In young adults vertebral artery dissection (VAD) is an important cause of brain infarction. Diabetes insipidus following a spontaneous bilateral vertebral artery dissection is uncommon. We report a case of 50-year-old female presented with subarachnoid hemorrhage (SAH) after rupture of an extradural vertebral artery dissecting aneurysm. She went on to develop central diabetes insipidus and was treated in a conservative way.
En adultos jóvenes, la disección de la arteria vertebral (DAV) constituye una importante causa de infarto cerebral. La diabetes insípida tras disección bilateral espontánea de esta arteria es poco frecuente. Describimos el caso de una mujer de 50 años de edad, que se presentó con una hemorragia subaracnoidea (HSA) tras la rotura de un aneurisma disecante extradural de la arteria vertebral. Evolucionó hasta el desarrollo de una diabetes insípida central que fue tratada de modo conservador.
VAD is an increasingly recognized cause of posterior circulation stroke in young and middle aged adults.1 The most frequent symptoms include unilateral neck pain that may be followed by transient or permanent ischemia of vertebrobasilar tributaries of the brain. Known mechanism is microtraumata due to abrupt head movements—for example, chiropractic maneuvers. In addition a pathogenetic role of connective tissue diseases, cystic media necrosis, fibromuscular dysplasia, migraine, and inflammatory diseases has been postulated. Lesions caused by VAD are cerebellar or brainstem infarcts, unilateral or bilateral thalamic infarcts (top of the basilar syndrome), or infarctions in the posterior cerebral artery territory due to intra-arterial embolism or hemodynamic decompensation when collaterals are insufficient. Vertebral artery dissection may lead to stroke or even death. Diabetes insipidus (DI) following spontaneous VAD has rarely been reported in the literature. Although placebo controlled studies on the treatment of vertebral artery dissection are missing, some authors suggest that early anticoagulation can prevent thrombembolic complications.
Case report50-Year-old female, a known hypothyroid on regular treatment since 10 years, with history of chronic migraine got admitted at outside hospital following loose stools, vomiting and worsening headache. She was treated with intravenous normal saline, ionotropes (dopamine and nor-adrenaline infusions) and antibiotics (piperacillin+tazobactum, ciprofloxacin and metronidazole). Following this her loose stools and vomiting got subsided and her blood pressure improved. Two days later she started having excessive urination (>7l per day). She was given intravenous normal saline at the rate equal to her previous hours urine output to avoid dehydration. She continued to pass 10–12l of urine per day for next 3 days. She was referred to us for further management.
After getting admitted to our hospital, she was complaining of severe occipital and nuchal pain. She denied any history of neck injury, yoga and spine surgery in the past. On physical examination, she was afebrile, conscious and oriented. She had a blood pressure of 140/80mm of Hg, pulse rate of 102/min and a central venous pressure of 10cm of water. Her systemic examination was unremarkable. She had a urine output of 400ml/h. Her laboratory investigations at the time of admission were as follows: BUN 5mg/dl, creatinine 0.29mg/dl, hemoglobin 11.5g/dl, total leukocyte count 11,500/mm3, platelet count 345,000/mm3, serum sodium 142meq/l, potassium 2.8meq/l, chloride 102meq/l, bicarbonate 29.9meq/l, uric acid 1.18mg/dl. Urine routine examination revealed a specific gravity 1.005, proteins – nil, WBC – occasional and RBC – nil. Sonography revealed normal sized kidneys with normal echotexture.
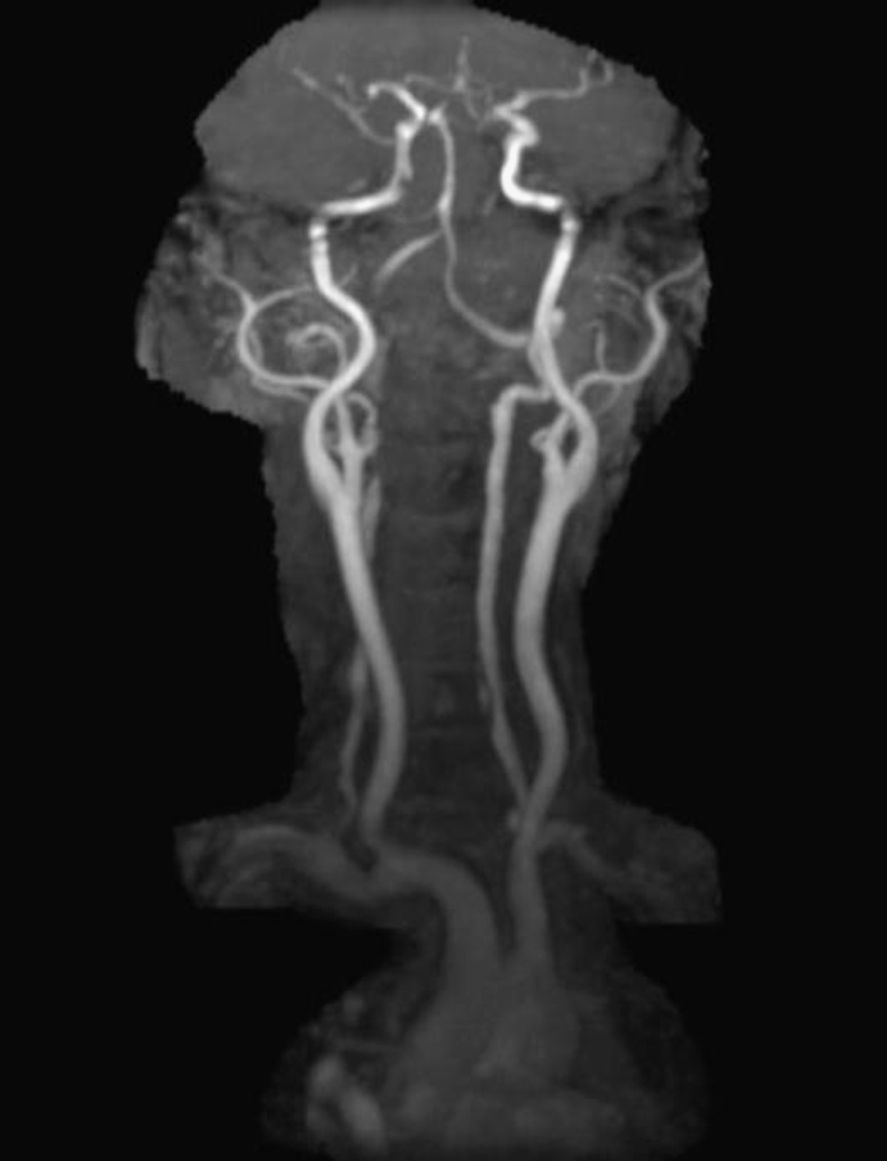
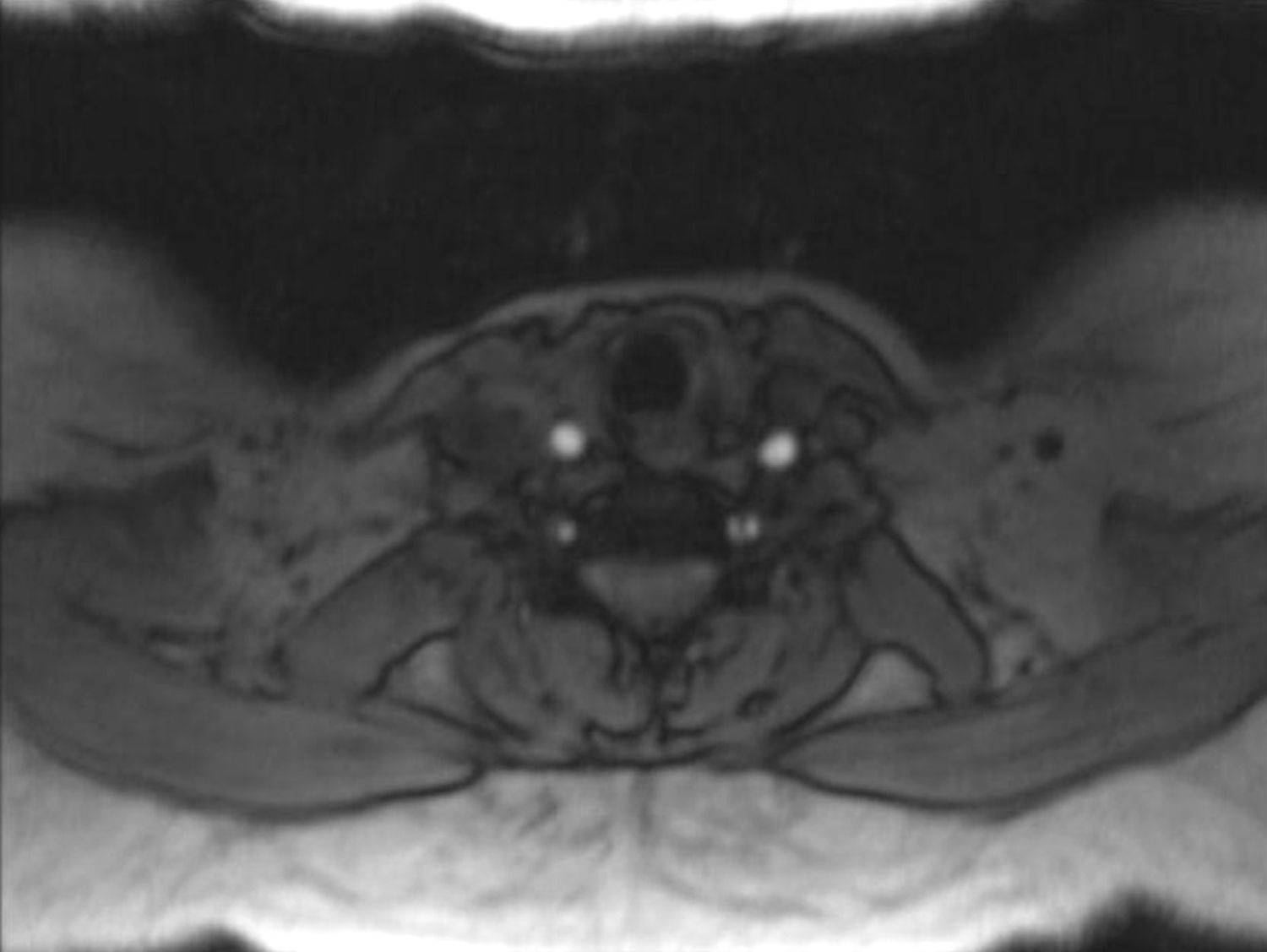
In order to find out the cause for her worsening headache MRI-Brain with MR-Angiography (MRA) was performed which revealed subarachnoid hemorrhage over bilateral parietal, occipital and in perimesencephalic cistern with severe vasospasm of bilateral posterior cerebral, middle cerebral and anterior cerebral arteries. She was then subjected to digital subtraction angiography which showed narrowed V2 segment of left vertebral artery, small dissecting aneurysm of V3 segment of left vertebral artery, severe narrowing of distal V2 segment of right vertebral artery and mild focal narrowing of intradural right vertebral artery suggesting bilateral multiple vertebral artery dissection. There was no intracranial aneurysm or arterio-venous malformations and venous system was normal. MRA of neck vessels confirmed dissection of bilateral vertebral arteries at multiple levels (Figs. 1 and 2). Vasculitis work-up was done to find out the cause for VAD, which came negative for ANA, dsDNA, c-ANCA, p-ANCA, anti-cardiolipin antibody, anti-phospholipid antibody and VDRL. As there was no history of trauma, a diagnosis of spontaneous VAD was made.
She was evaluated for polyuria, her urine examination revealed a specific gravity of 1.005, urine osmolality of 69mOsm/kg of water and serum osmolality of 300mOsm/kg. In view of low urinary specific gravity, low urine osmolality, polyuria and with no history suggestive of excessive water intake a diagnosis of DI was made. To differentiate between central and nephrogenic DI, she was treated with 10units of vasopressin subcutaneously, following which her urine osmolality increased from 110 to 262, confirming the diagnosis of central DI.
She was treated conservatively with intravenous normal saline, analgesics to reduce her headache and complete bed rest with restricted neck movements. Her urine output gradually decreased from 400ml/h on the day of admission to 100–125ml/h and urine osmolality gradually increased from 69 to 207mOsm/kg of water. She was discharged in a stable condition with anti-platelet drugs.
DiscussionVAD is an increasingly recognized cause of stroke in patients younger than 45 years. An expanding hematoma in the vessel wall is the route lesion in VAD. This intramural hematoma can arise spontaneously or as a secondary result of minor trauma, through hemorrhage of the vasa vasorum within the media of the vessel. It also can be introduced through an intimal flap that develops at the level of the inner lumen of the vessel. The incidence of VAD is estimated to be 2.6 per 100,000. VAD occurs in a much younger population with a female preponderance (female-to-male ratio is 3:1). VAD has been associated with a 10% mortality rate in acute phase. Death is the result of extensive intracranial dissection, brainstem infarction, or subarachnoid hemorrhage.2 Spontaneous VAD is the term used to describe all causes that do not involve blunt or penetrating trauma as a precipitating factor. Several risk factors have been associated with the development of VAD. These include spinal manipulation, minor neck trauma, yoga, hypertension, chronic headache syndromes/migraines,3 fibromuscular dysplasia, female sex and recent infection. VAD typically presents in a young person with severe occipital headache and posterior nuchal pain following a recent, relatively minor, head or neck injury.4 Focal neurologic signs attributable to ischemia of the brain stem or cerebellum ultimately develop in 85% of patients; however, a latent period as long as 3 days between the onset of pain and the development of CNS sequelae is not uncommon. Delays of weeks and years also have been reported. When neurologic dysfunction does occur, patients most commonly report symptoms attributable to lateral medullary dysfunction (Wallenberg syndrome). Rarely, patients may manifest the symptoms of medial medullary syndrome.
Central DI after spontaneous VAD treated clinically has been rarely reported in the literature. Vertebral arteries are not directly involved in the blood supply of pituitary gland. The anterior lobe is supplied via the superior hypophysial arteries, which derivated from internal carotid artery and traverses the diaphragma sellae along the pituitary stalk. The posterior lobe is supplied via the inferior hypophysial arteries, which also arise from internal carotid artery, nevertheless go down to the pituitary gland rather than within the stalk.5 The cause for central DI after a spontaneous VAD may be due to compression or destruction of the pituitary gland, interruption of the blood supply to the gland or to the pituitary stalk. Verees et al.6 postulated that DI occurred as a result of impingement on the intracavernous portion of the inferior hypophysial artery, which caused diminished perfusion to the posterior lobe. Alternatively, kinking or pressure on the infundibulum by edematous, hemorrhagic material, which impeded transit of antidiuretic hormone from the preoptic and paraventricular nuclei of the hypothalamus to the posterior lobe, might prove to be the originating cause of DI.7
The possible pathophysiology involved in our case is the intense vasospasm leading to diminished perfusion to the hypothalamus following subarachnoid hemorrhage, which occurred following rupture of bilateral extradural vertebral artery dissecting aneurysm. As subarachnoid hemorrhage got subsided, vasospasm to the hypothalamus got relived and patients transitory DI disappeared. In summary, central DI should be kept in mind while treating patients with VAD and should be treated effectively in order to prevent its further complications.