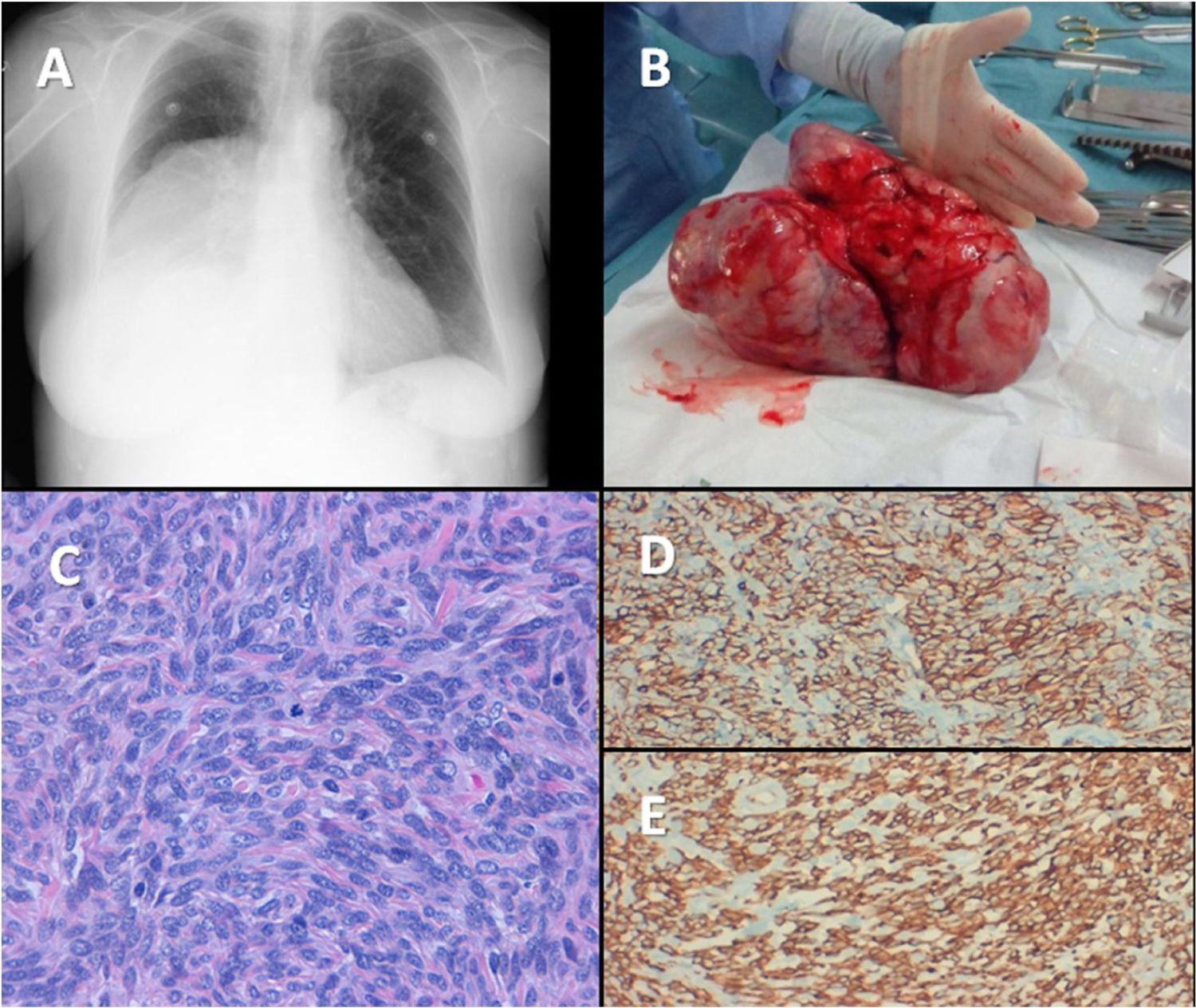
Non-islet cell tumor hypoglycemia is a rare paraneoplastic syndrome associated with large benign/malignant tumors, due to an overproduction of Big-IGF2.1 Below, we describe a case of this rare condition. An 83-year-old woman, with a previous history of type 2 diabetes mellitus (T2DM), dyslipidemia, depression and basal cell epithelioma resection, was admitted for further study of repetitive hypoglycemic events for the previous 3 months and 7kg weight loss within the last year. She had a well-controlled T2DM (HbA1c 5.9%) on metformin 850mg/daily. Her mother and 3 maternal cousins had been diagnosed with hepatocarcinoma. The physical examination was not relevant except for decreased vesicular murmur in the right lower hemithorax. Weight and body mass index were 52kg and 24kg/m2 respectively. During a hypoglycemic episode the following results were found: blood glucose 37mg/dl, C-peptide 0.09ng/ml (0.81–3.85), insulin <0.50μUI/ml (3–25), anti-insulin antibody <5U/ml (<5) and chromogranin A 59.8ng/ml (19.4–98.1). A chest X-ray showed opacity in the lower right lobe (Fig. 1A). Chest CT scan showed a non-invasive well-defined mass sized 10cm×10cm×20cm, displacing bronchial structures and a right pleural effusion with a maximum thickness of 15mm. This was suggestive of a primary pleural tumor, being a solitary fibrous tumor (SFT) or mesothelioma the most plausible diagnostic options. NICTH was suspected and lab tests revealed: IGF2 510ng/ml (350–1000), IGF1 <25ng/ml (54.60–204.4), IGFBP3 1.43ng/ml (2.2–4.5) and IGF2/IGF1 ratio 20.4 (<3). During the preoperative period, hypoglycemia was effectively prevented with a fractionated enriched diet, intravenous glucose infusion and prednisone 30mg/daily. A 1850g solid mass of 22.6cm×14.5cm×9.7cm solid mass was resected via a right posterolateral thoracotomy (Fig. 1B). The microscopic examination revealed a well-defined proliferation with highly-cellular focal areas that alternate with paucicellular areas, edema, focal necrosis, moderate atypia and a mitotic index of 4 mitosis/10 high-power fields (Fig. 1C). Immunohistochemistry was positive for CD34, BCL2, CD99 and IGF1R establishing the diagnosis of a non-invasive SFT (Fig. 1D and E). No radiological signs of disease recurrence were observed in the CT scan performed 2 months after surgery and glucose-lowering treatment (linagliptin/metformin 2.5/850mg BID) was resumed due to poor glycemic control. She did not receive postoperative radiotherapy and the tumor did not show recurrence for the first year after surgery. Then, a minimal pleural thickening with progressive growth was observed and radical fractionated radiotherapy was administered (total dose 5750cGy). There were no changes in glycemic control, T2DM treatment or hypoglycemic events during recurrence. In her last radiological control, 6 years after surgery, there were no signs of recurrence.
Non-islet cell tumor hypoglycemia is a rare cause of hypoglycemia, due to a tumor overproduction of pro-IGF-2 (Big-IGF2).1 Tumors of mesenchymal or hepatic origin are the most common casue, but hypoglycemia has also been described in other benign and malignant tumors: fibrosarcomas, mesotheliomas, hemangiopericytoma, Lymphoma/Leukemia, gastrointestinal stromal tumor (GIST), yolk cell tumor, plasmocytoma, Leydig cell tumor, phyllodes tumor of the breast and adrenocortical, pancreatic and medullary thyroid carcinoma.2,3 In a review, a total of 290 cases were identified in the literature between 1988 and 2013, hypoglycemia was de first manifestation in 48% of cases, age ranged from 2 to 87 years (mean 56.4), tumor diameter tended to be very large (between 10–20cm) and there was no gender preference.2 Other findings include hypokalemia and acromegaloid changes.2 Normally IGF2 is limited to the vascular space in the form of high molecular weight complexes, 80% as ternary complexes (With IGFBP3 and acid-labile subunit), 20% as binary complexes (With IGFBP3) and very little as free IGF2. Abnormal forms of IGF2 (Big-IGF2) cannot form these complexes resulting in higher amounts of low molecular weight complexes and free forms of IGF2, that can cross the capillary membranes to interact with insulin receptors causing hypoglycemia by inhibiting gluconeogenesis, glycogenolysis, and ketogenesis.4 Additionally, IGF2 suppresses insulin, GH (with resultant low IGF1) and glucagon secretion.3–5 Solitary fibrous tumor, first described in 1870 by Wagner, is a rare soft tissue neoplasm, with an incidence of 0.2 per 100,000/year, that causes less than 5% of primary pleural tumors. It can also be found in other locations such as retroperitoneal, hepatic or pelvic locations. Typically, it is usually diagnosed in the 5th–6th decades, there is no sex preference and they are benign in most cases, although patients may recur and metastasize on occasions.6 They usually present with respiratory symptoms (cough, dyspnea, chest pain) and though one third of tumors are diagnosed incidentally. Hypoglycemia is present in only 4% of patients (Doege-Potter syndrome), although most of them have an overproduction of Big-IGF2.6,7 NICTH is suspected when other most frequent causes of hypoinsulinemic hypoglycemia (low insulin/C-peptide/proinsulin/betahydroxybutyrate levels), in the absence of a hypoglycemic agent, are ruled out. Typically, high IGF2/IGF1 ratio (>3), low IGF1 and normal to high IGF2 levels are the main biochemical features. In some cases, IGF2 could be low, but the presence of higher amounts of high molecular weight IGF2 (Big-IGF2) confirms the diagnosis. Of note, false positive IGF2/IGF1 ratio could be present in patients with malnutrition and sepsis and false negative in those with renal failure (renal failure is associated with low levels of IGFBP3 that can influence IGF1 and IGF2 levels). Transparietal puncture biopsy is not enough as a diagnostic procedure and a complete resection is needed with a further pathological evaluation and demonstration of positive immunostaining for CD34 and signal transducer and activator of transcription 6 (STAT6).7,8 Recently, NAB2-STAT6 fusion genes, that convert a transcriptional repressor (NAB2) into a transcriptional activator (NAB2-STAT6) have been proposed for the pathogenesis of SFT, leading to overexpression of early growth response 1 (EGR1) target genes (IGF2, H19, and RRAD).9 A decreased activity of the enzyme PCSK4 (Proprotein Convertase Subtilisin/Kexin Type 4) that impairs the processing of pro-IGF2 may also contribute to the overproduction of big IGF2.10 The prognosis is good, even in malignant forms, when a complete resection is achieved. If there is a case of recurrence, local radiotherapy can be considered, but chemotherapy has very limited benefits.2 Hypoglycemia in NITCH improves with corticosteroid treatment, glucagon infusions and recombinant growth hormone (rGH) have been used as well with favorable results, but diazoxide and somatostatin analogs are not useful.2 Antibodies against both mature and pro IGF2, anti-IGF2 small interfering RNA and methods that enhance PCSK4 activity3 are therapies under investigation for the treatment of this rare form of hypoglycemia. In conclusion, based on this case report, NICTH should be suspected in patients with hypoglycemia of unclear etiology.
Financial supportThe authors state that they have not received funding for carrying out this study.