Chagas' disease has spread throughout Latin America because of the high rate of migration among these countries. Approximately 30% of Chagas' patients will develop cardiomyopathy, and 10% of these will develop severe cardiac damage leading to heart failure. Beta-blockade improves symptoms and survival in heart failure patients; however, its efficacy has not been well established in Chagas' disease. We evaluated the role of carvedilol in cardiac remodeling and mortality in a Chagas' cardiomyopathy animal model.
METHODS:We studied Trypanosoma cruzi infection in 55 Syrian hamsters that were divided into three groups: control (15), infected (20), and infected + carvedilol (20). Animals underwent echocardiography, electrocardiography, and morphometry for collagen evaluation in ventricles stained with picrosirius red.
RESULTS:The left ventricular diastolic diameter did not change between groups, although it was slightly larger in infected groups, as was left ventricular systolic diameter. Fractional shortening also did not change between groups, although it was slightly lower in infected groups. Collagen accumulation in the interstitial myocardial space was significantly higher in infected groups and was not attenuated by carvedilol. The same response was observed in the perivascular space. The survival curve showed significantly better survival in the control group compared with the infected groups; but no benefit of carvedilol was observed during the study. However, in the acute phase (up to 100 days of infection), carvedilol did reduce mortality.
CONCLUSION:Carvedilol did not attenuate cardiac remodeling or mortality in this model of Chagas' cardiomyopathy. The treatment did improve survival in the acute phase of the disease.
Chagas' disease was first described in the beginning of the 20th century by Carlos Chagas (1). However, the isolation of Trypanosoma cruzi DNA from the mummies of humans who lived in the Atacama Desert in Chile and Peru demonstrated that this disease may have affected human beings for 9,000 years (2).
Based on cross-sectional surveys, the prevalence of T. cruzi infection in Latin America is estimated to be as high as 17 million cases, and approximately 100 million people are at risk of being infected. Additionally, the incidence of Chagas' disease is estimated to be 700,000 new cases/year, and it causes approximately 45,000 deaths annually (3). Because of the high rates of migration from Latin America to nonendemic regions, such as North America and Europe, T. cruzi infection has spread through blood transfusions, organ transplantations, and congenitally (4,5). Based on this spread of the disease, the World Health Organization launched The Global Network for Chagas' Disease Elimination (6).
Chronic Chagas' cardiomyopathy may affect 30% of infected patients, and 10% of these patients will develop serious heart dysfunction with severe heart failure. The histopathological findings in the chronic stage are focal myocarditis that leads to myocyte loss, structural remodeling with intense fibrosis, geometric changes, and ventricular dysfunction. Several pathways have been proposed for the progression and development of chronic cardiomyopathy. Among them, in particular, is the sympathetic denervation of the heart in Chagas' patients. It is characterized by diffuse nervous tissue damage, parasitic invasion of preganglionic neurons, and degenerative abnormalities in Schwann cells and nerve fibers (7). Parasympathetic nerves and paravertebral sympathetic ganglia are also involved (8). Some studies have also demonstrated extremely decreased cardiac beta-receptor density in Chagas' cardiomyopathy (9). Moreover, a clinical study (10) demonstrated decreased norepinephrine content in myocardial tissue in Chagasic patients.
Several clinical trials using a variety of beta-receptor blockers have demonstrated improvements in symptoms, ventricular function, functional capacity, and survival in patients with heart failure (11-13). Based on these data, patients with Chagas' cardiomyopathy who develop heart failure should benefit from this treatment. However, none of these beta-blocker studies has included patients with Chagas' cardiomyopathy.
Therefore, because Chagas' cardiomyopathy is a sympathetic denervated heart disease with decreased adrenergic activities, we hypothesized that beta-blockers may not have the same benefits on cardiac structure, geometry, and function, as described for other causes of heart failure. We evaluated the effects of carvedilol on cardiac remodeling and mortality in an animal model of Chagas' cardiomyopathy.
MATERIAL AND METHODSAnimal InfectionWe studied 55 outbred six-week-old female Syrian hamsters after T. cruzi infection. These animals were divided into three groups: (1) normal control (c-15), (2) infected hamster (inf-20), and (3) infected hamster treated with carvedilol 10 mg/kg/day given by gavage (infcv-20), with the dose increased to 15 mg/kg/day after six months. The dosage adjustment was made because of unexpected but not significant heart rate decreases. The treatment began one week after T. cruzi inoculation. All of the infected animals were peritoneally inoculated with 3.5x105T. cruzi Y strain blood trypomastigotes and kept in a regular cage with water and food ad libitum (14). The protocol was performed in accordance with the recommendations of COBEA (the Brazilian College of Experimental Animal Studies) and the Guide for the Care and Use of Laboratory Animals (UFAW) (15,16). The study protocol was reviewed and approved by the ethical committee (SDC #2432/04/052; CAPPesq #0473/04) of the Heart Institute and University of São Paulo Medical School.
The animals were euthanized after 11 months of infection using intracardiac 3 M potassium chloride under 2,2,2-tribromoethanol anesthesia. The heart was removed through a median sternotomy and weighed. A portion of the cardiac tissue was fixed in 10% formalin, and another portion was frozen in liquid nitrogen and kept at -80°C. The tibia length (mm) was measured for normalization of the geometrical echocardiography parameter and heart weight because it was a long-term study, and body weight loss among infected animals could interfere with the results.
EchocardiographyAll of the animals underwent a 2-D echocardiography with M-mode and color Doppler before and at 4, 8, and 11 months after the infection. Briefly, the animal was peritoneally infused with 2,2,2-tribromoethanol (2 g/kg) and positioned as requested for easy accessibility of the transducer. Transthoracic echocardiography was performed using a Sequoia model 512 (Acuson, Mountain View, CA) equipped with a 13-MHz linear transducer. The heart was imaged in 2-dimensional mode in the parasternal long-axis, short-axis, and 4-chamber view. Left ventricular end diastolic diameter (LVEDD) and left ventricular systolic diameter (LVESD) were obtained, and the result was divided by tibia length, resulting in an index of LVEDD and LVESD. Left ventricle (LV) systolic function was assessed based on fractional shortening (FS). Global left ventricular function was evaluated by the myocardial performance index (MPI). For isovolumetric relaxation time (IRT), a 5-chamber view of the heart was used to obtain the sample volume position to straddle the left ventricle outflow tract and mitral orifice. The formula IRT/√RR gave the corrected IRT (CIRT) (17).
ElectrocardiographyAll of the animals underwent electrocardiography (ECG) before and at four, eight, and 11 months after infection at the same time as echocardiography. We used an automatic ECG EP-3 (Dixtal Biomédica, São Paulo, SP, Brazil) and connected the electrodes to the front and back paws, using the right and left legs and arms as references. We analyzed DI, DII, DIII, aVR, aVL and aVF with 2 N gain, 50 mm/s velocity and conventional acquisition for 20 sec. The heart rate, QRS duration, number of premature ventricular complexes, and cardiac rhythm were analyzed.
Collagen morphology and morphometrySerial paraffin sections (5 µm) of the formalin-fixed heart tissues were stained with fibrillar collagen-specific picrosirius red. Stained coronal sections from both ventricles were then viewed by light microscopy to identify sites of fibrosis, including those in the interstitial and perivascular space. The interstitial collagen volume fraction (ICVF) and perivascular collagen volume fraction (PCVF) for both ventricles were determined by video morphometry using a Quantimet 520 Image Analyses System (Leica, Inc.; Deerfield, IL, USA) (18). All of the measurements were taken by two independent and blinded examiners.
Statistical analysisData from each group were compared using the analysis of variance (ANOVA) or Kruskal-Wallis tests according to the normality of the sample. Tukey's test or Dunn's method was used to identify differences. Survival curves were constructed, and the log rank test was used to compare them. The data are presented as the mean±SEM and p-values<0.05 were considered significant.
RESULTSClinical evaluationThe animals in the infected group were sick, with some changes in their pile, and they remained clustered together in the cage. They tended to be prostrate and less active. In the 11th month, the body weight was higher in the infected group than in the control and carvedilol groups (C: 152±8 g; inf: 166±15 g; infcv: 142±20 g; p = 0.013). The numbers of survivors analyzed were 15 in the control group, six in the infected group, and eight in the carvedilol group.
ElectrocardiographyAt baseline, there were no differences in heart rate, QRS duration, or ventricular arrhythmias. The ECG at the fourth, eighth, and eleventh months showed a decreased heart rate in the carvedilol group, but this difference did not reach statistical significance. However, in the control group, the heart rate remained stable during the study, with an increase of 0.35%, while in the infected group, it increased by 11.5% (Figure 1). In contrast, in the carvedilol group, the heart rate increased only 4%, demonstrating the effects of the beta blockade. The sinus rhythm was 100% in all groups at baseline; at the end of the study, it was 100% in the control and infected groups and 90% in the carvedilol group (p = 0.33). The median QRS duration at baseline was 40 msec in all groups (p = 0.84); at the end of the study, it was 40 msec in the control and carvedilol groups and 60 msec in the infected group (p = 0.17). Ventricular premature complex at baseline was present in 3.7% of mice in the control group and no mice in the infected and carvedilol groups (p = 0.26); at the end of the study, it was present in 33% of mice in the control and infected groups and 25% in the carvedilol group (p = 0.37).
Myocardial remodelingThe mean ratio of heart weight over tibia length did not show significant differences between groups, despite slightly heavier hearts in the infected groups (C: 0.022±0.003; inf: 0.024±0.005; infcv: 0.026±0.011; p = 0.804).
The left ventricular diastolic diameter was similar in all groups at baseline. At the 11th month, there was an increase in the ratio of left ventricular diastolic diameter over tibia length ×100 in the infected and carvedilol groups, but this difference was not significant (C: 1.9±0.2; inf: 2.1±0.4; infcv: 2.1±0.2; p = 0.238). The ratio of systolic diameter over tibia length ×100 showed the same trend in the 11th month (C: 1.3±0.2; inf: 1.5±0.5; infcv: 1.6±0.2; p = 0.065) (Figure 2).
The collagen volume fraction of the interstitial and perivascular space in the left and right ventricle was increased in the infected groups compared with the control, and there were no differences between treated and nontreated groups (Table 1, Figure 3).
Collagen volume fraction in the interstitial and perivascular space in the left and right ventricles.
| Control | Infected | Carvedilol | p-value | |
|---|---|---|---|---|
| Left ventricle (%) | 0.81±0.36∗ | 4.24±1.43 | 3.60±1.90 | ∗p<0.001 |
| Right ventricle (%) | 1.29±0.60 | 5.20±2.50∗ | 4.42±2.35 | ∗p = 0.002vs control |
| Perivascular (%) | 0.81±0.49∗ | 1.50±0.40 | 1.41±0.60 | ∗p = 0.013 |
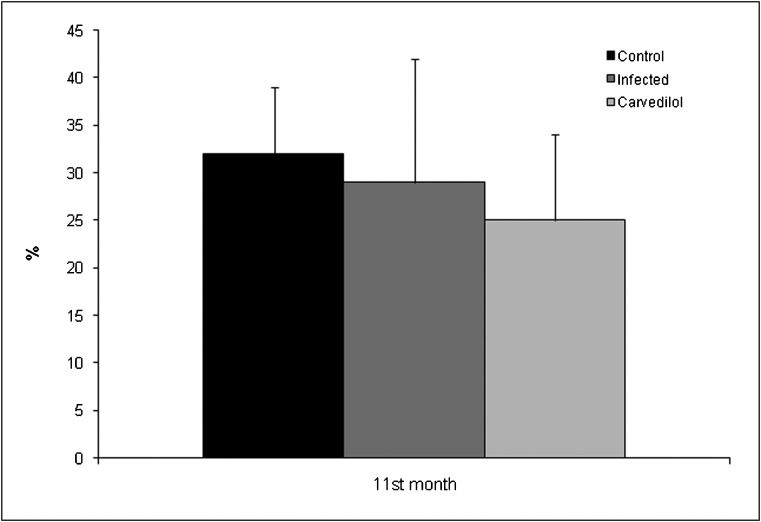
Despite the reduced fractional shortening in the infected groups, differences in the left ventricular systolic function did not reach statistical significance in the 11th month, although there was a slight decrease in the carvedilol-treated group (Figure 4). The myocardial performance index used to assess global cardiac function did not show any significant differences between groups at the 11th month (C: 0.67±0.12; inf: 0.58±0.11; infcv: 0.62±0.17; p = 0.365).
The left ventricular diastolic function was evaluated only by CIRT, which was not significantly different between groups.
SurvivalThe survival curves demonstrated significantly higher survival in the control group compared with the infected groups, with no differences between the treated and untreated groups (p<0.001, Figure 5A). However, in the acute phase (the first 100 days of infection), survival remained better in the control group compared with the infected groups, and the carvedilol group had better survival compared with the infected group (p<0.001, Figure 5B).
A Kaplan Meier survival curve for the total study period (A), showing no significant differences between infected groups but differences between the infected groups and the control and better survival in the carvedilol group compared with the infected group in the acute phase (B). ∗p<0.001 vs infected groups, ∗∗p<0.001 infected vs infected plus carvedilol.
Several clinical trials have demonstrated the benefits of beta-blockade on heart failure due to ischemic and idiopathic cardiomyopathies (11,12,13). However, these benefits have not been demonstrated for Chagas' cardiomyopathy. Some studies with beta-blockers included patients with Chagas' cardiomyopathy and showed benefits but had small sample sizes (19,20). One study concluded that beta-blockers were effective, not detrimental, and may improve survival in Chagas' patients with chronic heart failure and suggested that a randomized trial should be performed to confirm these findings (21). Another study suggested that the absence of beta-blocker use in the treatment of Chagas' cardiomyopathy was associated with poor prognosis (22), and this study was the basis for other studies (23). However, another study with small samples presented controversial results (24). Our experimental study was designed to evaluate the role of beta-blockade in the context of Chagas' disease, mainly its effects on attenuating cardiac remodeling.
In this study, the mean body weight in the first month was higher in the control group (p<0.003), most likely because the animals in the infected groups lost weight during the acute phase. However, by the 11th month, the infected group was heavier than the control and carvedilol groups, most likely because of a hydropic state.
Although other studies with rodents used carvedilol doses from 2 to 30 mg/kg/day, we used 10 mg/kg/day at the beginning and increased the dosage to 15 mg/kg/day thereafter (beginning at six months) to ensure safety because of the high incidence of AV blockage in Chagas' disease. Studies that used higher doses were mostly acute studies in animal models other than Chagas' cardiomyopathy. All of the measurements showed slower heart rates in the carvedilol group, although no significant differences were observed. There was no reduction in ventricular arrhythmias in the carvedilol group. Several studies have demonstrated a reduction in sudden death in heart failure patients using beta-blockers (11). However, it is possible that the pathophysiologic mechanisms of arrhythmias in Chagas' cardiomyopathy is related to intense myocarditis, extensive myocardial fibrosis, and sympathetic denervation, which may change the electrophysiological behavior of the myocardium and may not respond to the beta-blockade effect.
The left ventricular diastolic diameter at baseline was similar to that found in healthy Sirius hamsters (17). No significant dilation was observed in the infected groups, although infected animals had larger diameters than controls. The systolic diameter was also larger in the infected group, but with no significant differences (p = 0.065). This trend could represent an early stage of cardiac dilation and dysfunction. Neither diastolic nor systolic diameter was affected by carvedilol in the infected groups. Another study used the same animal model and demonstrated significant differences in ventricular diameter (14). This difference could be because the more dilated animals in our study died early, and not all animals will develop dilated cardiomyopathy, even with higher myocardial fibrosis.
The benefit of beta-blockers on myocardial remodeling has been described (25). However, it was not tested specifically in Chagas' cardiomyopathy, which produces intense fibrosis in myocardial tissue. Our results demonstrate that there was major collagen accumulation in the interstitial and perivascular space in the infected groups and that this accumulation was not attenuated by carvedilol. It is possible that the inflammation, autoimmune attack, and sympathetic denervation leading to myocardial fibrosis observed in Chagas' disease are different from other etiologies of cardiomyopathy. Other studies have already demonstrated that myocardial tissue from patients with Chagas' cardiomyopathy contain less norepinephrine than tissues from patients with ischemic disease.
Regarding cardiac function, although there was no significant difference between groups, the fractional shortening in infected animals was lower than previously described, and it was not affected by carvedilol.
The survival curve showed no benefits of carvedilol during the total period of the study. However, in the acute phase (up to 100 days), carvedilol significantly reduced mortality. Previously, a study of beta receptors and auto-antibodies with atenolol as the intervention was performed in a Chagas' model of BALB/c mice (26). The researchers demonstrated lower mortality in the treated group due to decreased antibody fixation over cardiac receptors. Another study demonstrated less parasitism in rats treated with propranolol (9). Therefore, we speculate that a decrease in myocarditis because of lower parasitism, together with a decrease in the injury caused by auto-antibodies, could explain the lower mortality observed in the acute phase. Carvedilol may have antioxidant and anti-inflammatory activities (27), which could also be part of this beneficial effect in the acute phase, which is characterized by inflammation and oxidative stress. The prevention of sudden death with beta-blockers has been described; this could be another plausible explanation for the decreased mortality in the acute phase.
We conclude that carvedilol did not attenuate cardiac remodeling or mortality in this model of Chagas' cardiomyopathy. The treatment did improve survival in the acute phase of the disease.
The publication was supported by FAPESP, Brazil (012/11438-4).
No potential conflict of interest was reported.
Pimentel WS conceived and designed the study, collected, analyzed and interpreted the data, critically reviewed the manuscript for important intellectual content and was also responsible for the statistical analysis, manuscript drafting and supervision of the study. Ramires FJ conceived and designed the study, analyzed and interpreted the data, critically reviewed the manuscript for important intellectual content and was also responsible for the statistical analysis, manuscript drafting and supervision of the study. Ianni BM, Bilate AM, Cunha-Neto E and Mady C conceived and designed the study. Salemi VM was responsible for the data acquisition. Oliveira AM was responsible for the data acquisition, analysis and interpretation. Fernandes F conceived and designed the study and was also responsible for the data analysis and interpretation.