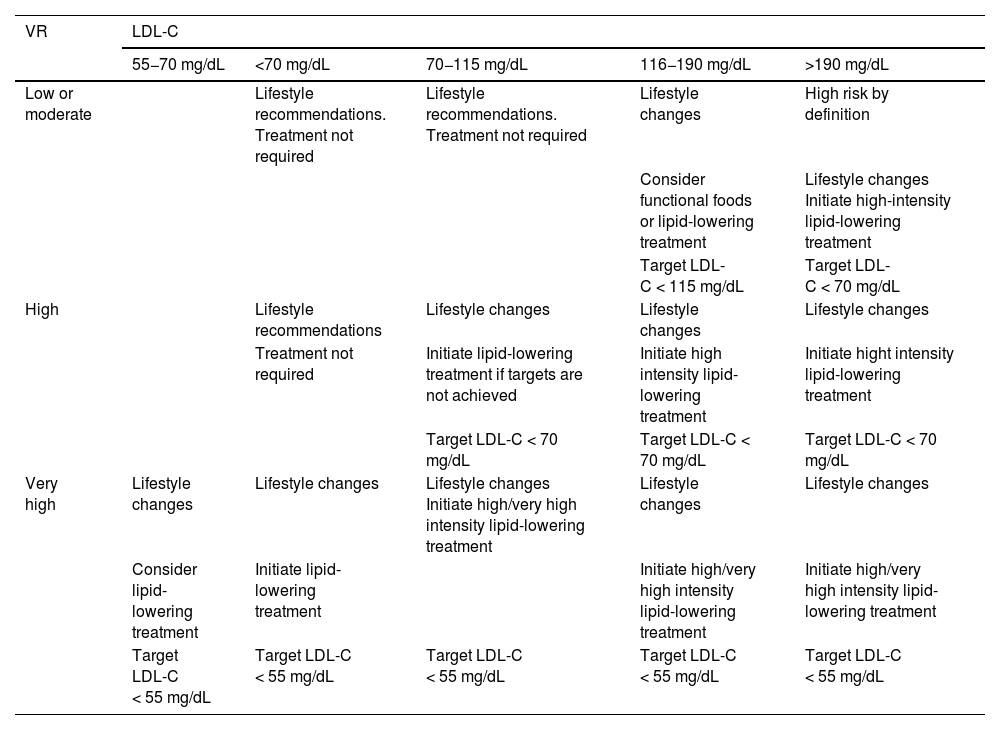
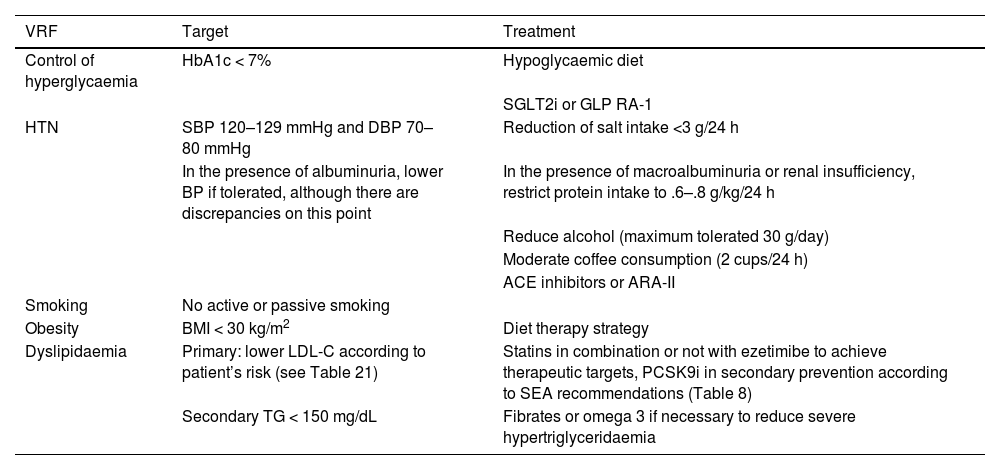
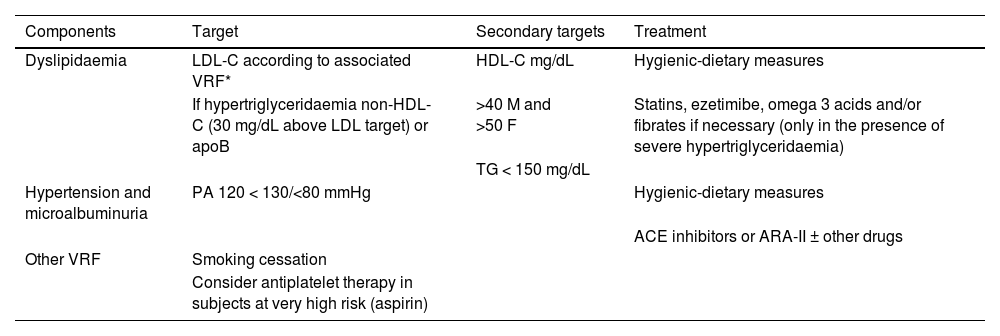
Overall calculation of vascular riskThe overall calculation of VR should be made by means of a global assessment of the patient that includes not only their risk score calculated with SCORE2, but also integrating risk modifying factors, TOD data, and the presence of AVD (Table 5).8,13,18
Global vascular risk estimation.
| Criteria | |||
|---|---|---|---|
| No TOD*, SVD*, or other risk-modulating factors** | With TOD*, SVD*, or other risk-modulating factors** | Blood pressure >180/110 mmHg or LDL-C >190 mg/dL (especially FH) | |
| • No AVD, DM, or CKD | Estimated by SCORE2 | Increase by one step the category obtained with SCORE2 | High |
| • Grade 3 CKD or | High | Very high | Very high |
| • T1DM or T2DM*** | |||
| • Clinical vascular disease or equivalent**** | Very high | Very high | Very high |
| • Grades 4 or 5 CKD | |||
| • DM with 3 or more VRFs; type 1 of more than 20 years’ duration | |||
* Go to Table 3 Diagnostic criteria.
** Increased risk depends on the number and intensity of modulating factors. In general, several modulating factors or extreme severity of modulating factors are required to elevate the risk category to the same level as the presence of SVD or TOD. The modulating factors would be:
• Obesity or sedentary lifestyle.
• Individuals in a situation of social exclusion.
• Glucose intolerance or impaired fasting blood glucose levels.
• Elevation of TG, Apo B, Lp(a).
• Family history of premature AVD.
• Diseases involving increased inflammatory-metabolic stress: autoimmune diseases, OSAHS, COPD, RA, MS, systemic lupus erythematosus, psoriasis, cancer, HIV.
• Severe psychiatric diseases.
• Non-alcoholic fatty liver disease.
*** Patients with T1DM under 35 years of age or type 2 under 50 years of age, and with less than 10 years’ duration, may have a moderate CV risk.
**** The following conditions are considered AVD or equivalent:
• Established clinical AVD:
◦ Coronary event (ACS, stable angina, revascularisation procedure).
◦ Cerebrovascular event: stroke or TIA.
◦ Symptomatic PAD.
◦ Abdominal aortic aneurysm.
◦ AVD evidenced by imaging techniques, i.e., presence of significant atheroma plaque:
◼ By coronary angiography or CT (multivessel disease with >50% of two epicardial arteries).
◼ By carotid or femoral ultrasound (stenosis >50%).
ACS: acute coronary syndrome; Apo B: apolipoprotein B; AVD: atherosclerotic vascular disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CT: computed tomography; CV: cardiovascular; DM: diabetes mellitus; EF: ejection fraction; FH: familial hypercholesterolaemia; HF: heart failure; HIV: human immunodeficiency virus; HTN: hypertension; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein (a); MS: metabolic syndrome; OSAHS: obstructive sleep apnoea-hypopnoea syndrome; PAD: peripheral arterial disease; RA: rheumatoid arthritis; SCORE2: Systematic Coronary Risk Estimation 2; SVD: subclinical vascular disease; T1DM: type 1 diabetes mellitus 1; T2DM: type 2 diabetes mellitus; TG: triglycerides; TIA: transient ischaemic attack; TOD: target organ damage; VR: vascular risk; VRFs: vascular risk factors.
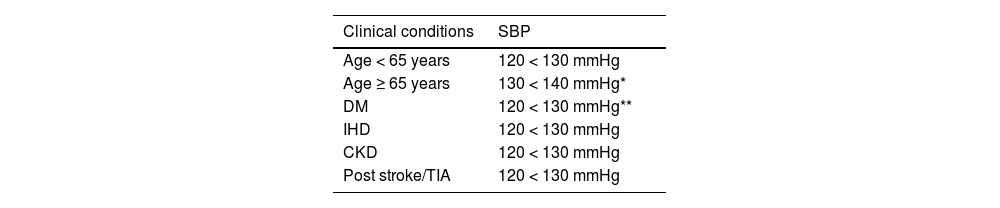
It is advisable to follow the strategy of the European guidelines on cardiovascular prevention and dyslipidaemia control, as well as those on HTN, which classify subjects into four risk categories: low, moderate, high, and very high.
There are situations that directly qualify as high or very high risk: grade 3 HTN, hypercholesterolaemia with LDL-C > 190 mg/dL, DM, TOD, stage 3, or higher chronic kidney disease (CKD), or established AVD. In the remaining situations we will use the SCORE2 system with the cut-off points indicated in the previous section. The presence of risk modifiers means increasing a risk category in the case of scores that are near a higher category.
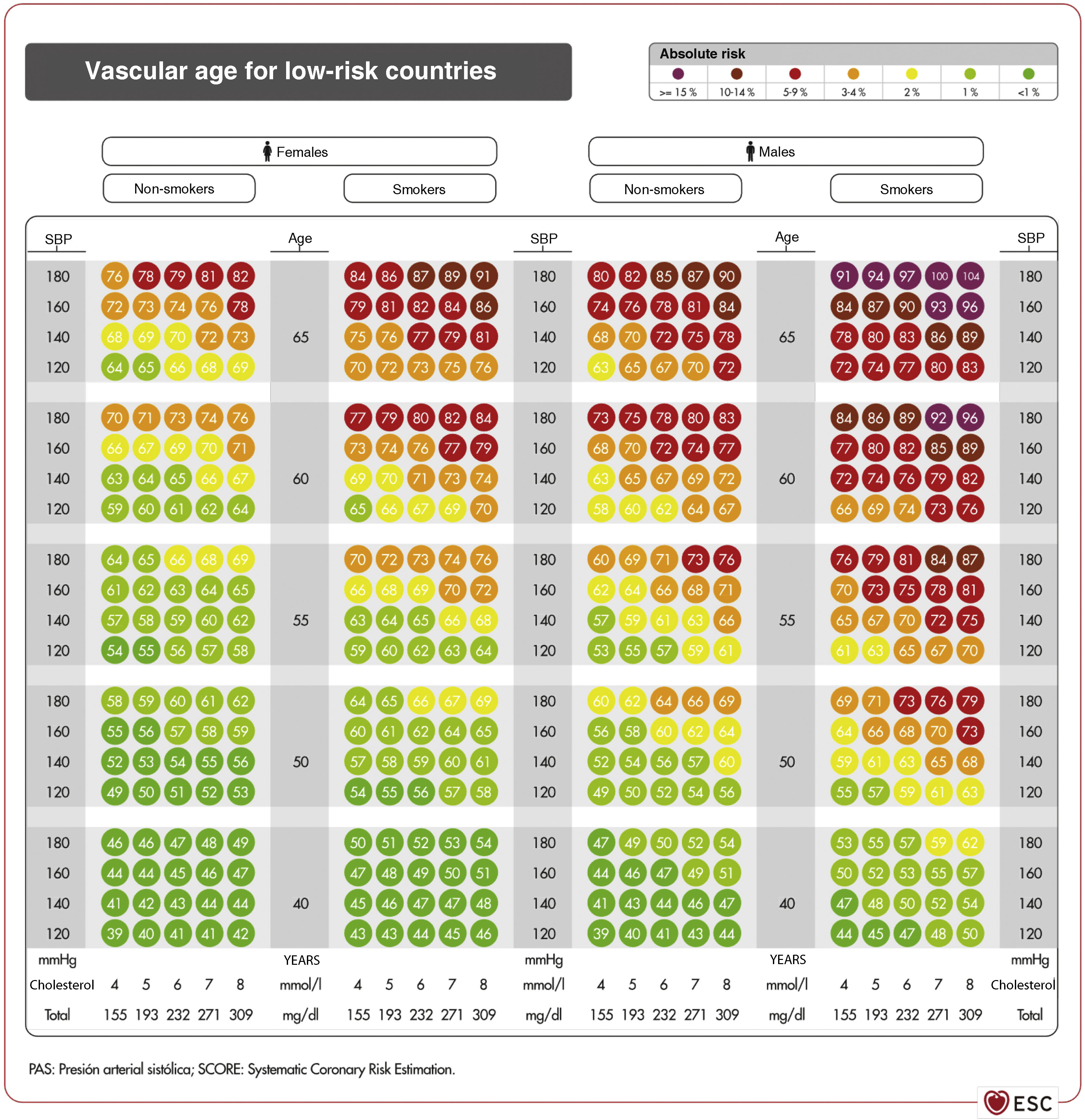
Vascular age and relative riskVascular age can be calculated in young adults with significant elevation of multiple VRFs (Fig. 3).59 Giving the patient this information on their VR status can be more easily understood than the mathematical score of absolute risk. Individuals should be made aware of their risk status so that they can adhere to therapeutic lifestyle measures and, if necessary, pharmacological measures.
Vascular age table according to SCORE for countries of low cardiovascular risk.59
Source: Cuende J.O., et al., How to calculate vascular age with the SCORE project scales: a new method of cardiovascular risk evaluation, European Heart Journal, 2010, 31, Issue 19, 2351–2358, doi:10.1093/eurheartj/ehq205. Translated and reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology. OUP and the European Society of Cardiology accept no responsibility for the accuracy of the translation. The licensee is solely responsible for the translation in this publication/reprint.59
The vascular age table derived from SCORE can be used to report absolute risk and vascular age. Calculation of the latter does not require calibration and can therefore be applied to any general population, with no territorial differences.
Both vascular age and relative risk can be used at any age, and are most clinically useful for subjects with a low short-term absolute risk. Consideration of absolute risk alone may lead to underestimation of controllable VRFs with important effects on lifetime risk.
Vascular ageing speed is derived from the concept of vascular age,60 which relates vascular and chronological age.
Vascular risk in patients with familial hypercholesterolaemiaSeveral specific tools have been developed for patients with FH, to whom the usual VR calculation tables do not apply. One is based on follow-up data from the Spanish Familial Hypercholesterolemia Cohort Study (SAFEHEART).61 This equation takes into account several factors such as age, smoking, LDL-C levels on treatment, BMI, BP, and Lp(a) levels and allows risk mapping in this population. The SEA registry provides another VR stratification tool for FH patients on statin therapy based on the presence of other VRFs (male sex, obesity, HTN, DM), peak LDL-C levels, and positive genetic testing for FH.62 Finally, a tool for risk calculation has been developed for patients with FH phenotype: the Catalan Primary Care System Database - Familial Hypercholesterolemia phenotype (SIDIAP-FHP) with better predictive capacity in both primary and secondary prevention.63
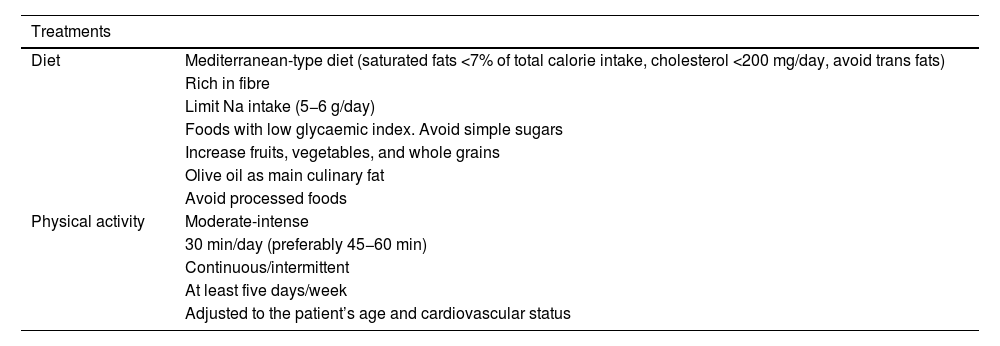
General recommendations to control vascular riskGeneral recommendations for the population to reduce their vascular riskThe Nutrition and Lifestyle working group of the SEA has a detailed consensus document,64 which provides useful and prioritised evidence to serve as a tool for health professionals to help their patients, based on the concept that healthy recommendations should be the same to control any VRF and for the primary and secondary prevention of AVD. It summarises the accumulated evidence on lifestyle components such as diet and physical exercise.64 In addition, it focusses on diet rather than isolated nutrients, and above all on the most important dietary patterns for vascular prevention. This concept of dietary pattern has become established in recent years as a model to examine the relationship between nutrition and health and to serve as an educational tool for the population, a change from the traditional paradigm that nutrients form the basic nutritional unit of the diet (e.g., fatty acids), it is rather the foods that contain them (oils, nuts, red meats, dairy products, etc.) because their matrices contain a multitude of components that interact synergistically or antagonistically on metabolic pathways that determine vascular health. We have compiled the main recommendations of this document in these standards.64
Different healthy diets have many components in common, some of which are recommended, such as fruits, vegetables, nuts, legumes, and fish, while others should be restricted, such as certain foods that are high in saturated fat, those with added sugar, high salt, or processed. There is strong evidence that plant-based dietary patterns, low in saturated fatty acids, cholesterol, and Na, high in fibre, K, and unsaturated fatty acids, are beneficial and reduce the expression of VRFs. In particular, the Mediterranean diet, the Dietary Approaches to Stop Hypertension (DASH) diet, the vegan-vegetarian diet, and the Alternative Healthy Eating Index (AHEI), all of which are plant-based and rich in complex carbohydrates. Data from large cohort studies and, for the Mediterranean diet, the randomised clinical trial PREDIMED, indicate that adherence to these dietary patterns clearly provides vascular benefit.65 In contrast, the low-fat diet is currently being questioned because of its low vascular protective potential. With regard to edible fats, virgin olive oil is the most effective culinary fat in the prevention of AVD.66 Nutritional intervention over about five years in the PREDIMED study showed that participants assigned to the Mediterranean diet supplemented with extra virgin olive oil or nuts experienced an average 30% reduction in major vascular events,65 in addition to other beneficial effects, including reduced risk of T2DM and AF.67 The results of the CordioPrev study68 were published recently, a randomised clinical trial including 1002 patients with established coronary heart disease who underwent a dietary intervention with a Mediterranean diet rich in virgin olive oil versus a low-fat diet rich in complex carbohydrates for seven years. The major adverse cardiovascular event of the study occurred in 198 participants, 87 in the Mediterranean diet arm (17.3%) and 111 in the low-fat diet arm (22.2%), resulting in a decrease in the rate of events of 26% in participants following the Mediterranean diet (hazard ratio (HR) of the different models from .719 (95% confidence interval (CI) 0.541–0.957) to .753 (.568–.998). These effects were most evident in males, where the difference between diets was 33% in favour of the Mediterranean diet. The results are relevant for clinical practice, supporting use of the Mediterranean diet to prevent the recurrence of vascular disease. It is important to note that most current margarines do not contain trans-fatty acids and provide n-6 and n-3 polyunsaturated fatty acids.
Consumption of fish or seafood at least three times a week, twice in the form of oily fish, reduces VR. Encouraging its consumption is therefore an important component of lifestyle changes for the prevention of AVD. A non-negligible benefit could be obtained if it replaces meat as the main dish at meals. Even so, the above-mentioned document stresses that, because of their high levels of marine pollutants, children and women of childbearing age should not consume large fatty fish (bluefin tuna, swordfish, shark), or mackerel because they contain more pollutants than smaller species. Evidence on meats indicates that consumption of white meat or lean meat (without visible fat), three to four servings per week, does not increase VR, unlike the consumption of processed meats (bacon, sausages, cured meats) that contain harmful additives, such as salt and nitrates, which increase total mortality and the risk of developing T2DM and AVD. The need is emerging to transform the food system to a new model that is healthy for the human population and for the planet. Red meat and its derivatives are a major source of global warming, overuse of land, and water consumption. Similarly, ultra-processed products, meat and non-meat, and the vast majority of pre-cooked foods, contain products such as added sugars or trans fats and should be kept out of our diet. They should therefore be avoided and consumption of foods rich in vegetable proteins should be increased over those rich in animal protein.69
At least two servings of dairy products should be consumed a day (milk, fermented milk, cheese, yoghurt, etc.), due to their important nutritional role in Ca metabolism and their richness in proteins of high biological quality. Skimmed dairy products are preferable and regular consumption of those with added sugars is not recommended. For vascular prevention, it is advisable to reduce consumption of concentrated dairy fats such as butter and cream. In the last decade, recommendations on egg consumption and health have been divergent, largely due to a lack of evidence. However, current scientific evidence suggests that egg consumption is not harmful in the context of a healthy diet. Both the general healthy population, as well as people with VRFs, previous AVD, or T2DM, can consume up to one egg per day without fear for their cardiometabolic health. In patients with T2DM there does not seem to be sufficient grounds for restricting egg consumption to reduce VR or improve metabolic control, although some series limit egg intake to a maximum of three eggs per week.70
Legumes and wholegrain cereals are seeds that contain multiple healthy nutrients and their frequent consumption is associated with reduced VRFs and AVD. To promote vascular health and lower cholesterolaemia, a serving of pulses at least four times a week is recommended. The recommended intake of wholegrain cereals is about four servings/day, including bread at all meals of the day, pasta two to three times/week, and rice two to three times/week. Based on the existing evidence, four to five servings per day of fruit and vegetables is recommended as this reduces overall and vascular mortality. Moreover, the beneficial effect of fruit and vegetables is dose-dependent and is more evident in cerebrovascular disease than in coronary heart disease. Consumption of tuber vegetables (especially potatoes) is not associated with an increase in VR, unless fried in non-recommended oils and salted.
Frequent consumption of nuts is associated with a reduction in AVD, especially coronary heart disease, and all-cause mortality.65 Frequent consumption (daily or at least three times a week) of a handful of nuts (equivalent to a 30 g serving) is highly recommended for cholesterol control and general health. It is advisable to eat them raw and unpeeled (not roasted or salted) if possible, as most of the antioxidants are in the skin. To maintain the satiating effect and avoid weight gain, they should be eaten during the day, not as a dessert. Recommended nuts include hazelnuts, walnuts, almonds, pistachios, cashew nuts, macadamias, pine nuts, etc. Although peanuts are legumes not tree nuts, their general composition and high unsaturated fatty acid content makes them similar to nuts, both nutritionally and in terms of their biological effects.
Cocoa is a nutrient-rich seed and consumption of its main derivative, chocolate, improves VRFs and is associated with reduced AVD and T2DM. There is information indicating that it has hypocholesterolaemic and antihypertensive effects, improving insulin resistance, therefore a healthy diet can include dark chocolate ≥ 70% without added sugar. Furthermore, it is advisable to eat it during the day and not in the evening after dinner, when the satiating effect cannot be compensated for by eating less food at the next meal.
Sugary drinks are a regular part of many people's diet and can account for up to 20% of daily calorie intake, leading to an increase in AVD, obesity, and T2DM. Replacing these drinks with water is very important in reducing energy consumption and the risk of these diseases and their complications. If the patient does not accept this, we can resort to drinks with artificial sweeteners, although their consumption causes alterations in the intestinal microbiota and an increase in insulin resistance that may favour the development of T2DM and increase VR.71
Consumption of alcoholic beverages of any type increases HDL-C and moderate intake (of non-distilled fermented beverages), compared to abstention or excessive consumption, is associated with a reduction in AVD and vascular mortality. Moderate consumption can always be allowed with meals and within the framework of a healthy diet such as the Mediterranean diet, with different recommendations for men and women, as women are more sensitive to the effects of alcohol. Alcohol consumption should not be encouraged in people who do not usually drink alcohol. Coffee (both regular and decaffeinated) and tea are rich in polyphenols and there is high-level evidence that regular consumption is associated with reduced CVR and reduced risk of developing T2DM.
There are numerous functional foods and nutraceuticals aimed at reducing CVR, mainly by reducing cholesterolaemia. The SEA has recently drawn up a position paper on their usefulness, identifying the following clinical scenarios in which these products could be used72:
- •
Lipid-lowering treatment in subjects intolerant to statins.
- •
Lipid-lowering treatment ‘à la carte’ in people in primary prevention.
- •
Long-term vascular prevention in people with no indication for lipid-lowering therapy.
- •
Patients with optimised lipid-lowering therapy who do not achieve therapeutic targets.
The SEA stresses that there are no vascular morbidity and mortality studies available with nutraceuticals or functional foods, and that long-term safety data are limited or non-existent. Both aspects should be discussed with the patient before recommending their use. They also believe that the nutraceuticals with the most scientific evidence are phytosterols and red yeast rice. There is also consistent evidence that omega-3 fatty acids at pharmacological doses lower plasma TG.
Excessive salt intake is associated with AVD and cardiometabolic mortality. A low-salt diet (<5 g/day) is recommended at the population level and especially in hypertensive patients and their relatives, remembering that the Na content of foods must be multiplied by 2.5 to calculate the amount of total salt. Limiting the consumption of salt-rich foods such as precooked foods, canned foods, salted meats, cured meats, and carbonated beverages is particularly effective for a low-salt diet. Lemon juice, garlic, or herbs are alternatives to salt.
It is reasonable to believe, and recent evidence shows, that there is no standard model for a healthy diet, but that the biological response varies between people, especially because of individual differences in the genome and microbiome. In the coming years, personalised and precision nutrition, together with other sciences such as chronobiology, in which everyone adopts the diet that is most beneficial for them personally, will be a challenge for the scientific community.73 Finally, adherence is one of the most complex problems in the relationship between people and their diet, which depends on very different factors, such as those of the patient, the family, the health team caring for the patient, and the health system itself. Therefore, strategies must be implemented to achieve adherence.
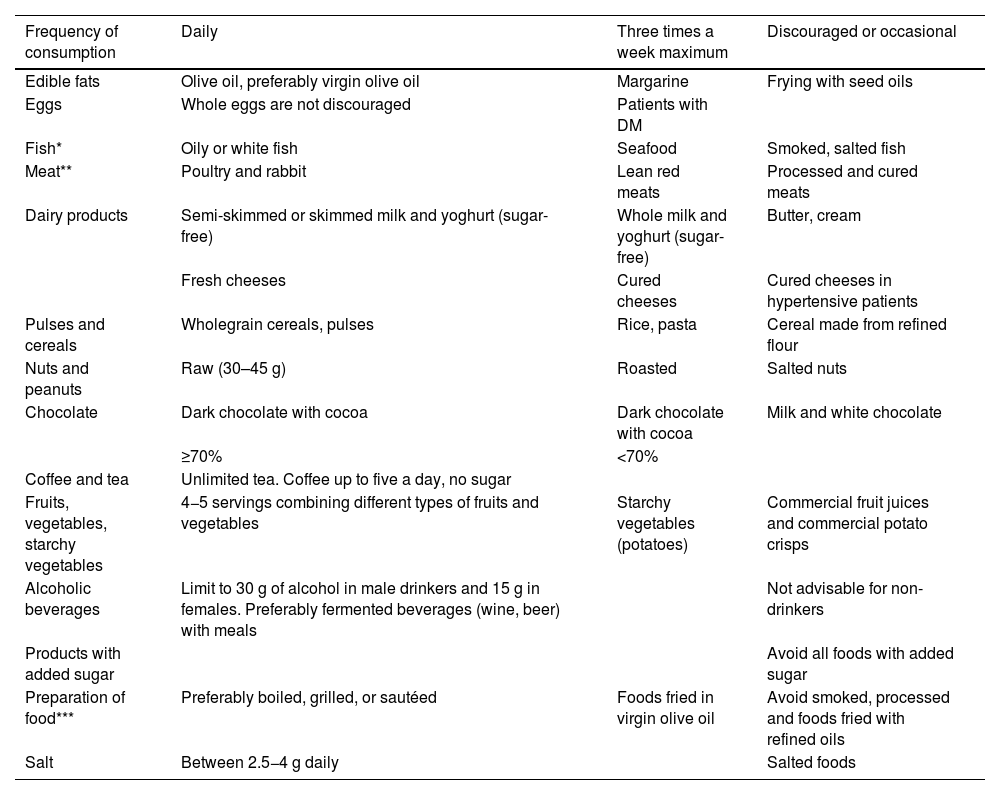
Table 6 taken from this document,64 shows the frequency in the form and quantity of food consumption in a practical way.
Frequency in form and quantity of food consumption.
| Frequency of consumption | Daily | Three times a week maximum | Discouraged or occasional |
|---|---|---|---|
| Edible fats | Olive oil, preferably virgin olive oil | Margarine | Frying with seed oils |
| Eggs | Whole eggs are not discouraged | Patients with DM | |
| Fish* | Oily or white fish | Seafood | Smoked, salted fish |
| Meat** | Poultry and rabbit | Lean red meats | Processed and cured meats |
| Dairy products | Semi-skimmed or skimmed milk and yoghurt (sugar-free) | Whole milk and yoghurt (sugar-free) | Butter, cream |
| Fresh cheeses | Cured cheeses | Cured cheeses in hypertensive patients | |
| Pulses and cereals | Wholegrain cereals, pulses | Rice, pasta | Cereal made from refined flour |
| Nuts and peanuts | Raw (30–45 g) | Roasted | Salted nuts |
| Chocolate | Dark chocolate with cocoa | Dark chocolate with cocoa | Milk and white chocolate |
| ≥70% | <70% | ||
| Coffee and tea | Unlimited tea. Coffee up to five a day, no sugar | ||
| Fruits, vegetables, starchy vegetables | 4−5 servings combining different types of fruits and vegetables | Starchy vegetables (potatoes) | Commercial fruit juices and commercial potato crisps |
| Alcoholic beverages | Limit to 30 g of alcohol in male drinkers and 15 g in females. Preferably fermented beverages (wine, beer) with meals | Not advisable for non-drinkers | |
| Products with added sugar | Avoid all foods with added sugar | ||
| Preparation of food*** | Preferably boiled, grilled, or sautéed | Foods fried in virgin olive oil | Avoid smoked, processed and foods fried with refined oils |
| Salt | Between 2.5−4 g daily | Salted foods |
DM: diabetes mellitus.
Published with permission of the Editor. Original source: adapted from Pérez-Jiménez F, et al.64
The MEDAS questionnaire is one way of assessing adherence to the Mediterranean diet (Appendix C Annex 3).
According to the World Health Organisation (WHO), physical activity is any bodily movement produced by skeletal muscles that requires energy expenditure. When performed on a regular and sustained basis, it protects against VR and improves VRFs. It should be adapted to the particularities of each individual, based on the principle that little is better than nothing and considered to encompass activities such as those performed during work, active forms of transport, household chores, or recreational activities. Physical exercise, on the other hand, is a variety of physical activity but is performed in a planned, structured, repetitive manner, with a goal related to improving or maintaining physical fitness. Both should be leisurely and moderate, rather than intense and concentrated.
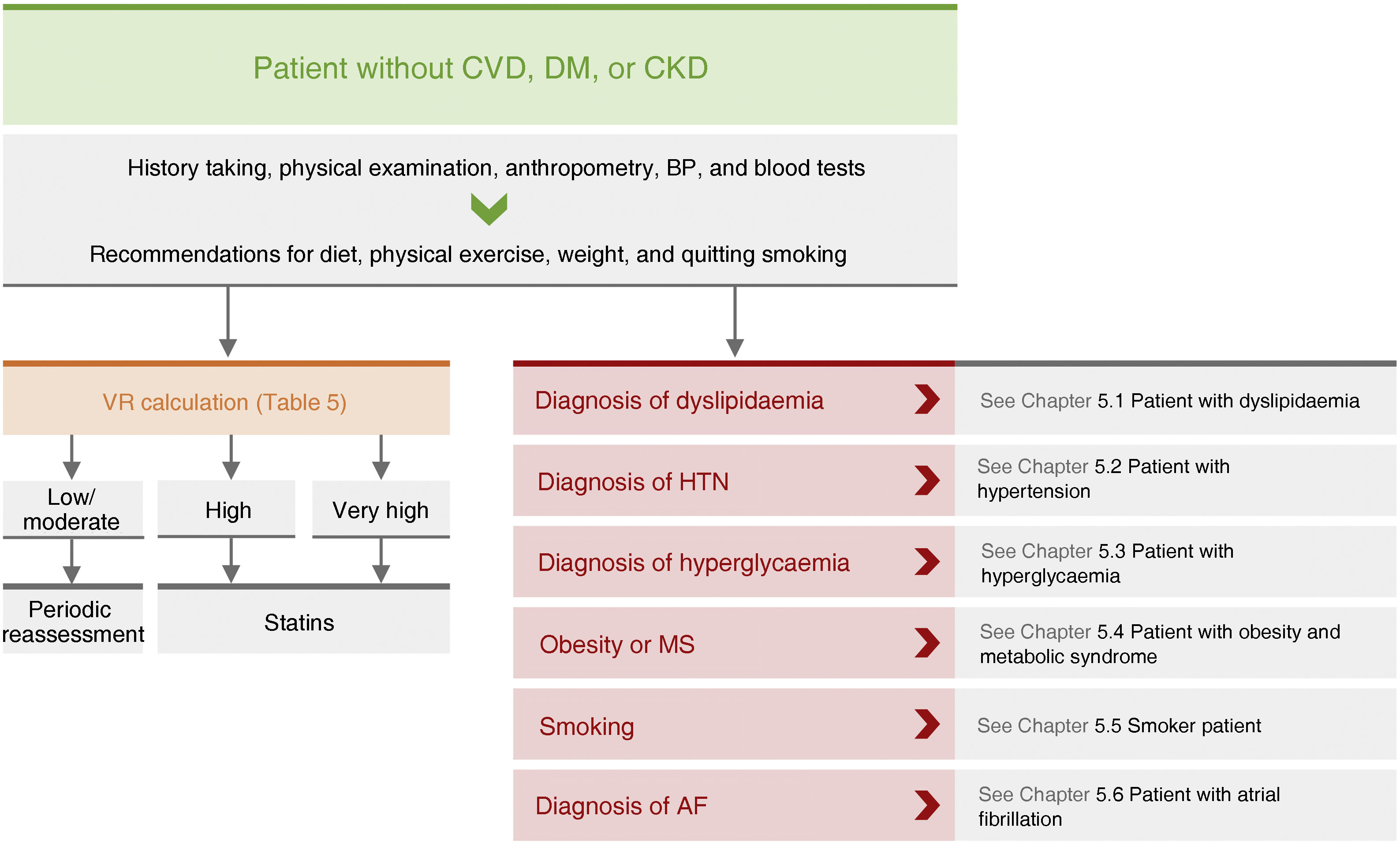
General pharmacological recommendations in primary prevention patientsRecommendations for clinical management of VR in patients without AVD, DM, or CKD are shown in Fig. 4.
Recommendations for clinical management of vascular risk in patients with no established vascular disease, diabetes mellitus, or chronic renal failure.
AF: atrial fibrillation; BP: blood pressure; CVD: cardiovascular disease; CKD: chronic kidney disease; DM: diabetes mellitus; HTN: hypertension; MS: metabolic syndrome; VR: vascular risk.
Treatment with low-dose aspirin has been shown to reduce the risk of vascular complications, mainly in middle-aged individuals, at the expense of a reduction in non-fatal myocardial infarctions, without affecting stroke risk or mortality. However, some of the benefit of aspirin is offset by its adverse effects, particularly those related to its bleeding potential, therefore, the balance of risks and benefits of low doses of aspirin have not been clearly established in primary prevention.
The US Preventive Service Task Force (USPSTF) guidelines74 recommend initiating low-dose (≤100 mg/day) acetylsalicylic acid for primary prevention of AVD in adults aged 50–59 years who have a 10-year risk of vascular morbidity and mortality greater than or equal to 10%, who are not at increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take this treatment daily for at least 10 years. The decision to initiate treatment in adults aged 60–69 years with a VR greater than or equal to 10% at 10 years should be individualised.74
However, the 2021 European guidelines for cardiovascular prevention do not systematically recommend antiplatelet therapy for patients without AVD because of the increased risk of bleeding.13 In this regard, several clinical trials have recently been published with acetylsalicylic acid in primary prevention, both in patients with and without DM, finding no clear benefit in its use in primary prevention of AVD,75–77 especially when existing VRFs are adequately controlled.
Lipid-lowering therapyIn numerous clinical trials and meta-analyses,78 statins have been shown to reduce vascular events in patients without AVD, even with non-elevated cholesterol concentrations. The relative risk reduction of AVD is independent of baseline VR and is approximately 22% for each mmol/l reduction in LDL-C. However, for treatment to be effective, the risk of vascular events must be reduced by at least 22% for each mmol/l reduction in LDL-C. However, for treatment to be efficient, it is important to select patients with a high baseline VR so that the absolute reduction in VR is greater (Table 5). Other drugs such as ezetimibe, proprotein convertase subtilisin/kexin proprotein convertase 9 inhibitors (PCSK9i), or bempedoic acid have been shown to reduce vascular events equivalent to statins in proportion to their lipid-lowering action, and therefore we believe that therapy in primary prevention should also be designed according to the LDL-C targets to be achieved based on the patient's VR, especially in patients with FH, taking into account the full therapeutic arsenal available. Indications for lipid-lowering therapy in primary prevention are outlined in the section ‘Specific therapeutic recommendations’.
Vitamin supplementsMany prospective observational case-control studies have described inverse associations between intake or serum concentrations of vitamins (A, group B, C, D, and E) and the risk of AVD. However, data from prospective studies and interventional clinical trials with vitamin and mineral supplementation have not demonstrated any vascular benefit.79 Therefore, the use of vitamin supplements is not indicated in the prevention of AVD.
General recommendations in patients with subclinical vascular disease and in secondary preventionPatients with SVD diagnosed by the presence of carotid or femoral artery plaques or by CAC testing have an intermediate risk of vascular complications between those in primary and secondary prevention, although many guidelines classify them as secondary prevention. In these circumstances, their management would not differ from that of patients in secondary prevention, although evidence of real efficacy of preventive treatment is limited in these populations.80 In any case, their risk level should be estimated according to Table 5.
In patients in secondary prevention, in addition to the previously mentioned hygiene and dietary measures (see section ‘General recommendations to the population to reduce their vascular risk’) and the treatments indicated for controlling VRFs, there are a series of treatments that have been shown to reduce the risk of new vascular events (Table 7).
Pharmacological measures that have been shown to reduce the rate of atherosclerotic vascular complications in patients in secondary prevention.
| Treatment | Potential indications |
|---|---|
| Antiplatelet drugs | • Low-dose aspirin or clopidogrel in patients with symptomatic coronary artery, cerebrovascular or peripheral arterial disease |
| • Aspirin plus clopidogrel after TIA or mild stroke.81,82 | |
| • Aspirin and dipyridamole in subjects with previous stroke or TIA | |
| • Aspirin plus a P2Y12i in subjects with ACS or stent placement, maintained for at least 12 months | |
| Lipid-lowering agents | • Statins with or without ezetimibe or bempedoic acid to reduce LDL-C at least 50% and below 55 mg/dL. PCSK9i if adequate reductions are not achieved with previous lipid-lowering therapy, and according to the criteria in Table 8 |
| • Ezetimibe and bempedoic acid, in patients with intolerance to statins and those in whom targets have not been achieved with other cholesterol-lowering therapies | |
| • Purified IPE 4 g/day for hypertriglyceridaemia >150 mg/dL that persists despite statin therapy in patients with high VR.83 Its protective effect is independent of their effect on TGs |
ACS: acute coronary syndrome; IPE: icosapent ethyl; LDL-C: low-density lipoprotein cholesterol; PCSK9i: proprotein convertase subtilisin/kexin 9 inhibitors; P2Y12i: platelet adenosine diphosphate receptor inhibitors; TGs: triglycerides; TIA: transient ischaemic attack.
Acetylsalicylic acid is the most studied antiplatelet agent for long-term vascular prevention in patients with acute myocardial infarction, ischaemic stroke, or symptomatic PAD. In a meta-analysis of 16 clinical studies with more than 17,000 patients, treatment with acetylsalicylic acid significantly reduced serious vascular events (coronary and cerebrovascular) and total mortality.84 Therapy with salicylic acid was also associated with a significant increase in major bleeds and development of anaemia even in the absence of apparent bleeding; however, the vascular benefits of acetylsalicylic acid clearly outweighed the risk of bleeding.
Clopidogrel has a similar effect to acetylsalicylic acid in patients with myocardial infarction or ischaemic stroke, but may be superior to it in subjects with symptomatic PAD. The combination of acetylsalicylic acid and clopidogrel in secondary prevention significantly reduces major cardiovascular events compared to acetylsalicylic acid monotherapy, but with a significant increase in the risk of bleeding.
In patients with non-cardioembolic ischaemic stroke (CVA) or transient ischaemic attack (TIA), acetylsalicylic acid can be used as monotherapy or in combination with dipyridamole, and clopidogrel can also be used in monotherapy. In subjects with a TIA or minor stroke, the benefit of dual antiplatelet therapy for up to 90 days outweighs the risks of increased bleeding.81,82 Protection occurs during the first 21 days, and therefore this is the most recommended interval for dual therapy.85
The standard treatment for a patient who has had acute coronary syndrome (ACS), with or without stent placement, is dual antiplatelet therapy (acetylsalicylic acid with an adenosine diphosphate receptor inhibitor [P2Y12i] for 12 months. In patients at high risk of bleeding, the time on dual antiplatelet therapy can be shortened to one to three months.
There is little evidence to support the use of antiplatelet therapy in patients with SVD. In subjects with low ABI, but without intermittent claudication, antiplatelet therapy has not been shown to be effective.80
Evidence is also very limited in subjects with asymptomatic carotid stenosis >50%, although the European Society for Vascular Surgery (ESVS) recommends the use of acetylsalicylic acid at doses of 75−325 mg or, if not tolerated, clopidogrel, with the aim of reducing the rate of coronary complications or complications in other vascular beds.86
There is interest in identifying a CAC threshold above which the benefit of antiplatelet therapy outweighs the bleeding risks, and clinical trials are likely to be conducted in this regard.87,88 It is reasonable that a high CAC score would help in the decision to use antiplatelet therapy in patients with a borderline indication, especially if there is no increased risk of bleeding.
Lipid-lowering agentsNumerous clinical trials and meta-analyses78 have shown that treatment with lipid-lowering drugs (resins, statins, ezetimibe, PCSK9i, bempedoic acid) in patients with established AVD decreases major vascular events and mortality.
Guideline data27 indicate that patients with established SVD (multivessel coronary artery disease demonstrated by >50% obstruction in at least two epicardial arteries on coronary CT or angiography, or by the presence of carotid plaques) should be considered at very high VR and treated as if they had previously had a vascular event. Recommendations for lipid-lowering therapy in these subjects are given in the section ‘Specific therapeutic recommendations’. Patients with multivessel coronary artery disease (especially if not revascularisable), or with involvement of multiple arterial beds, are at particularly high risk of vascular complications and more intensive lipid-lowering therapy (e.g., target LDL-C < 40 mg/dL) may be considered.89,90
Other drugsAlthough different drugs have been shown to reduce the rate of vascular complications in patients in primary and secondary prevention, we review here those that presumably reduce it through actions on atheromatous plaque. In patients in secondary prevention or with high-risk DM on statin therapy (mean LDL-C of 75 mg/dL and TG between 150−499 mg/dL), treatment with 4 g of IPE reduced the risk of serious vascular events by 25%.83 This drug has also been shown to reduce the progression of atheromatous plaque in patients with hypertriglyceridaemia treated with statins.91
The use of acetylsalicylic acid, a statin, and an angiotensin-converting enzyme inhibitor in the same tablet facilitates adherence to treatment in secondary prevention patients92 and has been shown to reduce the rate of vascular complications compared to standard treatment of the disease.93
Finally, specific interventions on inflammation have also been shown to reduce the number of vascular complications. The use of an anti-interleukin-1β (anti-IL-1β) monoclonal antibody, canakinumab, significantly reduced the recurrence rate of AVD, showing benefit despite an increase in severe and fatal infections.94 Colchicine is an anti-inflammatory drug that, at doses of .5 mg daily or every 12 h, has been shown to reduce the rate of vascular complications by 32%, with no significant differences in side effects.95
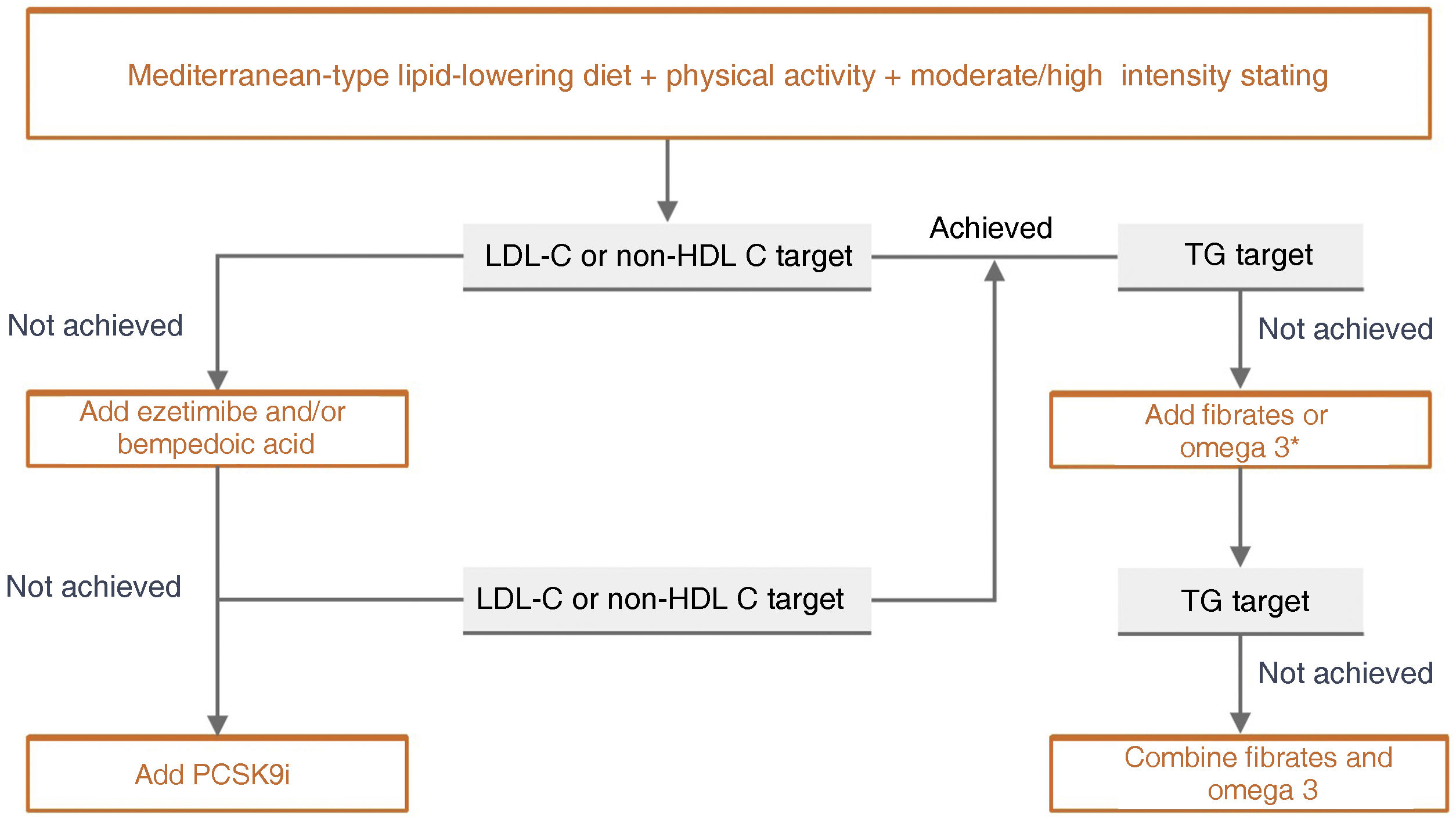
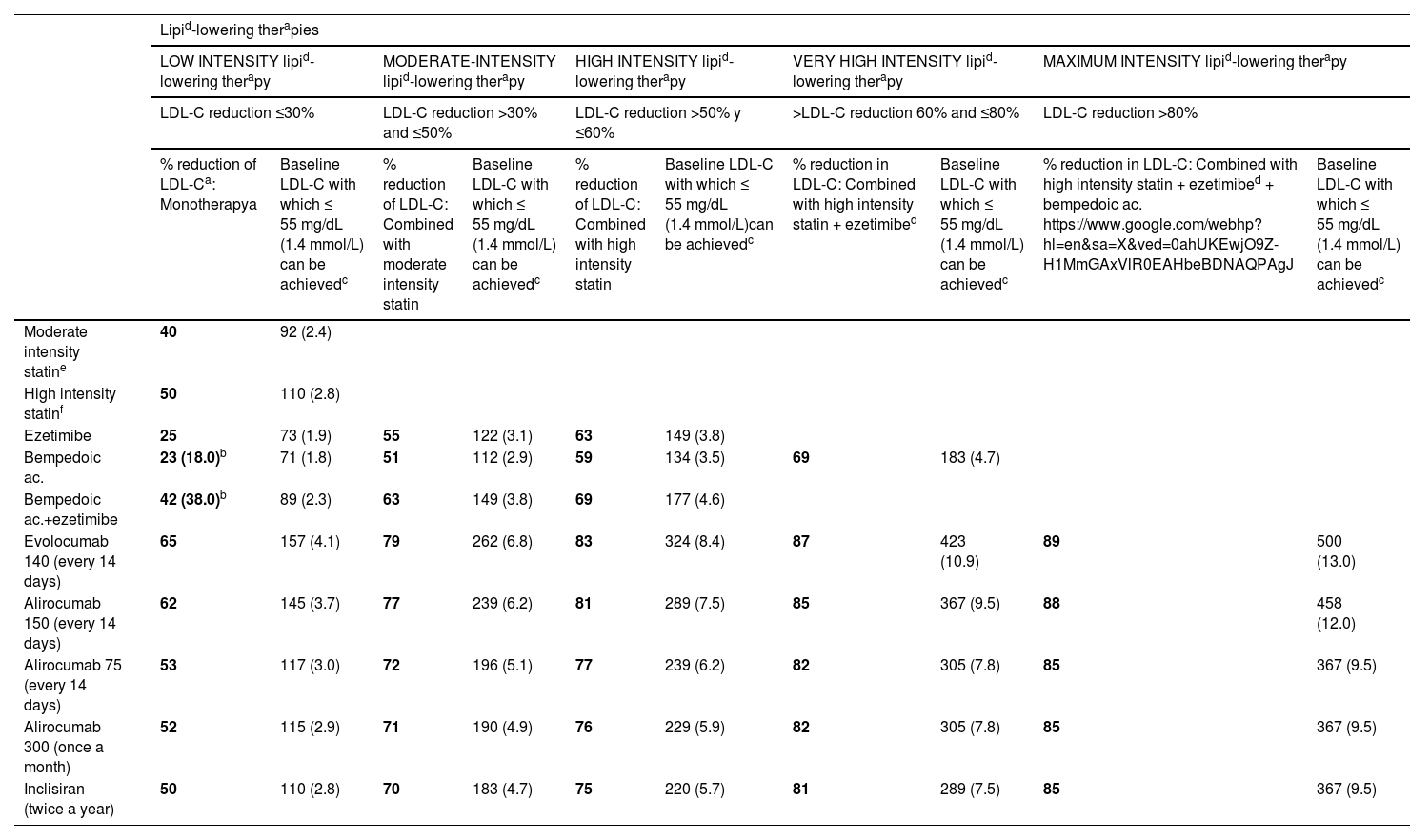
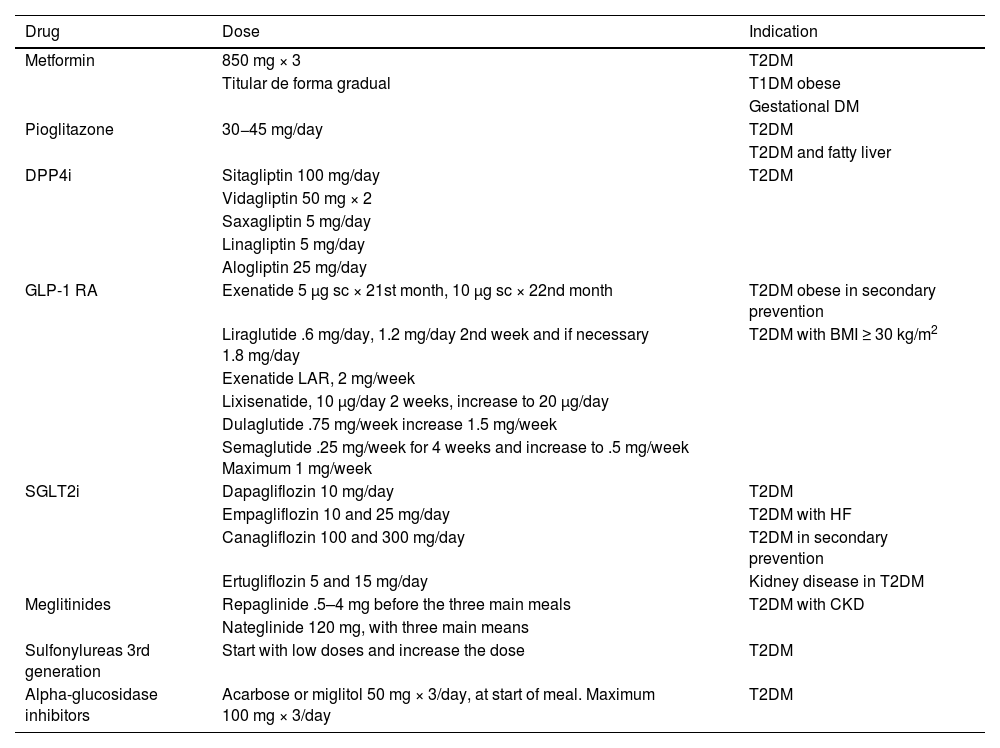
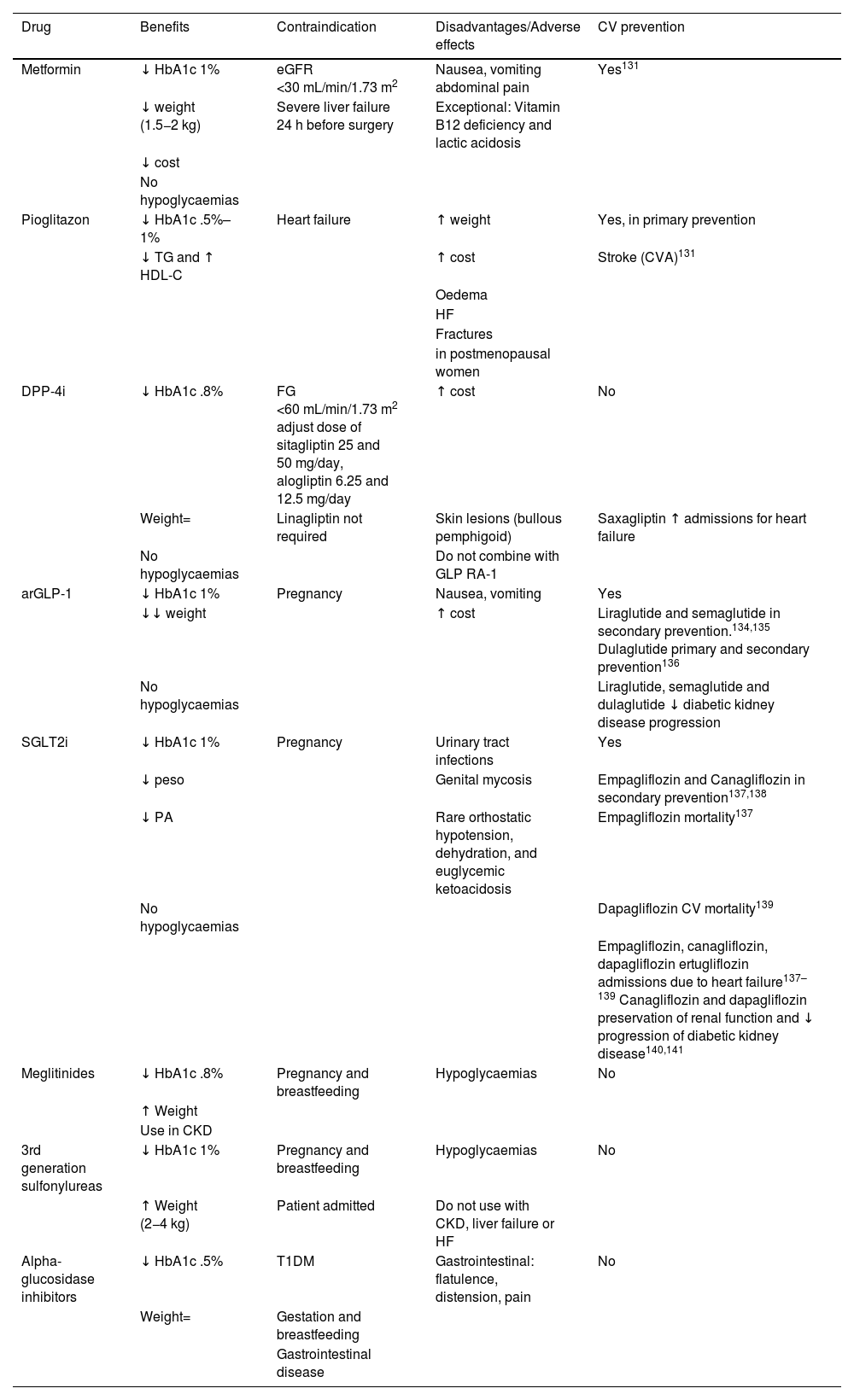
Specific therapeutic recommendationsPatient with dyslipidaemiaPatient with hypercholesterolaemiaThe Mediterranean-type diet, rich in vegetable products and low in animal fats, is recommended for cardiovascular prevention in the general population, and especially for patients with hypercholesterolaemia, as stated in the dietary recommendations of the SEA (Table 6). The indication for starting lipid-lowering therapy is based on both baseline LDL-C concentration and baseline VR. Lipid-lowering therapy should aim to achieve the LDL-C targets listed in the following sections. To achieve these targets, combinations of drugs are most often required, and therefore emphasis is placed on the use of high-intensity lipid-lowering therapies, in which statins should be included, as shown in Table 8.
Lipid-lowering therapies, in monotherapy or combination, according to their cholesterol-lowering strength.
| Lipid-lowering therapies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOW INTENSITY lipid-lowering therapy | MODERATE-INTENSITY lipid-lowering therapy | HIGH INTENSITY lipid-lowering therapy | VERY HIGH INTENSITY lipid-lowering therapy | MAXIMUM INTENSITY lipid-lowering therapy | ||||||
| LDL-C reduction ≤30% | LDL-C reduction >30% and ≤50% | LDL-C reduction >50% y ≤60% | >LDL-C reduction 60% and ≤80% | LDL-C reduction >80% | ||||||
| % reduction of LDL-Ca: Monotherapya | Baseline LDL-C with which ≤ 55 mg/dL (1.4 mmol/L) can be achievedc | % reduction of LDL-C: Combined with moderate intensity statin | Baseline LDL-C with which ≤ 55 mg/dL (1.4 mmol/L) can be achievedc | % reduction of LDL-C: Combined with high intensity statin | Baseline LDL-C with which ≤ 55 mg/dL (1.4 mmol/L)can be achievedc | % reduction in LDL-C: Combined with high intensity statin + ezetimibed | Baseline LDL-C with which ≤ 55 mg/dL (1.4 mmol/L) can be achievedc | % reduction in LDL-C: Combined with high intensity statin + ezetimibed + bempedoic ac. https://www.google.com/webhp?hl=en&sa=X&ved=0ahUKEwjO9Z-H1MmGAxVlR0EAHbeBDNAQPAgJ | Baseline LDL-C with which ≤ 55 mg/dL (1.4 mmol/L) can be achievedc | |
| Moderate intensity statine | 40 | 92 (2.4) | ||||||||
| High intensity statinf | 50 | 110 (2.8) | ||||||||
| Ezetimibe | 25 | 73 (1.9) | 55 | 122 (3.1) | 63 | 149 (3.8) | ||||
| Bempedoic ac. | 23 (18.0)b | 71 (1.8) | 51 | 112 (2.9) | 59 | 134 (3.5) | 69 | 183 (4.7) | ||
| Bempedoic ac.+ezetimibe | 42 (38.0)b | 89 (2.3) | 63 | 149 (3.8) | 69 | 177 (4.6) | ||||
| Evolocumab 140 (every 14 days) | 65 | 157 (4.1) | 79 | 262 (6.8) | 83 | 324 (8.4) | 87 | 423 (10.9) | 89 | 500 (13.0) |
| Alirocumab 150 (every 14 days) | 62 | 145 (3.7) | 77 | 239 (6.2) | 81 | 289 (7.5) | 85 | 367 (9.5) | 88 | 458 (12.0) |
| Alirocumab 75 (every 14 days) | 53 | 117 (3.0) | 72 | 196 (5.1) | 77 | 239 (6.2) | 82 | 305 (7.8) | 85 | 367 (9.5) |
| Alirocumab 300 (once a month) | 52 | 115 (2.9) | 71 | 190 (4.9) | 76 | 229 (5.9) | 82 | 305 (7.8) | 85 | 367 (9.5) |
| Inclisiran (twice a year) | 50 | 110 (2.8) | 70 | 183 (4.7) | 75 | 220 (5.7) | 81 | 289 (7.5) | 85 | 367 (9.5) |
Theoretical efficacy of lipid-lowering treatment with monotherapy and combination therapy. Mean LDL-C reduction by lipid-lowering monotherapy and combination therapies and baseline LDL-C sufficient to be reduced to target LDL-C of 55 mg/dL (1.4 mmol/L) by different therapies.91
Low-intensity statins with lipid-lowering effect of less than 30%–40%: simvastatin 10; pravastatin 10–20; lovastatin 10–20; fluvastatin 40; pitavastatin 1 (not included in Table).
Ac: acid; LDL-C: low-density lipoprotein cholesterol.
% LDL-C reduction by statins moderate intensity and high intensity by definition. The %LDL-C reduction by other drugs was taken from Toth et al.92
Efficacy of combination therapies was calculated according to the following formula: %A+%B (1-%A)+%C [1-(%A+%B (1-%A)]; where %A is the theoretical reduction in LDL-C induced by drug A, %B by drug B, and %C by drug C.93
This refers to patients in primary prevention, without DM, with preserved renal function, no FH, and VR of less than 5% at 10 years according to the SCORE tables, with no coexisting risk modulating factors, no SVD, and no TOD (Table 5). In this clinical situation, an LDL-C less than 115 mg/dLt would be considered ideal and no specific action would be required. Conversely, an LDL-C higher than 190 mg/dL would mean the patient would be considered high risk by definition and FH should be ruled out. The recommended LDL-C concentration, in these circumstances, would be LDL-C < 70 mg/dL and lipid-lowering treatment to achieve this target should be assessed together with the patient.
If LDL-C is between 115 and 190 mg/dL, treatment will be based on therapeutic lifestyle changes (TLC), which would include a diet according to Mediterranean dietary standards. The use of functional foods enriched in phytosterols, and fibre may be indicated to lower cholesterol together with increased physical activity, abstinence from smoking, and weight loss if necessary. The prescription of cholesterol-lowering drugs is not universally accepted in this population group and should be considered on an individual basis if a patient has two of the following VRFs: age (men > 45 years; women > 50 years); BMI > 30 kg/m2; smoking; HTN; family history of premature AVD; atherogenic dyslipidaemia; MS; or Lp(a) > 50 mg/dL or FH with a milder phenotype.
Patient at high vascular riskThe therapeutic goal is to reduce LDL-C < 70 mg/dL and at least a 50% decrease from baseline LDL-C values.
Initial treatment will be based on applying TLC. If LDL-C levels > 70 mg/dL persist, high-intensity cholesterol-lowering therapy is recommended to ensure an LDL-C decrease of at least 50% (Table 8). Initial treatment should be with statins and, if targets are not achieved, with ezetimibe or bempedoic acid. The combination should be considered initially in patients with baseline LDL-C > 140 mg/dL.
Patient at very high vascular riskThe therapeutic target is an LDL-C < 55 mg/dL and a decrease of at least 50% of baseline values.
Initial treatment should be based on the application of TLC and simultaneous high/very high intensity cholesterol-lowering therapy to ensure a decrease in LDL-C of at least 50% and to achieve the therapeutic target (Table 8). The common practice of initiating treatment with statins and then adding other lipid-lowering agents in a second phase has been shown to be insufficient for several reasons: a significant proportion of patients do not achieve >50% reductions, which, together with therapeutic inertia and lack of adherence, means that most patients do not achieve their therapeutic targets. Therefore, it is reasonable to consider the use of oral combination therapy (statins + ezetimibe/bempedoic acid) from the outset, which helps to achieve therapeutic targets in a shorter time, promotes adherence, and reduces the risk of side effects of the maximum dose of statins. The combination is mandatory from the outset in patients with baseline LDL-C levels above 110 mg/dL. The use of PCSK9i would be recommended according to the indications listed in Table 9, particularly in patients requiring LDL-C reductions greater than 60%. It should be noted that patients benefit from lower than target LDL-C concentrations. No adverse effects associated with extremely low LDL-C values have been detected. Furthermore, it has been shown that with very large decreases in LDL-C levels, atheromatous lesions are reduced and become more stable.
SEA criteria for the use of PCSK9i.
| Clinical situations | Additional conditions | LDL-C |
|---|---|---|
| Homozygous familial hypercholesterolaemia (HoFH) | – | >100 |
| Heterozygous familial hypercholesterolaemia (HeFH) | <4 associated VRFs | >160 |
| ≥4 associated VRFs | >130 | |
| With DM | >100 | |
| With AVD | >70 | |
| Secondary prevention | Stable | >130 |
| ACS (<1 year) | >100 | |
| DM + additional VRFs | >100 | |
| More than 2 uncontrolled VRFs | >100 | |
| Lp(a) > 50 mg/dL | >70 | |
| Recurrent coronary artery disease or non-revascularisable multivessel disease | >70 | |
| Isolated symptomatic PAD or polyvascular disease | >70 | |
| ACS < 1 year + DM | >70 | |
| CRI stage ≥ 3 + 1 VRF | >70 | |
| Primary prevention with very high risk | CRI stage ≥ 3b (not on dialysis) + DM | >130 |
ACS: acute coronary syndrome; AVD: atherosclerotic vascular disease; CRI: chronic renal insufficiency; DM: diabetes mellitus; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein (a); PAD: peripheral artery disease; VRFs: vascular risk factors.
Adapted from Ascaso JF, et al.96
A summary of the indications for lipid-lowering drug treatment according to the estimated risk in Table 5 is given in Table 10.
Indications for lipid-lowering treatment according to VR and LDL-C concentration.
| VR | LDL-C | ||||
|---|---|---|---|---|---|
| 55−70 mg/dL | <70 mg/dL | 70−115 mg/dL | 116−190 mg/dL | >190 mg/dL | |
| Low or moderate | Lifestyle recommendations. Treatment not required | Lifestyle recommendations. Treatment not required | Lifestyle changes | High risk by definition | |
| Consider functional foods or lipid-lowering treatment | Lifestyle changes Initiate high-intensity lipid-lowering treatment | ||||
| Target LDL-C < 115 mg/dL | Target LDL-C < 70 mg/dL | ||||
| High | Lifestyle recommendations | Lifestyle changes | Lifestyle changes | Lifestyle changes | |
| Treatment not required | Initiate lipid-lowering treatment if targets are not achieved | Initiate high intensity lipid-lowering treatment | Initiate hight intensity lipid-lowering treatment | ||
| Target LDL-C < 70 mg/dL | Target LDL-C < 70 mg/dL | Target LDL-C < 70 mg/dL | |||
| Very high | Lifestyle changes | Lifestyle changes | Lifestyle changes Initiate high/very high intensity lipid-lowering treatment | Lifestyle changes | Lifestyle changes |
| Consider lipid-lowering treatment | Initiate lipid-lowering treatment | Initiate high/very high intensity lipid-lowering treatment | Initiate high/very high intensity lipid-lowering treatment | ||
| Target LDL-C < 55 mg/dL | Target LDL-C < 55 mg/dL | Target LDL-C < 55 mg/dL | Target LDL-C < 55 mg/dL | Target LDL-C < 55 mg/dL | |
LDL-C: Low-density lipoprotein cholesterol; VR: vascular risk.
In patients with atherogenic dyslipidaemia, the main goal is to achieve LDL-C and non-HDL-C at therapeutic targets according to risk level, together with a healthy diet,97 statin therapy, or high-intensity therapeutic combinations (Fig. 5). Once this target has been reached, in patients in secondary prevention who, despite optimal lipid-lowering treatment, maintain a TG concentration > 150 mg/dL, the use of IPE 4 g/day should be considered, especially in those with DM.
Therapeutic scheme for the management of dyslipidaemia and vascular prevention in diabetes mellitus.
*The secondary target is a TG value < 200 mg/dL and pharmacological treatment is optional (see section ‘Patient with hypertriglyceridaemia’).
CV: cardiovascular; DM: diabetes mellitus; HDL: high density lipoprotein; LDL-C: low-density lipoprotein cholesterol; non-HDL C: non-HDL cholesterol; PCSK9i: proprotein convertase subtilisin/kexin type 9 inhibitors; TG: triglycerides.
Except in severe hypertriglyceridaemia, the therapeutic approach is to reduce VR by controlling LDL-C, with special attention in these cases to controlling non-HDL-C.
Triglycerides between 200 and 500 mg/dLIn these patients, action should be taken as mentioned above on their level of VR and their LDL-C and non-HDL-C levels. According to EAS recommendations, the use of IPE should be considered in very high-risk patients, in whom a TG concentration > 150 mg/dL persists after controlling LDL-C with statins in combination with ezetimibe/bempedoic acid. Despite the 2019 EAS/ESC recommendations in these situations, the results of the recent prominent study, do not favour the use of fibrates to reduce VR.98
Triglycerides between 500 and 1000 mg/dLAct as above for the patients’ VR level and non-HDL-C numbers (applying the same criteria as for LDL-C plus 30 mg/dL).
If, after applying the recommended therapeutic measures, TG levels >500 mg/dL persist, consider the combination of fenofibrate and/or omega-399 fatty acids, aimed at reducing the risk of pancreatitis.
Triglycerides >1000 mg/dLLowering TG with diet to prevent pancreatitis by reducing intake of alcohol, carbohydrates and, in resistant forms, the total amount of fat to less than 30 g per day is a priority. In severe and persistent cases, usually with familial chylomicronaemia syndrome (FCS) or multifactorial chylomicronaemia syndrome (MCS), the introduction of medium-chain TG oil (MCT) should be considered.
Initiate treatment with fenofibrate and/or omega-3 fatty acids (>3 g per day) and assess a combination in case of inadequate control.
Patient with genetic dyslipidaemiaFamilial hypercholesterolaemiaPatients with FH are by definition considered high risk and should achieve an LDL-C < 70 mg/dL, or <55 mg/dL in case of associated AVD. In addition to diet and healthy lifestyle, most patients should receive a combination of high-potency statins with ezetimibe100 also considering triple therapy with bempedoic acid. According to their baseline risk (which, as previously discussed, should be stratified), these patients can be treated with PCSK9i (Table 9). Early treatment is necessary to avoid the development of future vascular complications. Patients with the homozygous form of the disease should receive, in addition to conventional lipid-lowering therapy (high-dose statins and ezetimibe), PCSK9i if they have at least one defective (non-null) allele. While LDL apheresis has been considered the therapeutic gold standard in these patients, the new 2023 EAS recommendations101 recommend considering cholesterol-lowering therapies that act through pathways independent of the LDL receptor, such as lomitapide or evinacumab. Lomitapide is an orally administered small molecule that inhibits microsomal TG transfer protein (MTP) and limits the formation of very low-density lipoproteins (VLDL). It reduces LDL-C by more than 50%. Fat intake should be controlled, the diet should be supplemented with vitamin E and fatty acids, and hepatic fat gain should be controlled. Evinacumab is a monoclonal anti-protein 3 angiopoietin-like antibody (ANGPTL3), an inhibitor of lipoprotein lipase (LPL) and endothelial lipase, a deficiency of which is associated with generalised hypolipidaemia. It is administered by intravenous infusion once a month. The recommended LDL-C target in children is <115 mg/dL and in adults it is below 70 or 55 mg/dL depending on the presence of AVD.
DysbetalipoproteinaemiaThis is a rare hyperlipidaemia characterised by elevation of intermediate density lipoproteins, resulting in severe mixed hyperlipidaemia, the presence of palmar striatal xanthomas and an elevated risk of premature AVD, with a special predilection for PAD. It is usually caused by a combination of an E2/E2 genotype in the Apo E gene and one or more environmental factors (hypothyroidism, obesity, etc.). It should be suspected in any mixed hyperlipidaemia with low Apo B levels (<120 mg/dL).102 Treatment is aimed at controlling the coexisting environmental factor, especially obesity, and using statins in combination or not with ezetimibe and/or fibrates. Given the characteristics of dyslipidaemia, the main objective is to control non-HDL-C.103
Chylomicronaemia syndrome (CS)This is the accumulation of chylomicrons in the bloodstream due to a lack of LPL activity leading to insufficient TG catabolism with a severe increase in TG concentration.104 There is a group of chylomicronaemias with a severe genetic basis, FCS,105,106 and one that is much more frequent, due to the association of less pathogenic genetic variants with aggravating factors, MCS.107 FCS is a minority disease affecting 1/1,000,000 of the population. It is caused by homozygous or compound heterozygous recessive loss-of-function variants of the genes for LPL, apolipoprotein C2 (Apo C2), apolipoprotein A5 (Apo A5), lipase maturation factor 1 (LMF1), glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) or glycerol 3-phosphate dehydrogenase 1 (G3PDH1).108,109 Chylomicronaemia occurs even without aggravating factors and TG concentrations remain chronically very high, usually >10 mmol/l (885 mg/dL).110 The most serious complication is acute pancreatitis, which is more frequent and recurrent the higher the TG concentration, and can occur in early life.111 Pancreatitis may progress to chronic pancreatitis with pancreatic insufficiency and pancreoprivic DM. Eruptive xanthomas affecting the extensor surface of the extremities and trunk, hepatosplenomegaly, and lipaemia retinalis.112
MCS is due to the association of different genetic factors of minor pathogenicity, associated with entities responsible for secondary hypertriglyceridaemia.113 There is a risk of pancreatitis, but it is lower than in FCS due to the lesser severity and persistence of hypertriglyceridaemia.114 The response to diet, physical activity, and drugs for hypotriglyceridaemia is usually good. MCS usually appears in adulthood or mid-life.
In both situations, treatment is based on a diet with significant restriction of total fat (<30 g per day), use of MCT-rich oil, fibrates, and n-3 fatty acids at doses higher than 3 g per day, although in FCS the response to fibrates and omega-3 may be very poor or absent as LPL activity is not stimulable. Volanesorsen is available currently, an antisense oligonucleotide that blocks the synthesis of apolipoprotein C3 (Apo C3), a protein involved in the metabolism of TG-rich lipoproteins, hindering, among other effects, their clearance from plasma. It produces a TG reduction of more than 50%. Platelet count should be monitored as it may cause thrombopenia. There are several drugs in various phases of clinical trials for this rare entity for which information will be available in the short to medium term.
HypoalphalipoproteinaemiaThe most common causes of hypoalphalipoproteinaemia are those linked to hypertriglyceridaemia and what is termed atherogenic dyslipidaemia. Primary forms are rare and include Tangier disease, LCAT deficiency, and mutations in the Apo A1 gene.115 There are no specific treatments for these entities, and the management of these subjects should be aimed at strict control of the remaining VRFs and additional organ impairments, such as renal involvement in some of the diseases.116
HypobetalipoproteinaemiaThese are patients with very low values of Apo B-containing lipoproteins. They present clinically with very low (below the 10th percentile) LDL-C values. They include entities such as abetalipoproteinaemia (MTP deficiency), hypobetalipoproteinaemia (defective homo or heterozygous Apo B gene), PCSK9i and angiopoietin-like protein 4 [ANGPTL4] deficiency), chylomicron retention disease (secretion-associated Ras-related GTPase 1B [SAR1B] deficiency). Severe clinical manifestations, including intestinal malabsorption, fatty liver, haemolytic anaemia, and neurological disorders due to vitamin and essential fatty acid deficiencies, occur in the forms with total Apo B deficiency. They are treated with supplementation of these deficient elements. Moderate forms of hypobetalipoproteinaemia usually give rise to complications and should generally be considered benign and associated with lower VR. In those due to lower Apo B synthesis, an increased risk of fatty liver disease has been reported.117
Patients with hypertensionAdequate BP measurement is recommended to confirm the diagnosis of hypertension Table 3. A simultaneously measured BP difference between the two arms >15–20 mmHg is associated with an increased risk of AVD.
At the first visit it is necessary to measure BP lying down, after five minutes of rest, and then standing, after one to three minutes. Orthostatic hypotension is defined as a reduction in standing systolic BP > 20 mmHg, and should be considered when initiating or intensifying antihypertensive treatment.
ABPM and SMBP correlate more closely with prognosis and TOD than clinical measurement, and therefore are highly recommended.9,118
In addition to its value in diagnosing untreated subjects, and in identifying subjects with white coat HTN (elevated clinical BP, with normal ABPM BP values), ABPM is also a useful tool for monitoring treatment effects, assessing adherence, and detecting subjects with masked HTN (subjects with normal BP at consultation and elevated values during ABPM).
Table 11 shows the BP categories according to the figures obtained by clinical BP measurement and Table 12 the definition of HTN according to the threshold values of clinical, ambulatory, or self-measured BP at home.
Classification of blood pressure in adults.
| Category | SBP (mmHg) | DBP (mmHg) |
|---|---|---|
| Optimal | <120 | <80 |
| Normal | 120−129 | 80−84 |
| Normal high | 130−139 | 85−89 |
| Grade 1 hypertension | 140−159 | 90−99 |
| Grade 2 hypertension | 160−179 | 100−109 |
| Grade 3 hypertension | ≥180 | ≥110 |
| Isolated systolic hypertension | ≥140 | <90 |
The diagnosis of HTN shall be established by checking BP values in two or more measurements taken at each of two or more visits.
When SBP and DBP are in different categories, the higher category applies.
Isolated systolic HTN is classified into grades (1, 2 or 3) according to the SBP value.
DBP: diastolic blood pressure; HTN: hypertension; SBP: systolic blood pressure.
Definitions of hypertension according to clinical, ambulatory, or self-measured home blood pressure readings.
| Category | SBP (mmHg) | DBP (mmHg) |
|---|---|---|
| Office BP | ≥140 | ≥90 |
| Ambulatory BP | ||
| • Diurnal (or awake) | ≥135 | ≥85 |
| • Nocturnal (or sleeping) | ≥120 | ≥70 |
| • 24 h | ≥130 | ≥80 |
| Home BP | ≥135 | ≥85 |
BP: blood pressure; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Adapted from Mancia G, et al.8
BP readings obtained by ABPM can identify four phenotypes:
- •
Arterial normotension: normal BP readings in the clinic and on ABPM.
- •
White coat HTN: elevated clinical BP readings, but normal BP levels on ABPM.
- •
Masked HTN: normal clinical BP levels, but elevated ABPM readings.
- •
Established HTN: elevated clinical and ABPM BP readings.
BP values obtained by SMBP also correlate better with target organ involvement and with the incidence of AVD, but ABPM provides information on nocturnal BP, which is strongly correlated with prognosis. Its elevation is especially prevalent in certain clinical situations, such as resistant HTN, HTN associated with DM or advanced CKD, and HTN associated with obstructive sleep apnoea-hypopnoea syndrome (OSAHS).
Initiation of antihypertensive treatmentThe decision to initiate pharmacological treatment will depend not only on BP level, but also on the overall VR in relation to other associated VRFs, the presence of subclinical target organ damage (Table 4), which predicts cardiovascular mortality independently of SCORE,13,119 especially in the moderate risk group, and the presence of AVD or established kidney disease.
Initial treatment will be decided according to BP levels (grades 1−3) (Table 11) and according to the stage of HTN (1−3):
- •
Stage 1: uncomplicated HTN, without TOD, without DM, without AVD, and without stage ≥ 3 CKD, i.e., subjects with estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73 m2).
- •
Stage 2: HTN in the presence of TOD, or DM, or stage 3 CKD.
- •
Stage 3: presence of AVD, or CKD stages 4 (eGFR < 15−29 mL/min/1.73 m2) or 5 (eGFR < 15 mL/min/1.73 m2).
Initial treatment should always include lifestyle changes, as the only initial treatment in subjects with grade 1 HTN at stage 1, with low or moderate risk.120,121 In those with grade 1 but high-risk HTN or with TOD (stage 2 HTN), pharmacological treatment should be initiated early once the diagnosis of HTN has been confirmed.
In patients with stage 2 HTN (SBP between 160−179 mmHg and/or DBP of 100−109 mmHg) and in those with grade 3 HTN (SBP ≥ 180 mmHg or DBP ≥ 110 mmHg), pharmacological treatment should be added from the outset, and most will require combined antihypertensive treatment with at least two antihypertensives. Approximately 10%–12% of treated hypertensives will require more than three antihypertensives.122
The main benefit of antihypertensive treatment is in reducing BP,121 irrespective of the drug used, and treatment may be initiated with thiazide diuretics, or similar (indapamide, chlorthalidone), angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II AT1 receptor antagonists (ARA-II), Ca antagonists, or beta-blockers. The position of the latter as first-line drugs has been questioned, due to their lower effectiveness in stroke prevention or their possible unfavourable metabolic effects (although this may not apply to all beta-blockers), except in clinical situations with specific indications for these agents (coronary artery disease, heart failure [HF] with reduced ejection fraction [EF], etc.). They may also be indicated as initial treatment in young hypertensive patients with a hyperkinetic pattern and a tendency to tachycardia, or in women of childbearing age, in whom the use of renin-angiotensin system (RAS) inhibitors should be used with caution because they are contraindicated from the early stages of pregnancy.
A recent meta-analysis has shown that a 5 mmHg reduction in SBP reduces serious cardiovascular events by approximately 10% in both primary and secondary prevention.123
Therapeutic target of antihypertensive treatmentThe new 2023 ESH guidelines advise the following therapeutic targets, depending on age8: (Table 13)
- •
Age 18–64 years: SBP < 130 mmHg and DBP between 70−80 mmHg.
- •
Age 65–79 years: SBP between 130−139 mmHg and <130 mmHg, if tolerated. DBP between 70−80 mm Hg.
- •
Age > 80 years: SBP < 150 mmHg, and below 140 mmHg if tolerated. DBP between 70−80 mmHg. In subjects with isolated systolic HTN, it is usually necessary to intensify treatment if SBP is >160 mmHg, even if DBP is <70 mmHg.
Clinical SBP therapeutic targets in some subgroups of hypertensives.
| Clinical conditions | SBP |
|---|---|
| Age < 65 years | 120 < 130 mmHg |
| Age ≥ 65 years | 130 < 140 mmHg* |
| DM | 120 < 130 mmHg** |
| IHD | 120 < 130 mmHg |
| CKD | 120 < 130 mmHg |
| Post stroke/TIA | 120 < 130 mmHg |
TIA: transient ischaemic attack; DM: diabetes mellitus; IHD: ischaemic heart disease; CKD: chronic kidney disease; SBP: systolic blood pressure.
Adapted from Mancia G, et al.8
SBP < 120 mmHg or DBP < 70 mmHg is not recommended.
Treatment of hypertension in patients with comorbiditiesHypertension and diabetes mellitusThe prevalence of HTN in patients with DM is up to 80%, double that observed in the non-diabetic population of the same age and characteristics. The coexistence of HTN and DM increases the risk of developing AVD with a higher incidence of ischaemic heart disease (IHD), HF, PAD, stroke, and cardiovascular mortality.
It is recommended to initiate treatment with a combination of an RAS inhibitor plus a Ca antagonist or a thiazide or thiazide-like diuretic, except when eGFR is <30 mL/min/1.73 m2, in which case a loop diuretic would be indicated. The use of a combination of two RAS inhibitors is not recommended.
It is recommended to initiate drug treatment when SBP is ≥140 mmHg or DBP ≥ 90 mmHg, and the therapeutic target will be an SBP of <130 mmHg, but not lower than 120 mmHg, with a DBP < 80 mmHg, but not <70 mmHg.
Hypertension and chronic kidney diseaseThe prevalence of HTN in these patients is 67%–92%, and is their most frequent comorbidity. Moreover, the presence of HTN may accelerate renal damage, in addition to increasing VR. As in all patients with HTN, pharmacological treatment should be accompanied by lifestyle changes, with special emphasis on reducing Na intake. Loop diuretics should replace thiazide or thiazide-like diuretics when eGFR is <30 mL/min/1.73 m2.
A clinical trial published in 2021 has shown that in subjects with advanced CKD (eGFR of 23 ± 4.2 mL/min/1.73 m2), chlorthalidone at doses of 12.5−50 mg improves control in subjects with poorly controlled HTN compared to placebo at 12 weeks follow-up.124
Because BP lowering decreases perfusion pressure, a reduction in eGFR between 10%–20% at start of treatment is not uncommon. Electrolytes should be carefully monitored.
The therapeutic goal is an SBP < 130 mmHg, but not below 120 mmHg and a DBP < 80 mmHg, but not <70 mmHg.
An RAS inhibitor plus a Ca antagonist or a diuretic is recommended as initial treatment. Concomitant use of ACE inhibitors and ARA-II is not recommended.
Hypertension and stable coronary artery diseaseHypertension is a major VRF for IHD. Numerous clinical trials have demonstrated the benefits of antihypertensive treatment in reducing the incidence of IHD in both primary and secondary prevention.
Except in some cases, such as the frail elderly or those over 80 years of age, dual therapy with an RAS inhibitor (ACE inhibitor or ARA-II) plus a beta-blocker or Ca antagonist is generally recommended as initial treatment, although other combinations such as dihydropyridine Ca antagonist plus beta-blocker may be used. In a second step, if HTN is not controlled, the patient should be treated with triple therapy, usually with the addition of a diuretic to one of the above combinations.
If symptomatic angina is present, a combination of beta-blockers and dihydropyridine Ca antagonists is recommended.
Initiation of pharmacological treatment is advised when BP is ≥130/80 mmHg, and the therapeutic target would be an SBP of 130 mmHg or lower if tolerated, but not <120 mmHg, and a DBP < 80 mmHg, but not <70 mmHg. In subjects aged 65 years or older, a therapeutic target SBP between 130−140 mmHg may be adequate.
Hypertension and heart failureA history of HTN is present in 75% of patients with chronic HF. Antihypertensive treatment is recommended when clinical BP is ≥140/90 mmHg, both in patients with HF with reduced EF and in those with HF with preserved EF. In patients with HF and reduced EF, treatment with an RAS inhibitor (ACE inhibitor or ARA-II), a beta-blocker and a diuretic, associated with a Na-glucose cotransporter type 2 (SGLT2) inhibitor and a mineralocorticoid receptor antagonist, if not contraindicated, is recommended. If control of HTN is not achieved despite the above treatment, a dihydropyridine Ca antagonist could be added.
In patients with HF and preserved EF, ACE inhibitors or ARBs, beta-blockers, Ca antagonists, and thiazides or thiazide-like diuretics combined with the use of SGLT2i are recommended for control of HTN.
Hypertension and atrial fibrillationAHT is the most common modifiable VRF for the development of AF. The coexistence of hypertension and AF significantly increases the risk of ischaemic and haemorrhagic stroke. All first-line antihypertensive drugs would be indicated for the control of HTN, but the use of RAS inhibitors and beta-blockers could be considered in AF to prevent recurrence. The threshold for initiation of antihypertensive treatment and the therapeutic target would be the same as for the general population.
In patients with AF and SBP > 160 mmHg, improved control of HTN is recommended before initiating anticoagulant therapy.
Hypertension and strokeStroke is a major cause of mortality, disability, and dementia, and is independently associated with increased major vascular events in both sexes.105 Given that stroke is a heterogeneous group, in terms of causes and underlying haemodynamics, the control of HTN in these patients is complex and challenging. The therapeutic target is a BP < 130/80 mmHg.125 This target BP < 130/80 mmHg is recommended in TIA, as advised by a recent American Heart Association (AHA) document, as it reduces the risk of stroke recurrence by 22%.126
Resistant and refractory HTNThere are several causes of poor control of HTN in clinical practice, which should be considered before labelling a patient as having resistant HTN (Fig. 6).127
Resistant HTN represents approximately 10%–12% of treated hypertensives.122 HTN is considered resistant when BP < 140/90 mmHg has not been reduced despite optimal doses (or maximum tolerated doses) of three drugs and a treatment plan that includes a diuretic (typically ACE inhibitor or ARA-II plus a Ca antagonist and a diuretic).
Poor control should be confirmed by ABPM (preferable) or SMBP and causes of pseudoresistance (e.g. poor adherence) and secondary HTN should be excluded.
In recent years, a novel phenotype of refractory HTN has been described, when a BP < 140/90 mmHg is not achieved despite the use of ≥5 antihypertensive drugs.128,129 It is rare (1.4%), and also necessitates 24-h ABPM for confirmation, as well as ruling out causes of pseudoresistance, as mentioned above in resistant HTN. A recent prospective study130 has shown that patients with refractory HTN confirmed by 24-h ABPM have a higher risk of major cardiovascular events and mortality.
Follow-up of the patient with hypertensionAfter initiation of antihypertensive drug treatment, it is important to follow up at least once within the first two months to assess the effect on BP, detect possible adverse effects, and detect poor adherence. Then, a follow-up visit at three months is recommended to check BP control and assess adherence.
Poor adherence is a common cause of poor BP control. Nurses, community pharmacists, and other healthcare professionals play a key role in its assessment and detection and are also helpful in the education, support, and long-term follow-up of hypertensive patients and should be part of the overall strategy to improve BP control. Simplification of treatment and reduction of the number of tablets will undoubtedly help to improve adherence and BP control in the medium and long term.
In subjects with normal-high BP, even if not treated pharmacologically, lifestyle changes and regular follow-up (at least one visit per year) to measure clinical and ambulatory BP, and to reassess their VR, are recommended.
Patient with hyperglycaemia (pre-diabetes and diabetes mellitus)Classification and diagnostic testsHyperglycaemia is defined as having at least two fasting plasma glucose readings ≥100 mg/dL, and DM as having two fasting plasma glucose values ≥126 mg/dL. In addition to the fasting blood glucose criterion, preDM and DM can be diagnosed if at least one of the HbA1c criteria, the random blood glucose values, or the two-hour plasma glucose values of the oral glucose tolerance test (OGTT) described in Table 14 are met.131
American Diabetes Association (ADA) criteria for the diagnosis of prediabetes and diabetes mellitus.131
| Criteria for the diagnosis of prediabetes and DM* | Normal | Prediabetes | DM |
|---|---|---|---|
| HbA1c (%) o | <5.7 | 5.7−6.4 | ≥6.5 |
| Fasting blood glucose (mg/dL) or | <100 | 100−125 | ≥126 |
| 2 h OGTT with 75 g (mg/dL) or | <140 | 140−199 | ≥200 |
| random plasma glucose with hyperglycaemic symptoms (mg/dL) | ≥200 |
DM: diabetes mellitus; HbA1c: glycated haemoglobin; OGTT: oral glucose tolerance test.
The OGTT is performed by administering 75 g of glucose in an adult or 1.75 g/kg of weight (maximum 75 g) in children under standardised conditions. It is a very useful test to confirm the diagnosis of DM in patients whose fasting blood glucose or HbA1c values do not confirm the diagnosis, but who have a high probability of being diagnosed: obese subjects, MS, gestational DM, or those with pre-DM criteria.51,131 HbA1c should be tested using a DCCT reference assay.131
We recommend screening for DM in asymptomatic subjects to make an early diagnosis (Table 15). In this case, fasting blood glucose and HbA1c values are used to facilitate the diagnosis, and it is not necessary to use OGTT for screening.
Subjects to be screened for diabetes mellitus.51
| Age ≥ 35 years | Age < 35 years |
|---|---|
| In all cases | With a diagnosis of prediabetes or with a history of gestational DM or HIV or with a BMI ≥ 25 kg/m2, in Asians ≥ 23 kg/m2, with at least one of the following: |
| • DM in first-degree relatives | |
| • Belonging to an ethnic group at high risk for DM (African Americans, Latinos, etc.) | |
| • History of CVD | |
| • HTN (≥140/90 mmHg or on treatment) | |
| • HDL-C < 35 mg/dL, TG > 250 mg/dL, or both | |
| • POS | |
| • Sedentary lifestyle | |
| • Other conditions associated with insulin resistance (acanthosis nigricans, non-alcoholic fatty liver disease, familial combined hyperlipidaemia, etc.) |
BMI: body mass index; CVD: cardiovascular disease; DM: diabetes mellitus; HDL-C: high-density lipoprotein cholesterol; HIV: human immunodeficiency virus; HTN: hypertension; POS: polycystic ovary syndrome.
Patients with newly diagnosed DM are at elevated VR without necessarily having TOD or vascular disease. SCORE-2 DM should be used to assess VR in this group of patients.58
Control targets in prediabetes and diabetes mellitusThe general control targets are shown in Table 16.
General control targets for prediabetes and diabetes mellitus.
| Targets |
|---|
| • To establish, achieve, and maintain good metabolic control |
| • To prevent complications of DM |
| • To preserve the patient’s life and alleviate symptoms of hyperglycaemia |
| • To help the patient achieve a good quality of life (personal, family, work, and social) |
| • Individualise HbA1c value |
DM: diabetes mellitus; HbA1c: glycated haemoglobin.
To obtain good glycaemic control, it is first necessary to individualise the HbA1c target.131 In setting the HbA1c value, the following should be considered: the degree of motivation, the presence of chronic complications or severe comorbidities, the patient's age, estimated survival and the time course (Fig. 7). In young patients, without chronic complications or comorbidities, with short disease course and long estimated survival, we will seek to intensify treatment to achieve HbA1c between 6%–7%.131 To adequately treat patients with DM, it is necessary not only to control glycaemia and seek an individualised HbA1c value, but also to control BP values, lipids, stop smoking, and achieve an optimal weight. This is what is known as global treatment of DM. This treatment will always be individualised and early. The specific BMI, smoking, BP, and biochemical targets for the treatment of DM are listed in Table 17.
Specific clinical and biochemical targets of treatment in adult diabetics.
| Targets | Optimal | Acceptable | Undesirable |
|---|---|---|---|
| BBG (mg/dL) | 80−130 | 130−179 | ≥180 |
| 2 h-PG (mg/dL) | <180 | 180−199 | ≥200 |
| HbA1c (%) | <7.0 | 7.0−7.9 | ≥8.0 |
| LDL-C (mg/dL)* | Variable | Variable | Variable |
| BMI (kg/m2) | <25 | 25−26.9 | ≥27 |
| BP (mmHg) | <130/<80 | 130−139/80−89 | ≥140/≥90 |
| Smoking | NO SMOKING | ||
LDL-C according to CV risk: Very high-risk DM LDL-C < 55 mg/dl, high-risk DM LDL-C < 70 mg/dl, moderate risk DM LDL-C < 115 mg/dl (see Table 21).
BBG: basal blood glucose; BMI: body mass index; BP: blood pressure; DM: diabetes mellitus; HbA1c: glycated haemoglobin; LDL-C: low-density lipoprotein cholesterol; PG: postprandial blood glucose.
Treatment of preDM and DM is based on lifestyle changes, diet therapy, and the use of drugs to achieve the therapeutic goals discussed above.
Diet and lifestyle changesTreatment should aim to meet all of the above global goals. The therapy for preDM and T2DM is diet, lifestyle changes and physical activity.13,131,132 As most of those affected are obese, dietary management is similar to that described in the section on the obese patient.
Lifestyle modification in DM is an essential intervention to improve not only glycaemic control but also associated comorbidities such as dyslipidaemia, obesity, and HTN. Healthy lifestyle changes should include the following: smoking cessation and moderation of alcohol intake, weight loss (>5%) in case of overweight or obesity, personalised nutritional advice, and activity with continuous monitoring. Carbohydrate intake from vegetables, fruits, whole grains, legumes, and dairy products is recommended over other sources.13,132 Especially limit processed foods containing added fats, sugars, or Na. We recommend the consumption of n-3 polyunsaturated fatty acids found in fish, especially oily fish (sardines, salmon, tuna, mackerel, etc.). In summary, a Mediterranean-type diet rich in monounsaturated fatty acids (virgin olive oil and nuts) may be useful for the overall control of VR in these patients.13,132
Furthermore, different studies collected in meta-analyses on the effects of lifestyle interventions in people with pre-DM have found effective prevention for the development of DM.131 These studies support the use of a Mediterranean-type diet in subjects with preDM.133
The general recommendations for physical activity in people with preDM or DM are given in Table 18 below.
General recommendations for physical activity in people with pre-diabetes and DM.
| General recommendations |
|---|
| • Do at least 150 min per week of moderate to vigorous aerobic activity, spread over at least three days per week. Avoid more than two days without physical activity |
| • Do two to three resistance exercise sessions per week on non-consecutive days |
| • Reduce the amount of time spent in sedentary activities each day |
| • For older adults, do flexibility and balance exercises two to three times a week |
DM: diabetes mellitus.
In subjects with preDM, the ADA recommends treatment with metformin, in those with BMI > 35 kg/m2, a history of gestational DM, in subjects with fasting blood glucose ≥ 110 mg/dL and HbA1c ≥ 6.1%, and in obese subjects with visceral fat deposition.131
The treatment targets of patients with DM are to prevent and delay macro- and microvascular complications, and to reduce high vascular morbidity and mortality. The maximum benefit in cardiovascular prevention in patients with DM is obtained by intervening simultaneously in all the VRFs: smoking, dyslipidaemia, HTN, and hyperglycaemia.13,131
Focusing on the pharmacological treatment of hyperglycaemia, once the target HbA1c value has been established, the choice of drugs will depend on age, degree, and type of obesity, efficacy and tolerance of the drugs, cost, comorbidities (secondary prevention, HF, or diabetic kidney disease) (Fig. 7).
Table 19 shows the main drugs used for the treatment of hyperglycaemia in DM and their indication.
Main drugs used in the treatment of DM.
| Drug | Dose | Indication |
|---|---|---|
| Metformin | 850 mg × 3 | T2DM |
| Titular de forma gradual | T1DM obese | |
| Gestational DM | ||
| Pioglitazone | 30−45 mg/day | T2DM |
| T2DM and fatty liver | ||
| DPP4i | Sitagliptin 100 mg/day | T2DM |
| Vidagliptin 50 mg × 2 | ||
| Saxagliptin 5 mg/day | ||
| Linagliptin 5 mg/day | ||
| Alogliptin 25 mg/day | ||
| GLP-1 RA | Exenatide 5 μg sc × 21st month, 10 μg sc × 22nd month | T2DM obese in secondary prevention |
| Liraglutide .6 mg/day, 1.2 mg/day 2nd week and if necessary 1.8 mg/day | T2DM with BMI ≥ 30 kg/m2 | |
| Exenatide LAR, 2 mg/week | ||
| Lixisenatide, 10 μg/day 2 weeks, increase to 20 μg/day | ||
| Dulaglutide .75 mg/week increase 1.5 mg/week | ||
| Semaglutide .25 mg/week for 4 weeks and increase to .5 mg/week Maximum 1 mg/week | ||
| SGLT2i | Dapagliflozin 10 mg/day | T2DM |
| Empagliflozin 10 and 25 mg/day | T2DM with HF | |
| Canagliflozin 100 and 300 mg/day | T2DM in secondary prevention | |
| Ertugliflozin 5 and 15 mg/day | Kidney disease in T2DM | |
| Meglitinides | Repaglinide .5–4 mg before the three main meals | T2DM with CKD |
| Nateglinide 120 mg, with three main means | ||
| Sulfonylureas 3rd generation | Start with low doses and increase the dose | T2DM |
| Alpha-glucosidase inhibitors | Acarbose or miglitol 50 mg × 3/day, at start of meal. Maximum 100 mg × 3/day | T2DM |
BMI: body mass index; CKD: chronic kidney disease; DDP-4i: dipeptidyl peptidase 4 inhibitors; DM: diabetes mellitus; GLP RA-1: GLP-1 receptor agonists; T2DM: type 2 diabetes mellitus; HF: heart failure; SGLT2i: sodium-glucose cotransporter 2 inhibitors; sc: subcutaneous; T1DM: type 1 diabetes mellitus.
Table 20 summarises the main benefits, contraindications, adverse effects, and cardiovascular prevention capacity irrespective of their hypoglycaemic effect.
Benefits, contraindications, main adverse effects, and vascular prevention capacity of the main hypoglycaemic drugs used in the treatment of diabetes mellitus.
| Drug | Benefits | Contraindication | Disadvantages/Adverse effects | CV prevention |
|---|---|---|---|---|
| Metformin | ↓ HbA1c 1% | eGFR <30 mL/min/1.73 m2 | Nausea, vomiting abdominal pain | Yes131 |
| ↓ weight (1.5−2 kg) | Severe liver failure 24 h before surgery | Exceptional: Vitamin B12 deficiency and lactic acidosis | ||
| ↓ cost | ||||
| No hypoglycaemias | ||||
| Pioglitazon | ↓ HbA1c .5%–1% | Heart failure | ↑ weight | Yes, in primary prevention |
| ↓ TG and ↑ HDL-C | ↑ cost | Stroke (CVA)131 | ||
| Oedema | ||||
| HF | ||||
| Fractures | ||||
| in postmenopausal women | ||||
| DPP-4i | ↓ HbA1c .8% | FG <60 mL/min/1.73 m2 adjust dose of sitagliptin 25 and 50 mg/day, alogliptin 6.25 and 12.5 mg/day | ↑ cost | No |
| Weight= | Linagliptin not required | Skin lesions (bullous pemphigoid) | Saxagliptin ↑ admissions for heart failure | |
| No hypoglycaemias | Do not combine with GLP RA-1 | |||
| arGLP-1 | ↓ HbA1c 1% | Pregnancy | Nausea, vomiting | Yes |
| ↓↓ weight | ↑ cost | Liraglutide and semaglutide in secondary prevention.134,135 Dulaglutide primary and secondary prevention136 | ||
| No hypoglycaemias | Liraglutide, semaglutide and dulaglutide ↓ diabetic kidney disease progression | |||
| SGLT2i | ↓ HbA1c 1% | Pregnancy | Urinary tract infections | Yes |
| ↓ peso | Genital mycosis | Empagliflozin and Canagliflozin in secondary prevention137,138 | ||
| ↓ PA | Rare orthostatic hypotension, dehydration, and euglycemic ketoacidosis | Empagliflozin mortality137 | ||
| No hypoglycaemias | Dapagliflozin CV mortality139 | |||
| Empagliflozin, canagliflozin, dapagliflozin ertugliflozin admissions due to heart failure137–139 Canagliflozin and dapagliflozin preservation of renal function and ↓ progression of diabetic kidney disease140,141 | ||||
| Meglitinides | ↓ HbA1c .8% | Pregnancy and breastfeeding | Hypoglycaemias | No |
| ↑ Weight | ||||
| Use in CKD | ||||
| 3rd generation sulfonylureas | ↓ HbA1c 1% | Pregnancy and breastfeeding | Hypoglycaemias | No |
| ↑ Weight (2−4 kg) | Patient admitted | Do not use with CKD, liver failure or HF | ||
| Alpha-glucosidase inhibitors | ↓ HbA1c .5% | T1DM | Gastrointestinal: flatulence, distension, pain | No |
| Weight= | Gestation and breastfeeding | |||
| Gastrointestinal disease |
BP: blood pressure; CKD: chronic kidney disease; CV: cardiovascular; CVA: cerebrovascular accident; DDP-4i: dipeptidyl peptidase 4 inhibitors; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; GFR: glomerular filtration rate; GLP RA-1: GLP-1 receptor agonists; HbA1c: glycated haemoglobin; HDL-C: high-density lipoprotein cholesterol; HF: heart failure; SGLT2i: sodium-glucose cotransporter 2 inhibitors; T1DM: type 1 diabetes mellitus; TG: triglycerides.
Fig. 8 shows the treatment regimens according to the presence or absence of CVD, BMI, diabetic kidney disease, and HF. Metformin remains the drug of first choice in patients with T2DM.131 Although in subjects in secondary vascular prevention, high or very high VR, DM, or HF, SGLT2i, or glucagon-like peptide-1 receptor agonists (GLP-1 RA) could be used in the first step.
Therapeutic guidelines according to vascular status, body mass index, diabetic kidney disease, and presence of heart failure.
8A) Treatment regimen in patients with type 2 diabetes mellitus, with vascular disease or at high/very high vascular risk, or with heart failure, or diabetic kidney disease.
*if using metformin in the first step.
8B) Therapeutic regimen in patients with type 2 diabetes and moderate/low vascular risk, without established atherosclerotic vascular disease, without heart failure, or without diabetic kidney disease.
* use DDP4i in case of high risk of hypoglycaemia and in subjects with frailty, dementia, elderly.
CVD: cardiovascular disease; BMI: body mass index; DPP-4i: dipeptidyl peptidase-4 inhibitors; DM: diabetes mellitus; GLP RA-1: glucagon-like peptide-1 receptor agonists; HbA1c: glycated haemoglobin; SGLT2i: sodium/glucose cotransporter 2 inhibitors; SU: sulphonylureas.
As a second therapeutic step, we will choose the drug based on whether the patient is in primary or secondary prevention, presence of obesity, diabetic kidney disease, or HF (Fig. 8).131
There are numerous intervention studies that demonstrate the capacity of SGLT2i137–139 or GLP-1 RAs in vascular prevention134–136 and progression of diabetic kidney disease.140,141 Therefore, they should be priority drugs in the treatment of T2DM.
Depending on the HbA1c value, the initial treatment of hyperglycaemia will vary. If HbA1c is less than 7.5% we will opt for monotherapy, if it is between 7.5%–9% it will be dual therapy, and in cases of glycaemia greater than 9% with cardinal symptoms we will opt for insulinisation.
Insulinisation in T2DM with a long-acting or basal insulin is indicated if triple therapy fails (third therapeutic step, Fig. 8), always after having tried an GLP-1 RA. In cases where there is clear clinical evidence of hyperglycaemia with elevated HbA1c, insulinisation will also be necessary, as discussed in the previous paragraph. The total daily dose to start insulinisation is .1–.3 U/kg body weight, with modification of the dose every three days until targets are achieved. The real need to increase the dose should be assessed on each occasion, as high doses in obese and insulin-resistant subjects may increase weight and thus insulin resistance without improvement in glycaemic control.131
Cardiovascular prevention in diabetes mellitusTreatment and prevention of atherosclerotic vascular disease in the person with diabetes mellitusCardiovascular prevention in DM requires early, intensive, and sustained intervention in all VRFs: dyslipidaemia, BP, smoking, and abdominal obesity (Table 21).13,131,132
Treatment and prevention of AVD in the person with diabetes mellitus.
| VRF | Target | Treatment |
|---|---|---|
| Control of hyperglycaemia | HbA1c < 7% | Hypoglycaemic diet |
| SGLT2i or GLP RA-1 | ||
| HTN | SBP 120–129 mmHg and DBP 70–80 mmHg | Reduction of salt intake <3 g/24 h |
| In the presence of albuminuria, lower BP if tolerated, although there are discrepancies on this point | In the presence of macroalbuminuria or renal insufficiency, restrict protein intake to .6–.8 g/kg/24 h | |
| Reduce alcohol (maximum tolerated 30 g/day) | ||
| Moderate coffee consumption (2 cups/24 h) | ||
| ACE inhibitors or ARA-II | ||
| Smoking | No active or passive smoking | |
| Obesity | BMI < 30 kg/m2 | Diet therapy strategy |
| Dyslipidaemia | Primary: lower LDL-C according to patient’s risk (see Table 21) | Statins in combination or not with ezetimibe to achieve therapeutic targets, PCSK9i in secondary prevention according to SEA recommendations (Table 8) |
| Secondary TG < 150 mg/dL | Fibrates or omega 3 if necessary to reduce severe hypertriglyceridaemia |
ACE inhibitor: angiotensin 1-converting enzyme inhibitor; ARA-II: angiotensin II receptor antagonists type 1; AVD: atherosclerotic vascular disease; BP: blood pressure; BMI: body mass index; DM: diabetes mellitus; GLP-1: glucagon-like peptide-1 receptor agonists; HbA1c: glycated haemoglobin; LDL-C: low-density lipoprotein cholesterol; PCSK9i: proprotein convertase subtilisin/kexin 9 inhibitor; SEA: Spanish Society of Atherosclerosis; SGLT2i: sodium-glucose cotransporter type 2 inhibitors; TG: triglycerides; VRF: vascular risk factors.
In general, people with T2DM are considered to be at high VR.13,142 People with T2DM and multiple VRFs (dyslipidaemia, HTN, smoking), or SVD, or TOD, or who are in secondary prevention, are at very high VR.13 The primary objective for prevention is to obtain an LDL-C or non-HDL-C value as listed in Table 22, according to the patient’s risk classification.13
Primary LDL-C or non-HDL-C target for vascular prevention in people with diabetes mellitus13.
| Lipid profile | Moderate risk | High risk | Very high risk |
| DM of less than 10 years’ duration with no other VRF and no TOD | DM of | DM with AVD | |
| >10 years’ duration or poor glycaemic control and TOD in one site | DM with: | ||
| eGFR < 30 or | |||
| GFR 45−59 and ALB > 30 or | |||
| ALB > 300 | |||
| or TOD in three sites or more than 3 VRF or target organ involvement | |||
| T1DM of more than 20 years’ duration | |||
| LDL-C (mg/dL) | <100 | <70 | <55 |
| Non-HDL-C (mg/dL) | <130 | <100 | <85 |
| TG (mg/dL) | <200 | <200 | <200 |
| HDL-C (mg/dL) (M/F) | >40/>50 | >40/>50 | >40/>50 |
ALB: albuminuria; eGFR: estimated glomerular filtration rate; F: females; GFR: glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol: M: males; Non-HDL-C: non-HDL-cholesterol; TOD: target organ damage; VRF: vascular risk factors.
To achieve these targets, it will be necessary to use high-intensity statins, in most cases in combination with ezetimibe. Fig. 5 shows the strategy and outline of the treatment of dyslipidaemia in people with DM in order to achieve effective vascular prevention. Once the LDL-C or non-HDL-C value has been obtained, a secondary target of TG < 200 mg/dL should be sought in patients with DM and MS.13,51,143
Patient with obesity and metabolic syndromePatient with metabolic syndromeTable 23 defines MS.51 Metabolic disorders related to MS include the following51:
- •
Dyslipidaemia, essentially hypertriglyceridaemia, a decrease in HDL-C, and the presence of small, dense LDL-C particles, with an increase in plasma free fatty acids. This set of conditions is known as atherogenic dyslipidaemia.
- •
Hyperglycaemia or DM.
- •
HTN.
Diagnostic criteria for metabolic syndrome.
| Diagnostic criteria* | Description |
|---|---|
| Abdominal obesity | Elevated waist circumference (above iliac crests) according to sex and ethnicity (≥94 H and ≥80 M Caucasians) |
| Fasting blood glucose (mg/dL) | ≥100 or prior specific treatment |
| Plasma TG (mg/dL) | ≥150 or prior specific treatment |
| HDL-C (mg/dL) | <40 in. M or <50 in. F or on specific treatment |
| BP (mmHg) | Systolic ≥ 130 or diastolic ≥ 85 or with antihypertensive treatment |
BP: blood pressure; F: females; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; M: males; MS: metabolic syndrome; TG: triglycerides.
These disturbances, together with abdominal obesity, are the parameters that have been established for the diagnosis of MS. In addition, many other disorders not used for diagnosis are of great interest, such as hyperuricaemia, gout, hypercoagulability, and fibrinolysis defects often associated with elevated plasminogen activator inhibitor-1 (PAI-1), non-alcoholic hepatic steatosis, and hyperandrogenism. The clinical relevance of MS is related to its prevalence, 20%–40% of the general population and 80%–85% of subjects with T2DM. Patients with MS have an elevated risk of developing VAS and T2DM.
Data from different meta-analyses indicate that people with MS double their risk of vascular events and have a 1.5-fold increase in all-cause mortality compared to those without MS.51 Recent studies find a five to 10-fold increase in the relative risk of developing T2DM. Other no less important complications related to MS are OSAHS, respiratory failure, or idiopathic alveolar hypoventilation syndrome, and various types of cancer (breast, uterus, colon, oesophagus, pancreas, kidney, prostate, etc.).
Treatment of MS should seek to control all components of the syndrome (Tables 24 and 25). Table 25 shows the pharmacological treatment of MS. The mainstay of treatment is based on lifestyle changes aimed at weight reduction and control of atherogenic dyslipidaemia.51,132,144
Treatment of metabolic syndrome.
| Treatments | |
|---|---|
| Diet | Mediterranean-type diet (saturated fats <7% of total calorie intake, cholesterol <200 mg/day, avoid trans fats) |
| Rich in fibre | |
| Limit Na intake (5−6 g/day) | |
| Foods with low glycaemic index. Avoid simple sugars | |
| Increase fruits, vegetables, and whole grains | |
| Olive oil as main culinary fat | |
| Avoid processed foods | |
| Physical activity | Moderate-intense |
| 30 min/day (preferably 45−60 min) | |
| Continuous/intermittent | |
| At least five days/week | |
| Adjusted to the patient’s age and cardiovascular status | |
min: minutes; Na: sodium; SM: metabolic syndrome.
Treatment of other components of metabolic syndrome.
| Components | Target | Secondary targets | Treatment |
|---|---|---|---|
| Dyslipidaemia | LDL-C according to associated VRF* | HDL-C mg/dL | Hygienic-dietary measures |
| If hypertriglyceridaemia non-HDL-C (30 mg/dL above LDL target) or apoB | >40 M and >50 F | Statins, ezetimibe, omega 3 acids and/or fibrates if necessary (only in the presence of severe hypertriglyceridaemia) | |
| TG < 150 mg/dL | |||
| Hypertension and microalbuminuria | PA 120 < 130/<80 mmHg | Hygienic-dietary measures | |
| ACE inhibitors or ARA-II ± other drugs | |||
| Other VRF | Smoking cessation | ||
| Consider antiplatelet therapy in subjects at very high risk (aspirin) |
ACE inhibitors: angiotensin 1-converting enzyme inhibitors; ApoB: apolipoprotein B; ARA-II: angiotensin II receptor type 1 antagonists; BP: blood pressure; F: females; HDL-C: high-density lipoprotein cholesterol; IPE: icosapent ethyl; LDL-C: low-density lipoprotein cholesterol; M: males; non-HDL-C: non-HDL-cholesterol; MS: metabolic syndrome; TG: triglycerides; VRF: vascular risk factors.
MS is not a coronary equivalent, but it is a risk modulator (Table 4) in risk assessment to establish the LDL target.
Obesity is defined as a BMI ≥ 30 kg/m2.145 The classification of patients according to their BMI value is shown in Table 26.
Classification of people according to BMI. Definition of thinness and obesity.
| Classification | BMI |
|---|---|
| Under weight | <18.5 kg/m2 |
| Normal weight | 18.5−24.9 kg/m2 |
| Grade 1 overweight | 25−26.9 kg/m2 |
| Grade 2 overweight | 27−29.9 kg/m2 |
| Grade 1 obesity | 30−34.9 kg/m2 |
| Grade 2 obesity | 35−39.9 kg/m2 |
| Grade 3 or morbid obesity | 40−49.9 kg/m2 |
| Grade 4 or extreme obesity | ≥50 kg/m2 |
BMI: body mass index.
Waist circumference allows the diagnosis of the type of obesity (abdominal obesity) and cardiometabolic risk. Waist circumference differs according to ethnic group.145
Obesity is very prevalent in Spain. According to the European Health Survey in Spain (EESE) in 2020, 16.5% of men and 15.5% of women were obese and 44.9% of men and 30.6% of women were overweight. Obesity is the main VRF for developing T2DM and carries, especially abdominal obesity, a high VR.145
Treatment of obesity is complex and should be individualised.145 It is based on diet therapy strategies (Fig. 9),146 lifestyle changes, psychotherapy, and drugs (Tables 27 and 28).145
Indications for very low-calorie diets.
| Indications | Contraindications |
|---|---|
| • Patients with BMI > 35 kg/m2 in whom conventional treatment has failed and also have: | • BMI < 30 kg/m2 |
| ◦ Need for rapid weight loss, e.g., severe respiratory failure or orthopaedic surgery | • Pregnancy and breastfeeding |
| ◦ Severe-obesity-associated disease that responds to weight reduction, such as T2DM, HTN, dyslipidaemia, or OSAHS | • Severe systemic or organ disease, except in situations that are clearly aggravated by overweight, where individual risk-benefit assessment is recommended |
| • T1DM | |
| • Psychiatric disorders: eating disorder (ED), severe depression, psychosis, drug, or alcohol addiction | |
| • Water and electrolyte imbalances and orthostatic hypotension | |
| • Protein-losing diseases: Cushing’s disease, systemic lupus erythematosus, proteinuria, neoplasia, malabsorption, inflammatory disease, etc. | |
| • Acute cardiovascular diseases, cardiac arrhythmias, stroke | |
| • Major surgery or trauma in the last three months |
BMI: body mass index; T1DM: type 1 diabetes mellitus; HTN: hypertension; OSAHS: obstructive sleep apnoea-hypopnea syndrome.
Drug treatment of obesity.
| Liraglutide |
|---|
| Posology |
| Saxenda® 6 mg/mL solution for injection in prefilled pen |
| From start of treatment the dose should be increased weekly: |
| 1st week .6 mg/day; 2nd week 1.2 mg/day; 3rd week 1.8 mg/day; 4th week 2.4 mg/day |
| Maintenance 3 mg/day |
| Mechanism of action |
| GLP-1 analogue |
| Clinical effects |
| Weight loss of 8% maintained at three years |
| Decreased progression from prediabetes to DM |
| Adverse effects |
| Nausea and vomiting |
| Contraindications |
| Pregnancy and breastfeeding |
| Multiple endocrine neoplasia (MEN-2) |
| Medullary carcinoma of the thyroid |
| Advanced kidney disease |
| Advanced liver disease |
| Semaglutide | |
|---|---|
| Posology | |
| Ozempic (Wegovy®) | |
| Dose escalation | Weekly dose |
| Week 1–4 | .25 mg |
| Week 5–8 | .5 mg |
| Week 9–12 | 1 mg |
| Week 13–16 | 1.7 mg |
| Maintenance dose | 2.4 mg |
| Mechanism of action | |
| GLP-1 analogue | |
| Clinical effects | |
| Weight loss of 14.9% at 68 weeks | |
| Adverse effects | |
| Nausea and vomiting | |
| Contraindications | |
| Pregnancy and breastfeeding | |
| Not recommended in severe renal insufficiency (eGFR <30 mL/min/1.73 m2), including patients with end-stage kidney disease. | |
| Not recommended in severe liver insufficiency and should be used with caution in patients with mild to moderate liver insufficiency. | |
| Orlistat |
|---|
| Posology |
| Orlistat 120 mg/2−3 times daily at main meals (prescription required) |
| Orlistat 60 mg/2−3 times daily at main meals (no prescription required) |
| Clinical effects |
| ↓ 37% progression to T2DM |
| Dose 120 mg/3 times a day ↓ 3.1% initial weight at one year |
| ↓ SBP, DBP, TC, LDL-C |
| Adverse effects |
| 15%-25% gastrointestinal |
| There may be ↓ fat-soluble vitamin absorption |
| Oxalate nephrolithiasis (Orlistat ↑ urine oxalate and should be used with caution in patients with a history of oxalate nephrolithiasis) |
| Contraindications |
| Chronic malabsorption syndrome |
| Cholestasis |
| Pregnancy, breastfeeding |
| Naltrexone/Bupropion |
|---|
| Posology |
| Naltrexone/Bupropion 8 mg/90 mg extended-release tablets |
| On initiation of treatment, the dose should be increased over 4 weeks: |
| - Week 1: one tablet in the morning |
| - Week 2: one tablet in the morning and one in the evening |
| - Week 3: two tablets in the morning and one in the evening |
| - Week 4 and beyond: two tablets in the morning and two in the evening |
| The last dose is recommended to be taken in the evening to avoid insomnia if taken at dinner time. |
| Clinical effects |
| 50% of patients lose ≥5% of weight |
| Adverse effects |
| Nausea |
| Contraindications |
| Pregnancy and breastfeeding |
| Uncontrolled HTN |
| Patients currently suffering from seizure disorders or with a history of seizures |
| Known central nervous system neoplasm |
| Alcohol or benzodiazepine withdrawal syndrome |
| History of bipolar disorder |
| Any concurrent treatment containing bupropion or naltrexone |
| Current or previous diagnosis of bulimia or anorexia nervosa |
| Dependence on long-acting opioids or opioid agonists (e.g., methadone) or with acute opioid withdrawal syndrome |
| Concurrent treatment with monoamine oxidase inhibitors (MAOIs). A minimum of 14 days should elapse between discontinuing MAOIs and initiating treatment with naltrexone/bupropion |
| Severe liver insufficiency |
| End-stage kidney failure |
DBP: diastolic blood pressure; DM: diabetes mellitus; GLP-1: glucagon-like peptide type 1; eGFR: estimated glomerular filtration rate; LDL-C: low-density lipoprotein cholesterol; T2DM: type 2 diabetes mellitus; TC: total cholesterol; HTN: hypertension; SBP: systolic blood pressure.
Dietary intervention is mainly aimed at reducing intake. Different dietary patterns are effective in weight loss. Plans should be tailored to the clinical characteristics and preferences of the patient (Fig. 9). In general, a Mediterranean-type diet should be the basis of treatment in our setting.
A daily calorie reduction between 500−1000 kcal can produce a weight loss of between .5–1 kg/week. Weight reductions between 7%–10% produce significant health benefits and metabolic changes.
It is also important to prescribe appropriate physical exercise for each patient and psychological support with eating behaviour modification.
Pharmacological treatment of obesity should be individualised. Drug selection will depend on age, presence or absence of DM, and contraindications (Table 28).
Surgical treatment of obesity is reserved for a group of patients after therapeutic failure of other conservative options.147 Bariatric surgery (Table 29) is indicated in morbid obesity or severe obesity with multiple complications not controlled medically and where other therapeutic strategies have failed. It is an effective treatment that is not free of complications. The indications and contraindications are listed in Table 29.
Surgical treatment of obesity.
| Indications and contraindications | |
|---|---|
| Indications for bariatric surgery | • BMI ≥ 40 kg/m2 |
| • BMI ≥ 35 kg/m2 with one or more severe comorbidities: | |
| ◦ T2DM | |
| ◦ HTN | |
| ◦ Dyslipidaemia | |
| ◦ Respiratory disorders secondary to obesity | |
| ◦ Non-alcoholic fatty liver disease | |
| ◦ Arthrosis | |
| ◦ Urinary incontinence | |
| Requirements | • Age 18–60 years. In older people it must be individualised |
| • Rule out endocrinopathies | |
| • History of morbid obesity for at least 5 years | |
| • Inadequate response to medical-nutritional treatment | |
| • Failure or inadequate response to medical-nutritional treatment | |
| • Ability to adhere to recommendations and lifestyle changes post-surgery | |
| • The patient must understand the treatment they are about to undergo and the long-term consequences | |
| • Psychiatric assessment in case of relevant history or suspicion of mental illness | |
| Contraindications | • Severe psychiatric pathology: schizophrenia, major depression, intellectual disability |
| • Psychiatric instability | |
| • ED | |
| • Alcohol or other drug abuse | |
BMI: body mass index; ED: eating disorder; HTN: hypertension; T2DM: type 2 diabetes mellitus.
In 2020, smokers comprised 22% of the world's population, 37% of males and 8% of females. Every year, more than eight million people worldwide die from tobacco use and of these deaths, 1.2 million are due to passive smoking.148 Smoking is a lethal addiction and the number one cause of preventable death, doubling the risk of death from AVD and increasing it fivefold in those under 50 years of age. Tobacco smoking promotes the formation and rupture of atheromatous plaques; it also promotes inflammation, oxidation, and endothelial dysfunction that predisposes to arterial spasm, thrombosis, and vascular obstruction. Tobacco smoking is harmful in all its forms, in a manner proportional to the amount smoked.149 Smoking cessation is, among all preventive measures, the most cost-effective in terms of reducing VR. The benefit is observed in the first months after quitting. In Spain, in the period 2009–2012 the smoking population decreased by 3.13% and in the period 2009–2017 to 4.81%.150 However, tobacco continues to be promoted and once a smoking habit is acquired, it is difficult to stop, as the number of failed attempts before quitting smoking varies between five and 14 according to different reports.151 Advice on smoking cessation should be given to all smokers and in any interaction with healthcare professionals, which increases the likelihood of quitting by more than 50%. A person is considered to have quit smoking when six months have elapsed since they smoked their last cigarette.
Table 30 shows the recommendations on smoking cessation strategies contained in the European guidelines on cardiovascular prevention.13
Recommendations of strategy to manage smoking.
| Recommendation | Class | Level |
|---|---|---|
| All tobacco use should be discontinued, as tobacco use is strongly and independently associated with the development of vascular disease | I | A |
| Smokers should be helped to quit by offering nicotine substitutes and medications (varenicline and bupropion alone or in combination), when necessary | IIa | A |
| Smoking cessation is recommended despite weight gain, as weight gain does not reduce the vascular benefit of smoking cessation | I | B |
| Passive smoking should be avoided | I | B |
Adapted from Visseren, et al.13
Every medical VR control visit should include the following sections:
- •
Taking a history on smoking habits:
- o
Have you been a smoker at any time in your life (have you regularly smoked at least one cigarette per month) (No/Yes)
- o
How many cigarettes do you smoke per day?
- o
If you have quit smoking, how many months is it since you quit?
- o
How many serious attempts to stop smoking have you made in your lifetime?
- o
- •
Strongly advise to stop smoking. Information on the benefits of quitting smoking and cessation strategies. Also advise on the potential weight gain (3–5 kg on average) and its minor importance compared to the vascular preventive benefit and improvement of general health status.
- •
Assessment of the degree of addiction using the Fagerström test152 or an abbreviated questionnaire with only two questions153 (Appendix G Annex 7) to assess the need for pharmacological and nicotine substitution measures. The shorter the time between waking up in the morning and smoking the first cigarette and the greater the number of cigarettes smoked per day, the greater the nicotine dependence.
- •
Assessment of the patient’s attitude towards smoking. Three key questions can be asked:
- o
Do you think smoking harms you?
- o
Would you like to stop smoking?
- o
Do you think you will be able to stop?
- o
- •
If the patient has answered yes to all three questions above, they should be assisted with a planned strategy, including setting a date, behavioural/motivational therapy, and pharmacological support, or specific smoking counselling. If not, inform, motivate, and insist.
- •
Establish a follow-up programme.
Nicotine substitutes and various medications can be used in addition to communication-mediated therapies at the medical visit, including counselling and motivational interviewing.154 Nicotine substitutes, whether in the form of gum, patches, nasal sprays, or inhalers, are effective and increase the likelihood of giving up smoking.4 Although smoking cessation in individuals who respond to nicotine replacement therapy usually occurs within the first four weeks, extending treatment to eight to 12 weeks is associated with an increased likelihood of long-term cessation.155 Bupropion is an antidepressant for which there is a large clinical trial base that has demonstrated its efficacy in smoking cessation, increasing the chances of success by more than 50%. Its main drawback is a small risk (1/1000) of seizures, without increasing the risk of AVD or neuropsychiatric illness. Treatment can be prolonged to 12 weeks, although it can be extended to 52 weeks in patients at high risk of relapse. Varenicline is a nicotine receptor partial agonist for which there is also a large clinical trial base that has shown that its use more than doubles the likelihood of successful quitting. Its main side effect is nausea, which can be mitigated by increasing doses gradually and avoiding higher doses.156 However, in July 2021, the Spanish Agency for Medicines and Health Products (AEMPS) recommended that no new treatments with this drug should be started, as an impurity had been detected in varenicline tablets that forced the recall of three batches of this drug, and therefore patients could not be guaranteed their treatment would continue. Cytisine is a plant alkaloid which, like varenicline, is a partial nicotine receptor agonist, which mediates nicotine dependence through the release of dopamine and reduces the symptoms of tobacco withdrawal and also the level of satisfaction associated with smoking.157 Its efficacy in achieving smoking cessation may be somewhat lower than that of varenicline, but it is somewhat better tolerated.158 Cytisine has a complex dosing regimen and its prescription is restricted to clinicians involved in smoking cessation programmes. It is contraindicated in patients with recent ischaemic episodes, clinically relevant arrhythmias, pregnancy, and breast feeding. Future clinical trials should evaluate the efficacy of cytisine compared to varenicline and other drugs, as well as the most appropriate dosing regimens.
There is still insufficient information on the use of e-cigarettes and tobacco heating products. Use of the latter is increasing,159 over and above that of e-cigarettes, and this appears to have been influenced by the approval in 2020 by the US Food and Drug Administration (FDA) as a modified risk tobacco product.160 Switching from conventional heated tobacco to nicotine or smokeless tobacco products has been found to significantly decrease vascular risk, although it is still a high risk compared to quitting smoking.161 Many questions remain about e-cigarettes and heated tobacco products, most importantly their long-term health effects.
Patient with atrial fibrillationAF is the most common arrhythmia, affecting more than 40 million people worldwide.162 Due to increasing longevity and diagnosis, its global prevalence is estimated to be 15.9 million by 2050, with more than half of these patients aged ≥ 80 years.163 The prevalence of AF depends on population characteristics related to age, sex, race, and geographic area.
Age and sex. The age group with the highest prevalence of AF is patients over 75 years of age, at 9%, doubling (up to 18%) in patients over 85 years.164,165 In patients between 55 and 75 years of age, the prevalence ranges between 1% and 6%, while in patients under 55 years of age it is .1%.165 Prevalence is similar in both sexes, but slightly higher in men (1.1 vs. .8%).165
Race, ethnicity, and geographic area. Black and Hispanic adults have a lower risk of AF than non-Hispanic White patients.166 This difference is more pronounced at older ages. Developed countries have a higher prevalence of AF compared to developing countries, with the highest prevalence in North America and the lowest in Asia and sub-Saharan Africa.162
Risk factors that facilitate onset or recurrence of atrial fibrillationThe lifetime risk of AF increases with increasing VRF burden. Identifying, preventing, and treating these risk factors is key to reducing the prevalence of AF and its disease burden.
HypertensionHigh and uncontrolled BP can modify the structure of the myocardial wall, favouring the development of AF and increasing the likelihood of recurrent AF. Hypertensive patients have a relative risk of developing AF of 1.50 compared to normotensive patients.167 Furthermore, hypertension increases the risk of lacunar and haemorrhagic stroke. Therefore, BP control should be an integral part of the management of AF patients.167
Diabetes mellitusAlthough the influence of DM on myocardial remodelling and its predisposing to the development of AF is known, intensive glycaemic control does not decrease the rate of new-onset AF. Patients with diabetes and increased glycaemic variability have a 1.2 relative risk of developing AF.168 By contrast, long-standing DM predisposes to an increased risk of stroke and thromboembolic events in AF patients. However, metformin therapy in patients with DM appears to be associated with a lower long-term risk of AF.169 In this regard, although studies with SGLT2i have shown a reduction in left atrial volume, there are conflicting data on whether they reduce the incidence of AF.170
Obesity and metabolic syndromeObesity increases the likelihood of developing AF in parallel with an increase in BMI.171 This may be due to increased left atrial pressure and volume. Weight reduction, in the order of 10−15 kg, decreases recurrence of AF.171 Improved cardiorespiratory fitness may also further reduce the burden of AF in obese patients with AF.172 MS includes HTN, DM, and obesity, all of which, as we have seen, are associated with AF, therefore the combination of these conditions increases the risk.173
Heart failureThe prevalence of AF in patients with HF is slightly higher in those with depressed rather than preserved left ventricular ejection fraction (LVEF) (46% vs. 34%).174
Obstructive sleep apnoea-hypopnoea syndromeAtrial remodelling secondary to OSAHS-related hypoxia results in fibrosis and conduction slowing, leading to an increased prevalence of AF.175 The risk of AF in these patients increases with the severity of the OSAHS syndrome.176 The incidence and recurrence of AF is lower in patients treated with mechanical ventilation.
Chronic kidney diseaseThe incidence of AF is more frequent in patients with microalbuminuria and/or glomerular filtration rate (GFR) below 60 mL/min/1.73 m2, and is higher in those in end stages or on dialysis, the latter causing higher mortality and thromboembolic events.177
Diagnosis of atrial fibrillation and screening strategiesDiagnosis of AF is made by observing abnormal R-R intervals without P waves for at least 30 s on an ECG. Five temporal patterns are distinguished based on the presentation, duration, and spontaneous resolution of AF episodes:
- •
AF diagnosed for the first time.
- •
Paroxysmal AF: AF that reverses spontaneously/with intervention within <7 days.
- •
Persistent AF: persists for >7 days.
- •
Long-term persistent AF: continues for >1 year after adopting a rhythm control strategy.
- •
Permanent AF: AF that is accepted by both patient and physician, with no new medications adopted to restore or maintain sinus rhythm.
Recurrence of AF after a first cardioverted episode is found to be around 70%, influenced by several factors such as left ventricular dysfunction and left atrial diameter, and progression to persistent AF is 8%–25% during the first and fifth year.178,179 The terms ‘isolated AF’ (there is a cause of AF in each patient), ‘valvular/non-valvular AF’ (may create confusion), and ‘chronic AF’ (a contradictory definition) are in disuse.180 Depending on its clinical component, AF can be symptomatic, asymptomatic, or occult (becoming evident after an acute event or ECG).
Comprehensive management of the patient with atrial fibrillationComprehensive management of the AF patient includes patient information, education and self-care, and monitoring of VRFs. For this, we can use the CC to ABC (Atrial Fibrillation Better Care) pathway. After confirming AF by ECG, characterisation using the 4S-AF scheme has been proposed, in which we stratify according to stroke risk (using CHA2DS2-VASc), symptom severity (asymptomatic, mild, moderate, severe), AF burden (according to time pattern), and substrate severity. We will then follow the ABC where we will assess anticoagulation (A), symptom control (B), and comorbidities (C).180 However, there are situations that require a patient with AF to be referred to a specialist hospital emergency department, such as haemodynamic instability, high and uncontrollable HR, symptomatic bradycardia, severe angina, severe and irreversible dyspnoea, or the presence of TIA or stroke.
ScreeningAsymptomatic clinical AF has been independently associated with an increased risk of stroke and mortality compared to symptomatic AF, and therefore screening is recommended for patients with HTN, OSA, HF, or over 65 years of age by pulse palpation or 12-lead ECG.180 Although the standard ECG is considered the conventional diagnostic method, the role of new technologies, such as mobile apps and smartwatches, has been highlighted in recent years. Studies such as Apple Heart and Huawei Heart described a high ability to detect self-limiting AF episodes.181,182 Transthoracic echocardiography is recommended for the initial assessment and management of all patients with AF, where we can assess cardiac function and structure.
Prevention and non-pharmacological measuresIntensive weight reduction with comprehensive control of concomitant VRFs results in fewer recurrences and symptoms of AF. Excessive alcohol consumption is a risk factor for AF and bleeding in anticoagulated patients while abstinence from alcohol reduces recurrence in regular drinkers with AF.183 Regular caffeine consumption may be associated with a lower risk of AF, but caffeine intake may increase symptoms of palpitations unrelated to AF. Physical exercise (>150 min per week of moderate exercise or >75 min of vigorous exercise) has demonstrated vascular benefits. While the incidence of AF appears to be increased in elite athletes, moderate-intensity exercise should be advised to patients to prevent the incidence or recurrence of AF, and they should avoid excessive endurance exercise (marathons or triathlons), especially those over 50 years of age.184
Rhythm or heart rate control. Antiarrhythmic drugs, catheter ablation, and atrial fibrillation surgeryEarly rhythm control therapy is associated with a lower risk of events in patients with early AF and in patients at high risk,185 while in long-standing AF rate control is the most recommended therapy.186 Both rate control and rhythm control strategies aim to alleviate the pathophysiological consequences of AF and symptoms. In patients with new-onset AF, spontaneous restoration may be observed in about 75% during the first 24−48 h, so not initiating treatment during the first 24 h in haemodynamically stable patients without symptoms may be an alternative. The choice of rhythm control therapy will depend on patient characteristics, symptoms, and LVEF.
While we opt for electrical cardioversion in unstable patients, in stable patients the treatment will depend on the time of onset (over or under 48 h) or previous anticoagulation, discussing the possibility of electrical cardioversion with the patient if this is possible (in anticoagulant treatment, in which the presence of thrombus in the left atrium and atrial appendage has been ruled out, or with a duration of less than 48 h).
Heart rate controlThe target heart rate (HR) for AF patients is <100 bpm.
- •
Beta-blockers are the first-line drugs for heart rate control due to their rapid effect, especially in patients with depressed LVEF, inappropriate increase in ventricular rate during exercise, and recent infarction.
- •
Non-dihydropyridine calcium antagonists are an effective alternative in the absence of HF with depressed LVEF. Like beta-blockers, they are effective in reducing heart rate both at rest and during exercise.
- •
Digoxin should be used with caution due to its possible association with increased concentration-dependent mortality.187
- •
Amiodarone is useful in combination after failure of previous therapy, and should be avoided in those with thyroid disease.
Rhythm control therapy is recommended to improve symptoms and quality of life in symptomatic AF patients.180 While synchronised direct electrical cardioversion is the preferred method for AF patients with haemodynamic deterioration, it is interchangeable with pharmacological cardioversion in unstable patients.
- •
Flecainide and propafenone (class Ic): are indicated in patients without ischaemic heart disease (IHD), left ventricular hypertrophy, or systolic dysfunction and should be used with caution due to their proarrhythmogenic (less with propafenone) and bradycardic effect. Their effect is rapid (three to five hours) in more than 50% of cases. Use of the ‘pill-in-the-pocket’ with flecainide or propafenone is safe and recommended in patients with paroxysmal AF after training.188
- •
Amiodarone: the choice of intravenous amiodarone is made in patients with HF with depressed LVEF, and is the drug associated with the lowest rate of recurrences, but with a late cardioversion effect (eight to 12 h) and less than other antiarrhythmic drugs.189 Caution should be exercised if it is combined with another drug that prolongs QT interval and it should be discontinued when the QTc is >500 ms. Intravenous vernakalant has a faster cardioversion effect than amiodarone (one hour).180 It should not be used in hypotensive patients, with recent IHD, HF in functional class III-IV, severe aortic stenosis, or prolongation of QT interval.
- •
Dronederone: although it may be a drug used for rhythm control, it should not be used in patients with recent decompensated HF, NYHA functional class III-IV, permanent AF, or in conjunction with dabigatran or other drugs that prolong the QT interval.190
- •
Sotalol: useful for preventing recurrences, but contraindicated in LVH, HF with depressed LVEF, hypokalaemia, and prolonged QT interval due to the risk of ventricular arrhythmias and torsades de pointes. Dronederone and sotalol should not be administered with creatinine clearance below 30 mL/min.
Similarly, surgery can be used to control heart rhythm. This is based on the creation of isolation scars on the atria to prevent re-entrant phenomena which trigger and perpetuate arrhythmia, allowing the normal stimulus to be conducted from the sinus node to the atrioventricular node. Current techniques, based on energy transmission devices, mean it can be performed by thoracoscopy with minimal incision. In some studies, it has been shown to be more effective than catheter ablation, although with a greater likelihood of side effects and sometimes requiring subsequent pacemaker implantation.191 In symptomatic persistent AF, surgery may be indicated if catheter ablation has failed. It may also be considered in AF patients undergoing cardiac surgery.
Anticoagulation therapy for stroke preventionAnticoagulation is the treatment of choice for the prevention of ischaemic stroke, bearing in mind that the risk is similar in persistent and paroxysmal AF.180,192 Classically, antivitamin K (VKA) drugs, in an international normalised ratio (INR) range of 2−3, have been the drugs of choice, as they produce a reduction in stroke rate of 60%–80%. The indication for oral anticoagulation is established by prognostic models that have incorporated the patient's age and comorbidities, the most commonly used scale being Congestive Heart Failure, Hypertension, Age, Diabetes, Previous Stroke (CHA2DS2-VASc) (Table 31), so that patients with a score of 0 (1 in the case of women) do not require oral anticoagulation therapy. Anticoagulation would be indicated in men with CHA2DS2-VASc ≥ 1 or ≥2 in women. This recommendation applies to patients with non-valvular AF, provided there is no elevated risk of bleeding complications, estimated according to the Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, international normalized ratio scale, Elderly (>65 years), Drugs/alcohol concomitantly scale, (HAS-BLED) (Table 32).
CHADS2-VASc Score for Atrial Fibrillation Stroke Risk.
| VRF | Weight value |
|---|---|
| Congestive HF | 1 |
| Hypertension | 1 |
| Age ≥ 75 years | 2 |
| DM | 1 |
| Stroke/TIA/previous peripheral embolism | 2 |
| Vascular disease (peripheral artery disease, IHD, aortic plaque) | 1 |
| Age between 65 and 74 years | 1 |
| Female sex | 1 |
| Maximum score | 9 |
Interpretation: Low risk = 0; Moderate risk = 1; High risk ≥ 2.
AF: atrial fibrillation; CHADS2-VASc: Congestive Heart Failure, Hypertension, Age, Diabetes, Previous Stroke; HF: heart failure; IHD: ischaemic heart disease; TIA: transient ischaemic attack; VRF: vascular risk factors.
HAS-BLED bleeding risk score in atrial fibrillation.
| VRF | Weight value |
|---|---|
| Hypertension, poorly controlled BP | 1 |
| Abnormal renal/hepatic function | 1 or 2 |
| Stroke | 1 |
| Tendency or predisposition to bleeding | 1 |
| Unstable INR | 1 |
| Advanced age (>65 years, fragile condition) | 1 |
| Medication (aspirin or NSAIDs), excessive alcohol consumption | 1 or 2 |
| Maximum score | 9 |
AF: atrial fibrillation; BP: blood pressure; HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly; INR: International Normalized Ratio; NSAID: non-steroidal anti-inflammatory drug; VRF: vascular risk factors.
In the last decade, four large randomised studies have been conducted with direct-acting oral anticoagulants (DAOCs): dabigatran (RELY), rivaroxaban (ROCKET-AF), apixaban (ARISTOTLE), and edoxaban (ENGAGE-AF), and meta-analyses, which have shown equal or superior efficacy to AVKs, with lower incidence of bleeding complications, especially intracranial haemorrhage, making them the current alternative of choice in the initial treatment of AF (Fig. 10).193 Real-life studies also confirm that DAOCs have less bleeding risk than AVKs.194 Furthermore, DAOCs in combination with antiplatelet monotherapy may be a safe and effective alternative to triple therapy in patients with AF undergoing percutaneous coronary intervention.195 For all these reasons, the most recent clinical guidelines and meta-analyses consider DAOCs the preferred option for stroke prevention in AF patients.180,192,196
Algorithm for antithrombotic treatment in atrial fibrillation.
AVK: antivitamin K; CHA2DS2-VASc: Congestive Heart Failure, Hypertension, Age, Diabetes, Previous Stroke; HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly; DAOCs: direct acting oral anticoagulants.
Although European guidelines place DOACs above AVKs, their use is restricted in Spain. According to a therapeutic positioning report, the use of these drugs is only authorised, in general terms, in patients with poor therapeutic control, with a history of intracranial haemorrhage, or at risk of intracranial haemorrhage.197
In any case, it is important to involve the patient in the decision-making process about the different anticoagulation options and to consider polymedication and comorbidities that may cause bleeding. It is always important to consider age, frailty, weight, and renal function, as these may influence the choice of anticoagulant type and dose.
Anticoagulation in patients requiring cardioversionIt is important to initiate anticoagulation early in patients scheduled for cardioversion, as this procedure is associated with a risk of thromboembolism. Patients who have been in AF for more than 48 h should start anticoagulation at least 3 weeks before cardioversion, and then continue for four weeks (provided they do not require indefinite anticoagulation). Current guidelines recommend adequate anticoagulation with an AVK or dabigatran (both pre- and post-procedure). Other DOACs are being studied in prospective clinical trials.198
Organisation and functioning of the vascular risk consultation: professionals and devices. Quality criteriaIn recent years, several important national and international studies have confirmed a poor overall control of VRFs even in secondary prevention in patients with coronary heart disease, stroke, or PAD.199–206 This can be attributed to several factors, including poor adherence to treatment, therapeutic inertia, and also organisational models that, in general, show little capacity to improve these outcomes.
Multidisciplinary management of VR has clear advantages207:
- •
In standardising the approach and treatment of VRFs between the different levels of care, thus providing continuity of care in vascular prevention.
- •
In improving the detection of all VRFs in patients at high VR towards offering the most appropriate and early therapeutic intervention for each patient.
- •
In optimising healthcare resources, avoiding duplication of visits and complementary investigations.
- •
In defining and agreeing on referral criteria, to generate a bidirectional flow that facilitates, in most cases, the return of the patient to primary care after the assessments and interventions have been performed required by referral to specialist care. This ensures that this information reaches the primary care physician, and that the patient receives consistent health messages from both levels of care.
- •
In promoting teaching and research into VR.
A VR consultation, as an organisational unit within the programmed care setting, requires:
- •
Professionals from different specialties (internal medicine, endocrinology, cardiology, nephrology, neurology, endocrinology, clinical biochemistry), in coordination with other primary care professionals (family medicine, nurses, nutritionists) and other professionals such as smoking cessation units or mental health professionals, to facilitate the implementation of global vascular prevention strategies.
- •
Unified protocols based on clinical practice guidelines for the global control of the main VRFs.
- •
Basic structural requirements.208 This includes the possibility of adequate BP measurement with validated devices (Table 33).
Table 33.Resources of a hospital clinic for the overall management of the main vascular risk factors.
Architecture Material resources Computer resources Consultation room for each health professional and by work shift Fully furnished consulting room: desk and examination table Medical history, digital or otherwise, according to the health service’s model, accessible to the referral health area Space for nurses to measure BP, provide health education, and review adherence to treatment. Stethoscope, ophthalmoscope, torch, weighing scale, height measure, and tape measure Online access to risk scales and questionnaires Air-conditioning system in the centre and consulting rooms Validated BP measuring devices211 Electronic prescription, according to health service model It is advisable to have: Possibility of online consultation in primary care • Semi-automatic BP measuring device so that several programmed automatic measurements (generally 3) can be taken without the presence of an observer. It is recommended that on the first visit the three automatic measurements are taken simultaneously in both arms • Access to ABPM • Portable Doppler to determine ABI • Electrocardiograph ABI: ankle-brachial index; ABPM: ambulatory blood pressure monitoring; BP: blood pressure.
Adapted from Felip Benach A.208
As a basic tool for measuring the quality of care, indicators must be established (Table 34) to detect areas for improvement to optimise global control of VRFs, which must be used as a self-assessment system.209
Indicators to measure the quality of care in the overall management of patients at high vascular risk.
| Indicators | ||
|---|---|---|
| Structure or activity | Process or quality | Result |
| • Total number of patients referred to VR consultation | • Number of referrals to VR consultation that meet previously defined and agreed referral criteria/total number of referrals | • Number of patients visited who meet VRF control targets according to their risk category/total number of patients visited |
| • Number of sessions with the VR team | • Number of patients with a first visit to the VR clinic with a response time within the established margins/total number of first visits | • Adequacy of resource consumption (complementary examinations)/total number of patients seen |
| • Number of consulting sessions with primary care | • Number of patients visited with diagnosis coding (according to ICD-10/total number of patients visited) | • Number of VR consultation sessions closed by discharge to primary care with agreed care report/number of discharges from VR consultation |
| • Number of teleconsultations by members of VR team, virtual consultation, consulting sessions, telemedicine | • Number of patients in primary prevention and non-DM whose risk has been estimated by a scale/total number of patients in primary prevention and non-DM seen in the VR consultation | |
| • Scientific production: communications and publications shared by several members of the group | ||
CIE-10: International classification of diseases, 10th revision; CVR: cardiovascular risk; DM: diabetes mellitus; VRF: vascular risk factors.
Adapted from Armario P, et al.207
Consultations where patients at VR are seen must adapt to the organisational system of the corresponding health service, whether public or private. The incorporation in recent years of elements such digitised medical records, with the possibility of adding indicators and extracting information, electronic prescriptions that make it easier to ascertain the degree of adherence to treatment, and telemedicine options (both for patient-doctor and doctor-doctor interaction) can facilitate access to the patient's clinical information and better control of VRFs.
Moreover, the creation of alerts in clinical laboratories can also help in the detection, treatment, and monitoring of certain VRFs (dyslipidaemia, DM) in primary care, and facilitate effective contact between primary care and hospital professionals, through teleconsultations based on agreed referral criteria.210
The COVID-19 pandemic brought about substantial changes in medical care, including a growing role for teleconsultations, which have been incorporated alongside face-to-face consultations in many healthcare settings. In VR consultations, criteria for choosing face-to-face or teleconsultations have been proposed, as described in Tables 34 and 35. The minimum necessary parameters have also been proposed to facilitate the diagnosis and management of dyslipidaemia through e-consultations in VR units.12
Criteria for choosing the type of consultation.
| Preference for face-to-face consultation | Preference for teleconsultation |
|---|---|
| Suspicion of potentially serious or urgent problems, having to give bad news. Clinical changes, decompensation or worsening of the patient, need for interview with companions, first visit | Stable clinical situation |
| Difficulties in communicating with the patient (language, hearing loss, cognitive problems) | No communication difficulties |
| Requires physical examination | Does not require physical examination |
| Requires training in physical self-examination: | Is trained in physical self-examination |
| • Weight | |
| • BP measurement | |
| • SMBP | |
| Requires complementary tests in the short term: | Requires complementary tests in the medium to long term (manage requests via administrative channels) |
| • Blood test | |
| • ECG | |
| • Radiology | |
| • Ankle-brachial index | |
| • PWV | |
| • ABPM | |
| Requires more personalised health education training or major treatment changes or titration. | Will not require, a priori, changes in treatment |
| Uncontrolled CVD | No CVD or in stable condition |
| Presence of multiple comorbidities | No major comorbidities |
ABPM: Ambulatory blood pressure monitoring; BP: blood pressure; CVD: cardiovascular disease; ECG: electrocardiogram; PWV: pulse wave velocity; SMBP: self-measured blood pressure monitoring.
Adapted from Gijón-Conde T, et al.212
Patients with elevated VR are generally referred to consultations to control certain VRFs, essentially HTN, DM, and dyslipidaemia, either in the primary or secondary prevention setting. Thus, patients with established AVD (coronary, cerebrovascular, or peripheral arterial) are frequently referred for VR consultation. They are sometimes young, in whom no VRFs have been identified (for the diagnostic study of thrombophilia) or are unknown, or the VRFs detected during hospital assessment have not been adequately controlled.
It is not usual to take patients for smoking cessation, even though this is a major VRF; this task usually falls to specialist pneumology or PC units. In any case, each public health service usually establishes its own criteria for referral to specialist units, depending on their own agendas, availability of resources and level of care (regional hospitals versus tertiary referral hospitals).
The main reasons for referring patients with DM are shown in Table 36. The referral criteria for patients with HTN according to the practical guide of the Spanish Society of Hypertension - Spanish League for the Fight against Arterial Hypertension (SEH-LELHA) published in 2022 are as follows213:
- •
Suspected secondary HTN.
- •
Age of onset <40 years with grade 2−3 HTN (SBP ≥ 160 mmHg or DBP ≥ 100 mmHg).
- •
Repeated hypertensive crises in patients with previous normal BP or well-controlled HTN.
- •
Indication for complementary studies not available in primary care, particularly indication for advanced vascular explorations that may influence therapeutic decisions.
- •
Resistant HTN (HTN not controlled with three complementary drugs in adequate doses, one of them a diuretic), especially after ruling out pseudoresistant hypertension attributed to the white coat effect with ABPM, and ruling out non-adherence to non-pharmacological or pharmacological treatment.
- •
Difficult to control HTN in relation to multiple drug intolerances, multiple contraindications, constant non-adherence, or extreme variability of BP readings.
Reasons for referral of patients with diabetes mellitus.
| Diabetes mellitus |
|---|
| • T1DM |
| • Gestational DM. |
| • Diabetes not correctly identified |
| • Poor glycaemic control, unstable diabetes, hypoglycaemias |
| • Comorbidities (e.g., morbid obesity) |
| • Severe microvascular disease (diabetic foot polyneuropathy, CRF, advanced retinopathy) |
DM: diabetes mellitus; CRF: chronic renal failure; T1DM: type 1 diabetes mellitus.
The SEA has published criteria for the referral of patients with dyslipidaemia to its lipid units (Table 37).214
Criteria for referral to lipid units of the Spanish Society of Atherosclerosis.
| Dyslipidaemia | Thresholds | Clinical context | Diagnosis | Treatment |
|---|---|---|---|---|
| Hypercholesterolaemia | TC > 300 mg/dL | Tendinous xanthomas | FH cascade screening | Triple therapy |
| LDL-C > 200 mg/dL | Corneal arcus <45 years | Genetic testing | New treatments (PCSK9i) | |
| Lp(a) > 50 mg/dL | Family history +++ | Imaging techniques to detect SVD | Drug intolerance | |
| IHD or premature PAD | Refractoriness to treatment | |||
| Recurring IHD | Apheresis | |||
| Vascular disease without evident VRF | ||||
| Suspected FH | ||||
| Steatosis and/or cirrhosis | ||||
| Hypertriglyceridaemia | Fasting TG > 1000 mg/dL | Xanthomas | Secondary cause analysis | Exclude secondary cause |
| TG > 500 mg/dL despite treatment | Hepatomegaly | Biochemical tests | Ineffective treatment | |
| Hypertriglyceridaemia and TC > 350 mg/dL | Splenomegaly | Molecular diagnosis molecular | Special diets | |
| Lipemia retinalis | ||||
| Debut in infancy | ||||
| Pancreatitis | ||||
| HDL-C | HDL-C < 20 mg/dL | Hepatomegaly | Exclude secondary causes | Control secondary causes |
| HDL-C > 100 mg/dL | Splenomegaly | Biochemical tests | ||
| Tonsillar hypertrophy | Molecular diagnosis | |||
| Corneal opacity | ||||
| Renal insufficiency | ||||
| Hypocholesterolaemia | LDL-C < 50 mg/dL without treatment | Malabsorption | Exclude secondary causes | |
| Steatosis | Biochemical tests | |||
| Molecular diagnosis |
FH: familial hypercholesterolaemia; HDL-C: high-density lipoprotein cholesterol; IHD: ischaemic heart disease; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein (a); PAD: peripheral arterial disease; PCSK9i: proprotein convertase subtilisin/kexin proprotein convertase 9 inhibitor; SVD: subclinical vascular disease; TC: total cholesterol; TG: triglycerides. +++: Positive.
Adapted from Sánchez-Chaparro MA, et al.214
In the context of VR, the main type of DM to be considered is type 2, associated with dyslipidaemia, obesity, HTN, and MS; therefore, the most common cause will be lack of adequate glycaemic control, even though the therapeutic arsenal in the field of T2DM has become enormous in recent years.215,216
In the case of HTN in Spain, the most common reason for referring patients to an HTN unit is to rule out the cause of secondary HTN, followed by poor therapeutic control and resistant HTN,174 the latter being defined as the inability to control BP (<140/90 mmHg) despite three drugs, one of which is a diuretic.7
In the case of dyslipidaemia, the most common reasons for referral are poor control of serum cholesterol or TG levels, treatment and diagnosis of FH, adverse effects of medication, especially intolerance to statins (mainly due to muscle toxicity), or the need for combination therapy, especially with PCSK9i.214
Discharge criteria for patients at vascular riskIt is obvious that, once a definitive diagnosis has been made and the VRF that prompted the consultation has been controlled, patients should be referred from the specialist unit for follow-up by primary care. However, it is not uncommon for patients to be reviewed in consultations for various reasons; among others, failure to achieve therapeutic objectives, the need for unconventional procedures (bariatric surgery, LDL-C apheresis, or lysosomal enzyme replacement therapy by infusion in day hospitals), the need to prescribe and dispense certain drugs by the hospital pharmacy (as in the case of PCSK9i drugs), polymedication with a high risk of drug interactions (e.g., in subjects with HIV infection and after transplantation) and patients with medication-related adverse effects such as hypoglycaemias. Patients with monogenic hyperlipidaemia should maintain contact (at least annually in the absence of complications or comorbidities) with lipid units for shared follow-up with primary care. This reinforces adherence to medication and healthy lifestyles and favours the rapid incorporation of new therapeutic developments for this group of patients. Some patients with secondary HTN remain hypertensive despite the aetiological treatment of their disease, which suggests the prolonged effect of HTN on the vascular tree (remodelling) or that some of them may also have essential HTN.217
In any case, it would be advisable for each unit to establish its own discharge criteria, depending on its clinical activity and its relationship with primary care.
FundingThis work was funded by Laboratorios Ferrer. The authors are responsible for the content of the document. Laboratorios Ferrer was not involved in the design, drafting, and/or content of the manuscript.
Conflict of interestsLaboratorios Ferrer provided editing and administrative support in relation to the update of the document ‘SEA 2024 Standards for the Global Control of Vascular Risk’. Laboratorios Ferrer was not involved in its drafting or content.
Some authors have received honoraria from different pharmaceutical laboratories, including Laboratorios Ferrer, for their participation in conferences and consultancies, which are detailed in the corresponding section. The authors have not received any remuneration for this report and have no other direct conflict of interest to declare in relation to this work.
Arrobas-Velilla T has no conflict of interest to declare.
Armario P has no conflict of interest to declare.
Baeza-Trinidad R has no conflict of interest to declare.
Calmarza P has no conflict of interest to declare.
Cebollada J has received honoraria for conferences, presentations, manuscript or educational events from AstraZeneca, Daiichi-Sankyo, Pfizer, Ferrer, and Amgen.
Civera-Andrés M has no conflict of interest to declare.
Cuende Melero JI has received honoraria for conferences from Daiichi-Sankyo Spain.
Díaz-Díaz JL has received honoraria for conferences, presentations, manuscript or educational events from Sanofi, MSD, Amgen, Viatris, and Ferrer; support for meetings from Viatris, Sanofi, Amgen, MSD, and Ferrer; and participation on advisory boards from Amgen, Sanofi, and Ferrer.
Espíldora-Hernández J has no conflict of interests to declare.
Fernández Pardo J has received honoraria for presentations from Servier, Mylan, and Daiichi-Sankyo.
Guijarro C has received consultancy honoraria from Sanofi, Amgen, and Daiichi Sankyo, for conferences, presentations, speakers bureaus, manuscript writing, or educational events from Rubio, Sanofi, Amgen, Ferrer, Daiichi Sankyo, Amarin, Servier, and Novartis, meeting support from Laboratorios Ferrer and for advisory boards from Sanofi, Amgen and Daiichi Sankyo.
Jericó C has received honoraria for conferences, presentations, speakers bureaus, manuscript writing, or educational events from Amarin, Amgen, Daichii-Sankyo, Novo Nordisk, Sanofi, and Viatris.
Laclaustra M has no conflict of interest to declare.
Lahoz C has no conflict of interest to declare.
López-Miranda J has received consultancy fees from Amgen and Sanofi; for conferences, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, Sanofi, MSD, Ferrer, Novartis, and Laboratorios Esteve; and meeting support from Amgen and Sanofi.
Martínez-Hervás S has no conflict of interest to declare.
Masana L has received consultancy honoraria from Amryt, Amarin, Daiichi-Sankyo, Ferrer, Novartis, Sanofi, Ultragenix; and for conferences, presentations, speakers bureaus, manuscript writing, or educational events and meeting support from Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Ferrer, MSD, Ultragenix, Viatris.
Mostaza JM has received honoraria for conferences, presentations, consultancies, or educational events from Sanofi, Amgen, Novartis, Daiichi-Sankyo, Servier, Viatris, Ultragenix, and Ferrer.
Muñiz-Grijalvo O has received honoraria for conferences and educational activities from Amgen, Sanofi, MSD, Sobi, Viatris, Amarin, and Daichii-Sankyo as well as consulting honoraria, advisory boards and meeting support from Amgen, Sanofi, Sobi, and Ultragenix.
Páramo JA has no conflict of interest to declare.
Pascual V has received honoraria for conferences and educational activities from Adamed, Amarin, Almirall, Astra-Zeneca, Daichii-Sankyo, Esteve, Ferrer, Novartis, Sanofi-Events, Servier, and Viatris.
Pedro-Botet J has received honoraria for advisory boards from Amgen, Daiichi-Sankyo, Ferrer, Mylan (Viatris) and Sanofi and conferences from Amarin Pharma, Amgen, Daiichi-Sankyo, Esteve, Ferrer, Organon, Mylan (Viatris), Sanofi, and Servier.
Pérez-Martínez P has received fees for conferences, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, Ferrer, Esteve, Daiichi-Sankyo, and Viatris.
Pintó X has received consultancy honoraria from Menarini, Ultragenyx, and Viatris; conferences, presentations, speakers bureaus, manuscript writing, or educational events from Novartis, Daiichi-Sankyo, Sobi, Almirall and Ferrer; and participation on a data safety monitoring board or advisory boards from Amgen, Sanofi and Novartis.
Puzo J has received honoraria for conferences and talks from Amgen, Sanofi, and Beckman Coulter, and for board, society, committee, or advocacy group leadership from SEQC (Spanish Society of Laboratory Medicine).
Real JT has no conflict of interest to declare.
Valdivielso P has received honoraria for consultancy, expert witness fees, conferences, presentations, manuscript, or educational event fees from Amgen, Sanofi, Novartis, Ferrer, and Daiichi-Snakyo, and meeting support from Amgen; Novartis; Ferrer; MSD; Daiichi-Sankyo; Sanofi and grants or contracts from Ferrer, SOBI and Lipigon, Ionis and Capenergy.
The authors have no other conflict of interest to declare.
IVC: intermittent vascular claudication
Figure subject to copyright. Original source: Manzano L, Mostaza JM, Suárez C, Cairols M, Redondo R, Valdivielso P, et al. Figure subject to copyright. Original source: Manzano L, Mostaza JM, Suárez C, Cairols M, Redondo R, Valdivielso P, et al. Value of the ankle-brachial index in cardiovascular risk stratification of patients without known atherotrombotic disease. MERITO study. Med Clin (Barc) 2007; 128:241–6.218
The target population is the general male population.
- 1
In the last 6 months, with what frequency was penetration possible for you?
- -
Did not even attempt it
- -
Never
- -
Fewer than half of the times
- -
Approximately half of the times
- -
Over half of the times
- -
Nearly always or always
- -
- 2
In the last 6 months, with what frequency were you able to maintain an erection and complete intercourse?
- -
Did not even attempt it
- -
Never
- -
Fewer than half of the times
- -
Approximately half of the times
- -
Over half of the times
- -
Nearly always or always
- -
- 3
When you attempted to have sex, with what frequency was it satisfactory for you?
- -
Did not even attempt it
- -
Never
- -
Fewer than half of the times
- -
Approximately half of the times
- -
Over half of the times
- -
Nearly always or always
- -
The score ranges between 0–15. A score of 12 or less suggests erectile dysfunction. The scores for each response option are as follows:
- -
Did not even attempt it (0)
- -
Never (1)
- -
Fewer than half of the times (2)
- -
Approximately half of the times (3)
- -
Over half of the times (4)
- -
Nearly always or always (5)
Published with permission of the Editor. Original source: Martín-Morales A, Meijide Rico F, García González JI, Manero Font M, García-Losa M, Artés Ferragud M; ESTIMA. Development and psychometric validation of a new screening questionnaire for erectile dysfunction (SQUED questionnaire). Actas Urol Esp. 2007 Feb;31(2):106-12 Copyright © 2007 Spanish Urology Association. Published by Elsevier España,S.L.U. all rights reserved.2
| 1. Do you use olive oil as the main source of fat for cooking? |
| Yes = 1 point |
| 2. How much olive oil do you consume per day (include that used in frying, salads, meals eaten away from home, etc.)? |
| Four or more tablespoons = 1 point |
| 3. How many servings of vegetables do you consume per day? |
| (Garnish and side serving = ½ serving) 1 serving = 200 g. |
| Two or more (at least one in a salad or raw) = 1 point |
| 4. How many pieces of fruit (including fresh-squeezed juice) do you consume per day? |
| Three or more per day = 1 point |
| 5. How many servings of red meat, hamburger or sausages do you consume per day? |
| (serving: 100–150 g) |
| Fewer than one serving per day = 1 point |
| 6. How many servings of butter, margarine or cream do you consume per day? |
| (One serving: 12 g) |
| Fewer than one per day = 1 point |
| 7. How many carbonated and/or sugar-sweetened beverages (soft drinks, colas, tonic waters) do you consume per day? |
| Fewer than one per day = 1 point |
| 8. Do you drink wine? How much do you consume per week? |
| Seven or more glasses per week = 1 point |
| 9. How many servings of pulses do you consume per week? |
| (one plate or serving of 150 g) |
| 3 or more per week = 1 point |
| 10. How many servings of fish/seafood do you consume per week? |
| (100–150 g of fish, 4−5 pieces or 200 g of seafood) |
| Three or more per week = 1 point |
| 11. How many times do you consume commercial (not homemade) pastry such as cookies or cake per week? |
| Fewer than two per week = 1 point |
| 12. How many times do you consume nuts per week? |
| (serving 30 g) |
| Three or more per week = 1 point |
| 13. ¿Do you prefer to eat chicken, turkey, or rabbit instead of beef, pork, hamburgers, or sausages? |
| (chicken: 1 piece or serving of 100–150 g) |
| Yes = 1 point |
| 14. How many times per week do you consume boiled vegetables, pasta, rice, or other dishes with a sauce of tomato, garlic, onion, or leeks sautéed in olive oil)? |
| Twice or more per week = 1 point |
| TOTAL SCORE |
| <9 = Low adherence |
| ≥ 9 = Good adherence |
Reproduced from A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. 141, Issue 6, 1140–5. Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, et al. The Journal of Nutrition, 2011, doi.org/10.3945/jn.110.135566. Copyright (2024), with the permission of Elsevier.42
| 1. During the last seven days, on how many days did you do vigorous physical activities like heavy lifting, digging, aerobics, or fast bicycling? |
| Days per week (indicate number) |
| No vigorous physical activities (skip to question 3) |
| 2. How much time did you usually spend doing vigorous physical activities on one of those days? |
| Indicate hours per day |
| Indicate minutes per day |
| Don’t know/not sure |
| 3. During the last 7 days, on how many days did you do moderate physical activities like carrying light loads, bicycling at a regular pace? Do not include walking |
| Days per week (indicate number) |
| No moderate physical activities (skip to question 5) |
| 4. How much time did you usually spend doing moderate physical activities on one of those days? Indicate hours per day |
| Indicate minutes per day |
| Don’t know/not sure |
| 5. During the last seven days, on how many days did you walk for at least 10 min at a time? |
| Days per week (indicate number) |
| No walking (skip to question 7) |
| 6. How much time did you usually spend walking on one of those days? |
| Indicate hours per day |
| Indicate minutes per day |
| Don’t know/not sure |
| 7. During the last 7 days, how much time did you spend sitting on a week day? |
| Indicate hours per day |
| Indicate minutes per day |
| Don’t know/not sure |
| TEST SCORE |
| 1. Walking: 3.3 METs* × walking minutes × days in the week (e.g. 3.3 × 30 min × five days = 495 MET) |
| 2. Moderate Physical Activity: 4 METs* × minutes × days in the week |
| 3. Vigorous Physical Activity: 8 METs* × minutes × days in the week |
| Then add up the three values obtained: Total = walking + moderate physical activity + vigorous physical activity |
| CLASSIFICATION CRITERIA: |
| Moderate Physical Activity: |
| • Three or more days of vigorous physical activity for at least 20 min per day |
| • Five or more days of moderate physical activity and/or walking at least 30 min per day |
| • 5 or more days of any combination of walking, moderate or vigorous physical activity achieving at least a total of 600 METs* |
| Vigorous Physical Activity |
| • Vigorous Physical Activity at least three days per week achieving a total of at least 1500 METs* |
| • Seven days of any combination of walking, with moderate physical activity and/or vigorous physical activity, achieving a total of at least 3000 METs * |
| * The test’s unit of measurement |
| RESULT: ACTIVITY LEVEL (tick as appropriate) |
| HIGH LEVEL |
| MODERATE LEVEL |
| LOW OR INACTIVE LEVEL |
International Physical Activity Questionnaire (IPAQ) [Internet]. Regional Government of Andalusia. Available at: https://www.juntadeandalucia.es/export/drupaljda/salud_5af958727c664_cuestionario_actividad_fisica_ipaq.pdf.44
| Pre-preanalytical phase (test ordering): |
| 1. Comprehensive testing of atherogenic lipoproteins should include tests to assess the risk conferred by LDL particles, remnant particles and, in selected cases, Lp(a). |
| Pre-analytical phase (test sampling) |
| 1. Fasting is not routinely required for assessing the lipid profile. |
| 2. Consider fasting sample when nonfasting TG are ≥400 mg/dL |
| 3. Take 2–3 serial blood specimens, at least 1 week apart, to allow to average for biological variation (importantly when test results are near the treatment decision thresholds) |
| Analytical phase |
| 1. Follow-up of measured or calculated LDL-C and non-HDL-C of a patient, from baseline to on-treatment measurements, should be ideally performed with the same method (and preferably the same laboratory). |
| 2. Clinicians should be notified when the laboratory test changes from a method to another. |
| 3. Calculation of LDL-C in patients with low LDL-C concentration < 70 mg/dL and/or TG concentration 175–400 mg/dL, and in nonfasting samples may be inadequate and introduce errors above 10%. |
| 4. Direct LDL-C assays should be used for calculation of RemnantC and for assessment of LDL-C when TG concentration is ≥400 mg/dL. |
| 5. Lp(a)-corrected LDL-C should be assessed at least once in patients with suspected or known high Lp(a), or if the patient shows a poor response to LDL-C-lowering therapy. |
| 6. ApoB assays currently provide the most accurate measurement of overall burden of atherogenic particles |
| Postanalytical phase (test reporting) |
| 1. Laboratories should automatically calculate and report non-HDL-C on all lipid profiles. RemnantC could also be reported in selected patients = CT- (HDL-C + LDL-C) (measured by the direct method) |
| 2. Laboratory reports should flag abnormal concentrations based on decision thresholds. |
| 3. Extremely high concentrations beyond the reference limits should alert clinicians (interpretative commenting on test report). |
| Post-postanalytical phase (test interpretation and use) |
| 1. LDL-C is the primary target of lipid-lowering therapy. |
| 2. When the LDL-C target is not achieved or cannot be adequately assessed, non-HDL-C or apo B should be preferred as secondary treatment targets in patients with TG 2–10 mmol/L (175–880 mg/dL), diabetes, obesity, or metabolic syndrome. |
Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin Chem Lab Med 2020; 58(4):496–517.11
| Family history | |
| - First-degree relative with known premature IHD and/or | 1 |
| - First-degree relative with known LDL-C > the 95th percentile | |
| - First-degree relative with xanthomata and/or corneal arcus, and/or | 2 |
| - Children < 18 years with LDL-C > the 95th percentile | |
| Clinical history | |
| - Evidence of premature coronary artery disease | 2 |
| - Evidence of premature cerebral or peripheral vascular disease | 1 |
| Physical examination | |
| - Tendinous xanthomata | 6 |
| - Corneal arcus < 45 years | 4 |
| Cholesterol levels | |
| - LDL-C > 330 mg/dL (>8.5 mmol/L) | 8 |
| - LDL-C 250−329 mg/dL (6.5–8.4 mmol/L) | 5 |
| - LDL-C 190−249 mg/dL (5.0–6.4 mmol/L) | 3 |
| - LDL-C < 190 mg/dL (>5.0 mmol/L) | 1 |
| DNA analysis | |
| - Functional mutation in the LDLR gene | 8 |
| SCORE | |
| - Definite FH | >8 |
| - Probable FH | 6−8 |
| - Possible FH | 3−5 |
| - FH unlikely | <3 |
FH: Familial hypercholesterolaemia; IHD: ischaemic heart disease; LDL-C: low density lipoprotein cholesterol.
Austin, M. A., C. M. Hutter, R. L. Zimmern, and S. E. Humphries, 2004, Genetic Causes of Monogenic Heterozygous Familial Hypercholesterolemia: A HuGE Prevalence Review: American Journal of Epidemiology, v. 160, no. 5, p. 407–420, doi:10.1093/aje/kwh236.22 Translated and reproduced by permission of Oxford University Press on behalf of the American Journal of Epidemiology. OUP and American Journal of Epidemiology take no responsibility for the accuracy of the translation. The licensee is solely responsible for the translation in this publication/reprint.
Interpretation of degree of nicotine dependency: Total score: 0–2 low; 3–4 moderate; 5–6 high.
Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 1989; 84:791-800. https://doi.org/10.1111/j.1360-0443.1989.tb03059.x.153