Obesity is correlated with the development of chronic metabolic conditions such as type 2 diabetes mellitus (T2DM), hypertension and hyperlipidaemia. Bariatric surgery in an effective treatment for obese T2DM patients.1 Nowadays, obesity also affects type 1 diabetes mellitus (T1DM) patients. In fact, 13% of youth T1DM patients suffer from obesity and up to half of the patients are either overweight or obese.2 However, evidence about the effect of bariatric surgery on T1DM remains unclear.3
Four recent systematic reviews described data from about one hundred obese T1DM patients treated with bariatric surgery.4–7 These reports confirmed bariatric surgery as a tool for significant weight loss and insulin requirements reduction, although benefits in other glycaemic endpoints were not consistent. Hypoglycaemic episodes have been described after bariatric surgery by most authors. Comorbidity resolution was satisfactory and a reduction in medications needed to treat hypertension and dyslipidaemia was an additional benefit.
The rising incidence of obesity in T1DM patients is related to the use of intensive insulin therapy to maintain tight glycaemic control.8 Continuous subcutaneous insulin infusion (CSII) allows attach tight glycaemic objectives without an increased risk of hypoglycaemia. Therefore, CSII use in routine clinical practice is increasing in the last two decades.9 However, there is a lack of information about the results of bariatric surgery in obese T1DM patients on CSII therapy.
The objective of the present study is to describe the impact of bariatric surgery on glycaemic control, hypoglycaemia, insulin requirements and metabolic outcomes in obese T1DM patients treated with CSII. The initiative came from the Working Group of Diabetes and Technology of the Spanish Diabetes Association. All members of the group were asked to perform a retrospective analysis of T1DM patients on CSII therapy submitted to bariatric surgery from January 2001 to January 2016, with at least 2 years of follow up. The study protocol was approved by each Ethic Institutional Review Board and all patients signed informed consent. T2DM or T1DM patients treated with other insulin regimens different from CSII were excluded. All patients met the Spanish Endocrinology and Nutrition Society criteria for bariatric surgery: (1) body mass index (BMI) ≥40kg/m2, (2) BMI 35–39.9kg/m2 and a serious weight-related health problem consequence of obesity, such as diabetes mellitus, high blood pressure, dyslipidaemia or severe sleep apnoea. The diagnosis of T1DM was verified in all patients by the presence of autoantibodies known to be associated with T1DM (islet cell cytoplasmic antibodies, ICA; antibodies against IA-2 protein, IA2; 65-kDa glutamate decarboxylase, GAD65) and/or absence of C-peptide and/or diabetes ketoacidosis at diabetes diagnosis. Data were analysed from pre and post-surgical (24 months) visits. Glycated haemoglobin A1c (HbA1c) was measured by a method approved by the National Glycohemoglobin Standarization Program. Hypoglycaemia events and severe hypoglycaemia were defined in order to standardized concepts.10 Hypoglycaemia frequency was estimated from self-monitoring blood glucose values during the previous month to each visit. Changes in weight, BMI, total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), systolic blood pressure (SBP), diastolic blood pressure (DBP), improvements in hypertension and hyperlipidaemia were gathered.
Six T1DM obese patients treated with CSII during 9.5±3.1 years (duration of CSII at surgery time 5.0±2.4 years) undergone bariatric surgery in the 30 reviewed hospitals during the analysed period of time. Patients had a male-to-female ratio of 1:5, a mean age of 43.2±11.5 years, a median duration of T1DM of 24.2±4.9 years. Surgical procedures were Roux-en-Y gastric bypass (RYGB) in 3 patients and sleeve gastrectomy in other 3. Total follow-up from surgery time was 4.5±1.4 years.
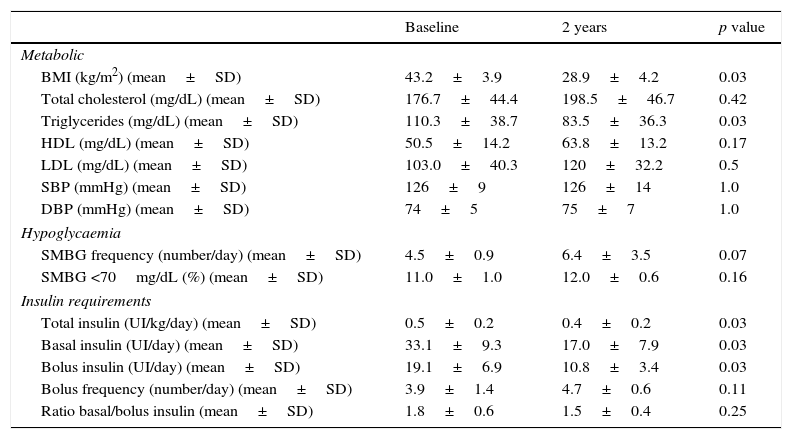
Although we could not detect significant HbA1c improvement after surgery (7.9±0.8% vs. 7.3±0.8%, p=0.08), we found a daily insulin requirement reduction (mean difference in change, −0.10±0.04UI/kg/day; 95% CI, −0.06 to −0.15; p<0.05). Insulin decrease proceeded from both basal rate (mean difference in change −16.1±5.5UI/day; 95% CI, −10.4 to −21.8; p<0.05) and insulin boluses (mean difference in change −8.3±4.8UI/day; 95% CI, −3.3 to −13.3; p<0.05). No differences in the proportion of basal/bolus during the follow-up were detected. Hypoglycaemic frequency and severe hypoglycaemia frequency did not significantly change after surgical procedure. In fact, only one patient experienced one severe hypoglycaemia in the first two years after bariatric surgery. Daily carbohydrate intake showed a reduction at the end of follow up (mean difference in change −53.8±25.2g/day; 95% CI, −27.4 to −80.3; p<0.05).
A significant body weight reduction was observed at the end of the follow-up (mean difference in change, −38.1±13.4kg; 95% CI, −24.1 to −52.2; p<0.05). This weight difference was also representative for a BMI reduction (mean difference in change, −14.2±3.9kg/m2; 95% CI, −10.1 to −18.3; p<0.05). There were also favourable changes in triglycerides following the surgery (mean difference in change, -26.9±15.1mg/dL; 95% CI, −11.0 to −42.7; p<0.05). Cardiometabolic risk factor assessment did not show other benefits. Lipid-lowering medication was discontinued in three of four (75%) patients. Hypertension resolved in one of three patients (33%). Other metabolic and diabetes-related results are shown in Table 1.
Metabolic and diabetes results at baseline and after 2 years follow-up.
| Baseline | 2 years | p value | |
|---|---|---|---|
| Metabolic | |||
| BMI (kg/m2) (mean±SD) | 43.2±3.9 | 28.9±4.2 | 0.03 |
| Total cholesterol (mg/dL) (mean±SD) | 176.7±44.4 | 198.5±46.7 | 0.42 |
| Triglycerides (mg/dL) (mean±SD) | 110.3±38.7 | 83.5±36.3 | 0.03 |
| HDL (mg/dL) (mean±SD) | 50.5±14.2 | 63.8±13.2 | 0.17 |
| LDL (mg/dL) (mean±SD) | 103.0±40.3 | 120±32.2 | 0.5 |
| SBP (mmHg) (mean±SD) | 126±9 | 126±14 | 1.0 |
| DBP (mmHg) (mean±SD) | 74±5 | 75±7 | 1.0 |
| Hypoglycaemia | |||
| SMBG frequency (number/day) (mean±SD) | 4.5±0.9 | 6.4±3.5 | 0.07 |
| SMBG <70mg/dL (%) (mean±SD) | 11.0±1.0 | 12.0±0.6 | 0.16 |
| Insulin requirements | |||
| Total insulin (UI/kg/day) (mean±SD) | 0.5±0.2 | 0.4±0.2 | 0.03 |
| Basal insulin (UI/day) (mean±SD) | 33.1±9.3 | 17.0±7.9 | 0.03 |
| Bolus insulin (UI/day) (mean±SD) | 19.1±6.9 | 10.8±3.4 | 0.03 |
| Bolus frequency (number/day) (mean±SD) | 3.9±1.4 | 4.7±0.6 | 0.11 |
| Ratio basal/bolus insulin (mean±SD) | 1.8±0.6 | 1.5±0.4 | 0.25 |
BMI, body mass index; SD, standard deviation; T1DM, type 1 diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; SMBG, self-monitoring blood glucose.
Limited information is available about bariatric surgery results in T1DM CSII treated patients (five studies reported a total of 22 cases).4–6 All studies described data from mixed intensive insulin regimens (multiple daily injections and CSII). These small cohorts showed significant improvement in weight and insulin requirements, although other glycaemic benefits, such as HbA1c reduction, were not consistent. This was also demonstrated in our study. Improved insulin resistance following weight loss and decreased caloric intake due to the restrictive component of bariatric surgery have been postulated to explain the reduction in insulin requirements. Our patients showed carbohydrate intake and insulin bolus reductions that could explain this hypothesis.
Our study was limited by small sample size and could not be representative for all T1DM patients treated with CSII. Nevertheless, our findings suggest that bariatric surgery induces weight loss but does not improve glycaemic control in T1DM patients on CSII. Otherwise, a negative impact on glucose control has been not observed. Thus, the role of bariatric surgery in such patients will require larger and longer studies.