Advanced glycation end-products (AGEs) are a marker of metabolic memory. Their levels increases when oxidative stress, inflammation, or chronic hyperglycemia exists. The role of morbid obesity in AGE levels, and the impact of bariatric surgery on them are unknown.
Patients and methodAn observational study with three sex- and age-matched cohorts: 52 patients with obesity, 46 patients undergoing bariatric surgery in the last 5 years, and 46 control subjects. AGE were measured using skin autofluorescence (SAF) in the forearm with an AGE Reader™ (DiagnOptics Technologies, Groningen, The Netherlands). Presence of metabolic syndrome was assessed.
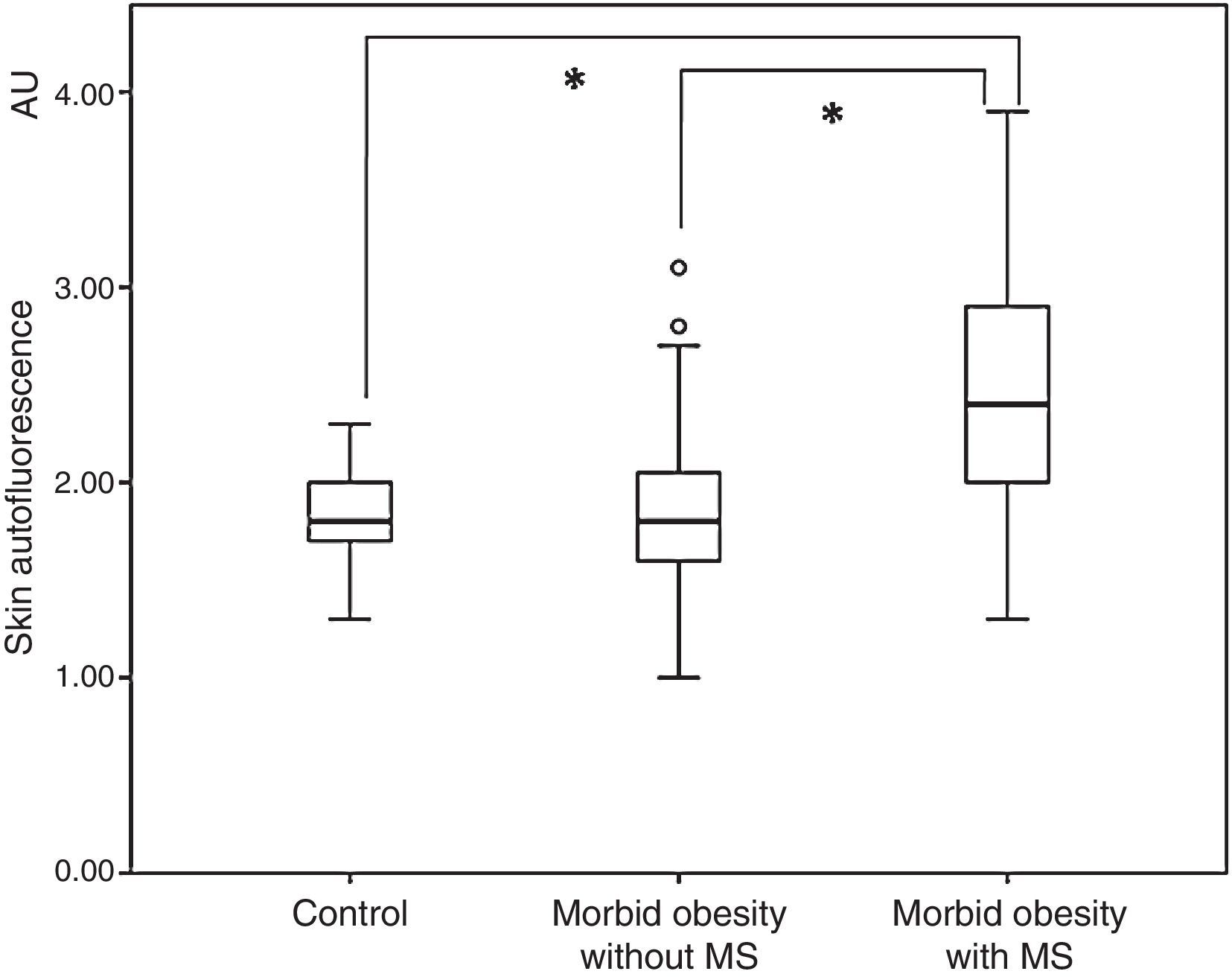
ResultsPatients with morbid obesity had higher SAF levels (2.14±0.65AU) than non-obese subjects (1.81±0.22AU; P<.001), which was mainly attributed to obese subjects with metabolic syndrome (2.44±0.67 vs. 1.86±0.51AU; P<.001). After bariatric surgery, SAF continued to be high (2.18±0.40AU), and greater as compared to the non-obese population (P<.001). A multivariate analysis showed that age and presence of metabolic syndrome (but not sex or body mass index) were independently associated to SAF (R2=0.320).
ConclusionSAF is increased in patients with morbid obesity and metabolic syndrome, mainly because of the existence of type 2 diabetes mellitus. In the first 5 years following bariatric surgery, weight loss and metabolic improvement are not associated with a parallel decrease in subcutaneous AGE levels.
Los productos finales de glicación avanzada (AGE) son un indicador de memoria metabólica. Su concentración se incrementa cuando existe estrés oxidativo, inflamación o hiperglucemia crónica. Se desconoce el papel de la obesidad mórbida en su concentración, así como la influencia que la cirugía bariátrica ejerce sobre ellos.
Pacientes y métodoEstudio observacional con 3 cohortes equiparadas por sexo y edad: 52 pacientes con obesidad, 46 sometidos a cirugía bariátrica en los últimos 5 años y 46 sujetos control. La determinación de los AGE se realizó mediante autofluorescencia cutánea (SAF) del antebrazo con un AGE Reader™ (DiagnOptics Technologies, Groningen, Países Bajos). Se evaluó la presencia de síndrome metabólico.
ResultadosLos sujetos con obesidad mórbida presentaron una SAF (2,14±0,65AU) superior a la de la población no obesa (1,81±0,22AU; p<0,001). Este incremento fue a expensas de aquellos sujetos obesos con síndrome metabólico (2,44±0,67 vs. 1,86±0,51AU; p<0,001). Tras la cirugía bariátrica, la SAF se mantuvo elevada (2,18±0,40AU) y superior a la de la población no obesa (p<0,001). El análisis multivariante mostró que la edad y la presencia de síndrome metabólico (pero no el sexo, ni el índice de masa corporal) se asociaron independientemente con la SAF (R2=0,320).
ConclusionesEn la obesidad mórbida acompañada de síndrome metabólico existe un incremento de la SAF, a expensas principalmente de la presencia de diabetes tipo 2. En los primeros 5 años tras la cirugía, la pérdida ponderal y la mejoría metabólica no se acompañan de un descenso paralelo de la concentración tisular de AGE.
Advanced glycation end-products (AGEs) are a heterogeneous group of compounds which are formed through non-enzymatic glycation of proteins after exposure to sugars.1 AGE accumulation increases physiologically with age, but accelerated AGE production has been reported in type 2 diabetes mellitus (T2DM) associated to chronic hyperglycemia,2 and also in advanced chronic renal disease, related to both lack of elimination and to increased oxidative stress.3 However, little is known about the influence of morbid obesity on AGE levels.
Obesity affects virtually a fourth of the Spanish population, similarly to what occurs worldwide, and is associated to significant metabolic comorbidity.4,5 Because of the recurrent failure of low-calorie diets and the lack of adequate drugs, bariatric surgery has become the only effective procedure to achieve and maintain a significant weight loss in subjects with morbid obesity.6 The relationship between AGEs and body mass index (BMI) has mainly been assess in people with overweight or mild obesity, and a correlation has been found between AGEs and: (i) BMI in the general population, (ii) BMI in patients with T2DM, (iii) BMI in the end stages of chronic kidney disease, and (iv) presence of abdominal obesity.2,7–9 However, we do not know the relationship between AGEs and severe obesity, or whether weight loss associated to bariatric surgery has some impact on AGE levels.
The possibility of measuring AGE levels by skin autofluorescence (SAF) allowed for overriding the initial difficulty of AGE testing in tissue, thus facilitating measurement in large populations.10 Our study objective was to investigate accumulation of AGEs, estimated by SAF in patients with morbid obesity waiting for bariatric surgery, and also after the weight loss induced by surgery during a five-year follow-up period.
Patients and methodsStudy designAn observational, cross-sectional study on 144 patients divided into three groups matched by sex and age: (i) 52 subjects with morbid obesity on a bariatric surgery protocol, (ii) 46 patients who had undergone bariatric surgery in the past five years, and (iii) a control group of 46 subjects with BMI <30kg/m2 and one cardiovascular risk factor. The study objectives and design were approved by the clinical research evaluation committee of our hospital. All patients enrolled voluntarily agreed to participate in the study after receiving the necessary information and signing the informed consent.
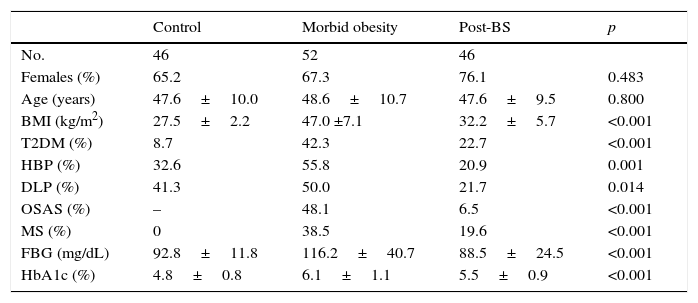
Study populationCaucasian patients routinely monitored at the obesity unit of Hospital Universitario Arnau de Vilanova in Lleida. Patients were recruited from June 2015 to February 2016. Obesity was diagnosed and categorized based on the standards established by the SEEDO,11 while diagnosis of metabolic syndrome (MS) was based on the criteria of the World Health Organization.12 The surgical procedure used included Roux-en-Y gastric bypass and vertical gastrectomy. Follow-up after surgery was five years, which is the time since the bariatric surgery program was started at our hospital. Patients in the control group were recruited at the unit for detection and treatment of cardiovascular diseases. These were non-obese patients (BMI <30kg/m2) of both sexes with no or a single cardiovascular risk factor (T2DM, high blood pressure, or dyslipidemia). Patients in any group with renal failure (glomerular filtration rate ≤60mL/min/1.73m2, calculated by the CKD-EPI formula), minors, or over 65 years of age, non-caucasians, pregnant women, and subjects with severe disease conditioning survival were excluded from the study. Table 1 shows the main characteristics of the study population. Clinical and laboratory data were collected from the computer clinical records of all patients. Anthropometric data were collected using standardized procedures at the time of SAF.
Main clinical characteristics and metabolic data of the population.
| Control | Morbid obesity | Post-BS | p | |
|---|---|---|---|---|
| No. | 46 | 52 | 46 | |
| Females (%) | 65.2 | 67.3 | 76.1 | 0.483 |
| Age (years) | 47.6±10.0 | 48.6±10.7 | 47.6±9.5 | 0.800 |
| BMI (kg/m2) | 27.5±2.2 | 47.0 ±7.1 | 32.2±5.7 | <0.001 |
| T2DM (%) | 8.7 | 42.3 | 22.7 | <0.001 |
| HBP (%) | 32.6 | 55.8 | 20.9 | 0.001 |
| DLP (%) | 41.3 | 50.0 | 21.7 | 0.014 |
| OSAS (%) | – | 48.1 | 6.5 | <0.001 |
| MS (%) | 0 | 38.5 | 19.6 | <0.001 |
| FBG (mg/dL) | 92.8±11.8 | 116.2±40.7 | 88.5±24.5 | <0.001 |
| HbA1c (%) | 4.8±0.8 | 6.1±1.1 | 5.5±0.9 | <0.001 |
BS: bariatric surgery; DLP: dyslipidemia; T2DM: type 2 diabetes mellitus; FBG: fasting blood glucose; HbA1c: glycosylated hemoglobin; HBP: high blood pressure; BMI: body mass index; OSAS: obstructive sleep apnea syndrome; MS: metabolic syndrome.
Data are means±standard deviation or percentages.
An AGE Reader™ (DiagnOptics, Groningen, The Netherlands) was used to measure SAF. This is a noninvasive, fully automated device that measures AGE levels in the dominant forearm using an ultraviolet A light spectrum. The device has previously been validated in patients with T2DM and on hemodialysis.10,13 SAF is calculated as the ratio between the fluorescence emitted in a wavelength ranging from 420 to 600nm and the light received as the consequence of the excitation produced in subcutaneous AGEs, using a spectrophotometer and dedicated software. The wavelength received ranges from 300 to 420nm. The mean of three readings was used in all subjects, and the whole test could be completed in each patient in just over one minute (coefficient of variation, 0.24). Finally, as SAF cannot be reliably measured in people with a dark skin because of excess light absorption, only caucasian subjects were recruited.
Statistical analysisData are given as mean±standard deviation for continuous variables. Categorical variables are given as percentage. Normal distribution of variables was confirmed using a Kolmogorov–Smirnov test. Comparisons between groups were performed using a Chi-square test for categorical variables, and Student's t and ANOVA tests for continuous variables. The relationship between continuous variables and SAF was studies using a Pearson's linear correlation test. Stepwise multiple linear regression was also performed to explore the variables independently related to SAF. The independent variables included in the analysis were age, sex, BMI, and presence of MS in the first model. In a second model, MS was replaced by its components (T2DM, high blood pressure, and dyslipidemia). All p values were based on a two-sided test of statistical significance. A value of p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software (SPSS Chicago, IL, USA).
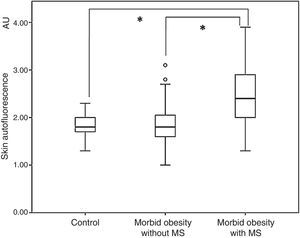
ResultsOverall, subjects with morbid obesity had a significantly higher SAF (2.14±0.65 arbitrary units, AU) as compared to the non-obese population (1.81±0.22AU; p<0.001. When SAF levels in the group of obese subjects were assessed as a function of the presence or absence of MS, they were significantly higher in the first subgroup (2.44±0.67 vs. 1.86±0.51AU; p<0.001) (Fig. 1). In addition, SAF levels were similar in obese patients with no MS and the control group (1.86±0.51 vs. 1.81±0.22AU; p=0.540).
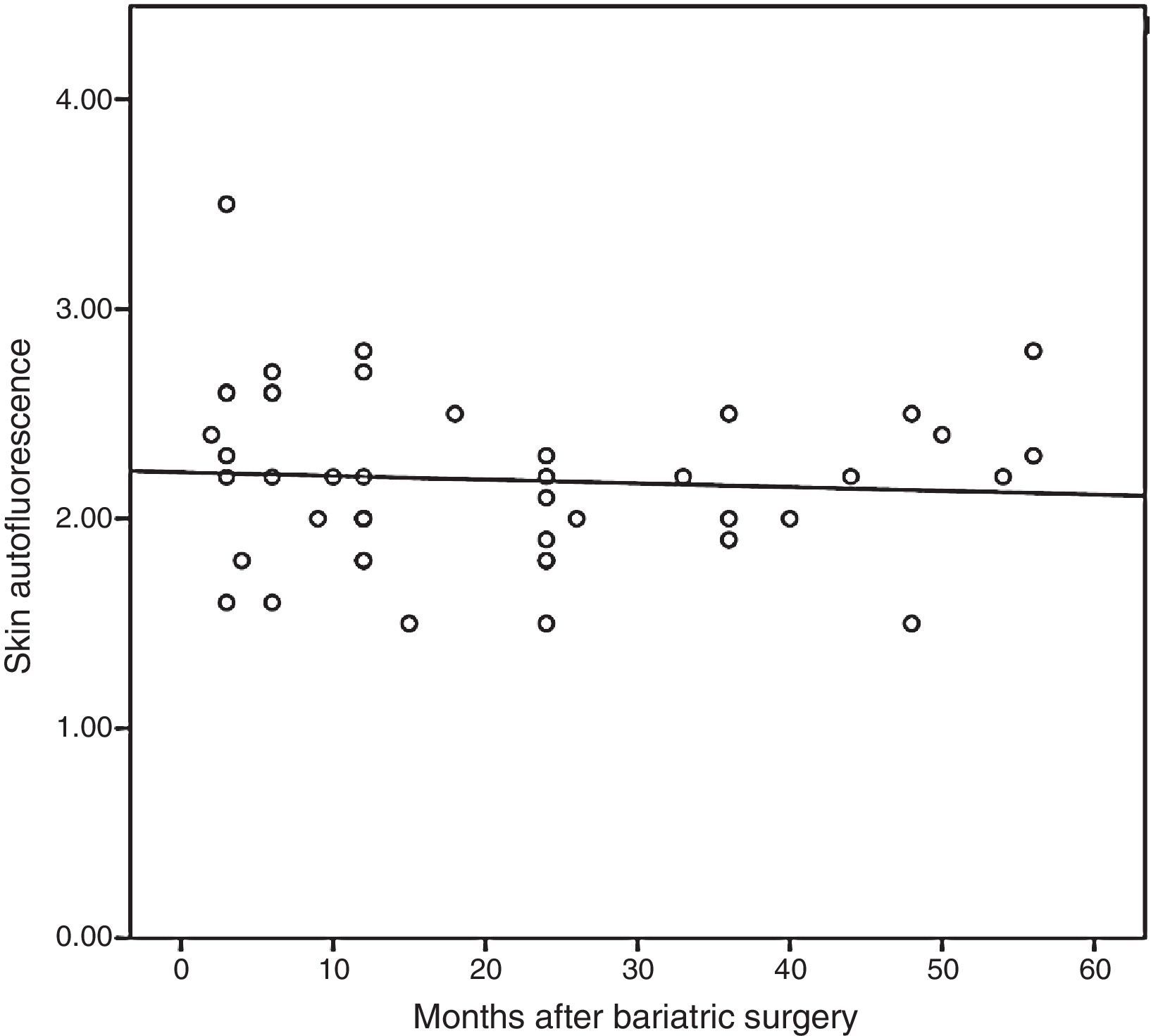
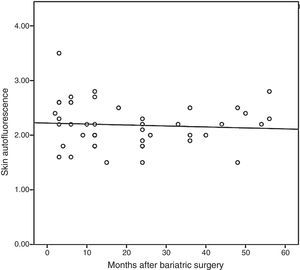
On the other hand, subjects undergoing bariatric surgery also had higher SAF levels than the non-obese population (2.18±0.40 vs. 1.81±0.22AU; p<0.001), but similar to those of patients with morbid obesity (p=0.732). When SAF was measured in this population of patients undergoing bariatric surgery as a function of presence or absence of MS, some difference was still found, but it did not reach statistical significance (2.13±0.37 vs. 2.43±0.48AU; p=0.051). Finally, Fig. 2 shows that SAF does not significantly decrease during follow-up (r=−0.072; p=0634). Thus, comparison of SAF levels of subjects undergoing surgery before and after maximum weight loss showed that the values remained relatively stable (2.32±0.48 vs. 2.11±0.34AU; p=0.099).
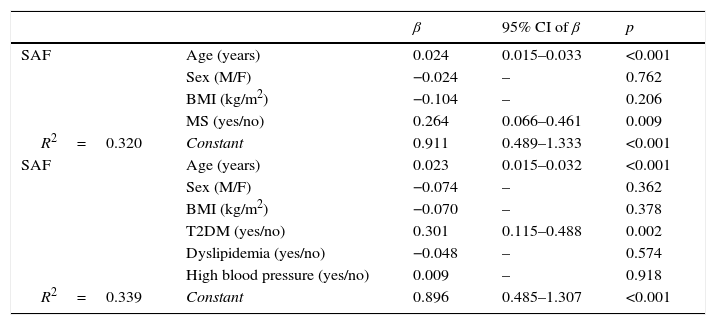
Joint assessment of all study subjects showed no correlation between SAF and BMI (r=0.089; p=0.288), but a positive correlation was found with age (r=0.451; p<0.001), basal fasting blood glucose (r=0.295; p<0.001), and HbA1c level (r=0.496; p<0.001). Finally, the stepwise multiple linear regression analysis (Table 2) showed that age and presence of MS (but not sex or BMI) were independent predictors of SAF (R2=0.320). When MS was replaced by its components, age and presence of T2DM (but not sex, BMI, or presence of high blood pressure or dyslipidemia) where the variables independently predicting for subcutaneous AGE levels. Thus, SAF was significantly higher in patients with T2DM as compared to subjects without diabetes in both the groups with morbid obesity (2.51±0.65 vs. 1.88±0.52AU; p=0.001) and previous surgery (2.50±0.41 vs. 2.09±0.35AU; p=0.004).
Stepwise multiple linear regression analysis of variables associated to skin autofluorescence.
| β | 95% CI of β | p | ||
|---|---|---|---|---|
| SAF | Age (years) | 0.024 | 0.015–0.033 | <0.001 |
| Sex (M/F) | −0.024 | – | 0.762 | |
| BMI (kg/m2) | −0.104 | – | 0.206 | |
| MS (yes/no) | 0.264 | 0.066–0.461 | 0.009 | |
| R2=0.320 | Constant | 0.911 | 0.489–1.333 | <0.001 |
| SAF | Age (years) | 0.023 | 0.015–0.032 | <0.001 |
| Sex (M/F) | −0.074 | – | 0.362 | |
| BMI (kg/m2) | −0.070 | – | 0.378 | |
| T2DM (yes/no) | 0.301 | 0.115–0.488 | 0.002 | |
| Dyslipidemia (yes/no) | −0.048 | – | 0.574 | |
| High blood pressure (yes/no) | 0.009 | – | 0.918 | |
| R2=0.339 | Constant | 0.896 | 0.485–1.307 | <0.001 |
β: standardized partial regression coefficient; T2DM: type 2 diabetes mellitus; M: male; CI: confidence interval; BMI: body mass index; F: female; SAF: skin autofluorescence; MS: metabolic syndrome.
The first study assessing measurement of subcutaneous AGEs in patients with morbid obesity, in whom higher levels were found as compared to non-obese subjects, is reported here. Previous studies have also reported a positive correlation between SAF levels and BMI, but in populations with lower degrees of obesity. Thus, in a recent population study on 9009 subjects from the LifeLines Cohort Study, SAF level was independently associated not only to age and HbA1c, but also to BMI.7 Similarly, BMI was independently associated to SAF in a cohort of 973 subjects with T2DM, together with other parameters such as time since diabetes onset, age, smoking, and HbA1c.2
On the other hand, and confirming our results, higher serum levels of the soluble receptor for AGEs have also been found in subjects with morbid obesity.14 However, our study also demonstrated that in subjects with morbid obesity, increases in AGEs occur in those in whom excess weight leads to the occurrence of metabolic comorbidities. Thus, patients with morbid obesity but no MS had SAF similar to those of non-obese subjects. That is, increase in SAF levels in the group of patients with morbid obesity should be attributed to the metabolic consequences associated to weight increase. The Engelsen et al. study, where SAF was used to assess AGEs as a function of the degree of visceral obesity, supports our results.9 Thus, mean SAF levels were higher in the 816 subjects with central obesity than in the 431 with no central obesity, and even higher in the subgroup of subjects with central obesity and comorbidities as compared to those with no metabolic impact. In this study, a progressive and significant increase was seen in SAF levels from the group with no obesity or risk factors (1.63±0.37AU) to subjects with central obesity only (1.74±0.44AU) and, finally, to those with both central obesity and comorbid conditions (1.87±0.43AU; p<0.001). In this setting, if these findings are confirmed, study of subcutaneous AGEs using SAF could be considered as a useful tool for the study and identification of “metabolically healthy” obese subjects. Although there are clinical criteria for adequate diagnosis of this condition, differentiation of this population requires time and supplemental tests.15–19 Availability of a small, easy to use, reliable, noninvasive tool that gives a result in just over one minute may provide an added value when making clinical and therapeutic decisions for obesity management.
The benefit of the weight loss achieved through bariatric surgery on metabolic comorbidity is widely known.20 However, changes in AGE tissue deposit during this period are unknown, and reports on change over time of the soluble receptor for AGEs are conflicting. On the one hand, Lorenzi et al. studied in 69 patients with morbid obesity undergoing gastric bypass the changes in levels of soluble receptors for AGEs more than one year after surgery and found significant decreases.14 Although this result may be interpreted as a reflection of the metabolic improvement induced by weight reduction, it is in sharp contrast to the finding reported by Brix et al. two years before21 of a significant increase in the levels of soluble receptors for AGEs after bariatric surgery.
Our study is the first to use SAF measurement to assess changes in subcutaneous AGE deposit after bariatric surgery, and has shown that AGE levels do not appear to decrease after a five-year follow-up following surgery. This result suggests that the rate at which protein turnover occurs is a determinant factor for tissue deposition of AGEs. Skin collagen turnover may take up to 14.8 years,22 and it therefore seems logical to think that five years is not a period long enough to achieve a marked decrease in AGE tissue levels. Because of this offset between resolution of MS and normalization of SAF, measurement of AGEs, a helpful parameter to assess cardiovascular risk before surgery,23 loses its clinical validity in the first few years after surgery. A doubt also arises as to whether the rapid metabolic improvement reported after bariatric surgery, even before a significant weight loss is achieved in the case of T2DM, actually results in biological improvement at tissue level too in these first few years.24 Based on our data, it may be suggested that tissue damage is not corrected so fast as laboratory values or blood pressure levels.
Joint analysis of all study patients showed that both age and presence of MS, especially when T2DM is one of its components, independently condition subcutaneous AGE levels.
It should be noted that our study had some limitations. First of all, this was a cross-sectional study, and no causal relationship can therefore be established between presence of MS and AGE levels tested using SAF. Second, the same patients were not studied before and after bariatric surgery. Thus, although we assume that the weight loss achieved through gastrointestinal surgery does not induce a decrease in SAF, there are no studies following up the same patient cohort over time that support this hypothesis. Third, patients enrolled with a few months of follow-up after surgery still show a high prevalence of T2DM, which may contribute to make AGE decrease difficult in this group. Fourth, the characteristics of SAF measurement only allow for evaluating subjects with a clear skin, because the variation in skin reflectance in subjects with a darker skin invalidates reading. Finally, although the populations were age-matched, we have no other variables that should be considered when deposition of AGEs is assessed, such as their long half-life, glomerular filtration rate, smoking, or coffee consumption.7
In conclusion, the increase in subcutaneous AGE levels in the population with morbid obesity and metabolic syndrome occurs at the expense of subjects with T2DM. After surgery, despite the weight loss and resolution of comorbidities it induces, SAF levels remain elevated for at least the first five years. Thus, measurement of subcutaneous AGE levels using SAF may help us differentiate obese subjects who have greater metabolic involvement at baseline, but the procedure would no longer be clinically useful after surgery.
FundingThis study was supported by Instituto de Salud Carlos III (action plan II14//00008). This managing body had no role in study design, data collection, analysis and interpretation, report preparation, or the decision to submit the article for publication.
Conflict of interestThere are no conflicts of interest regarding this article.
We thank the whole multidisciplinary team of the Obesity Unit of Hospital Universitario Arnau de Vilanova, which allowed for the conduct of this study. Our special thanks to J.A. Baena-Fustegueras, M.C. de la Fuente, and S. Ros, who performed the bariatric surgery procedures and many follow-up visits, and to L. Gutiérrez and M. Bueno for data collection.
Please cite this article as: Sánchez E, Baena-Fustegueras JA, de la Fuente MC, Gutiérrez L, Bueno M, Ros S, et al. Productos finales de glicación avanzada en la obesidad mórbida y tras la cirugía bariátrica: cuando la memoria glucémica empieza a fallar. Endocrinol Diabetes Nutr. 2017;64:4–10.