Children and adolescents with type 1 diabetes mellitus (T1DM) are at high risk for the development of celiac disease (CD) because of the common genetic characteristics of both conditions. The study objectives were to investigate the frequency of the human leukocyte antigen system (HLA) for CD in pediatric T1DM patients and to determine whether HLA testing is suitable for CD screening in that population and is cost-effective as compared to serological screening for CD.
Patients and methodsA retrospective, descriptive study was conducted in 296 patients (148 girls; 148 boys) with T1DM aged <18 years who attended a Madrid hospital. Data on the frequency of genotypes DQ2/DQ8 in a subgroup of 92 patients and the additional cost of performing HLA typing for screening CD were collected. Only when the risk HLA haplotype (DQ2/DQ8) is negative no further serological screening for CD is required.
ResultsTwenty-three patients with T1DM (7.77%) also had CD. Alleles DQ2 or DQ8 were found in 91.3% of patients in whom the HLA haplotype was studied. Thus, only 8.7% with a negative haplotype would have benefited from HLA testing. The additional cost of HLA typing was € 105.2 for each patient with positive DQ2 or DQ8 in our population.
ConclusionsHLA typing is not a cost-effective screening method for CD in T1DM because of the frequent association of T1DM with risk genotypes for CD.
Los niños y adolescentes con diabetes mellitus tipo 1 (DM1) presentan un alto riesgo de desarrollar enfermedad celiaca (EC), ya que ambas entidades tienen características genéticas comunes. Los objetivos son investigar la frecuencia de los genotipos HLA (sistema de histocompatibilidad de antígenos leucocitarios humanos) de EC en pacientes pediátricos con DM1 y establecer si el estudio HLA es un método adecuado para el cribado de EC en esa población y su coste-efectividad comparándolo con la estrategia del cribado serológico de EC.
Pacientes y métodosEstudio retrospectivo y descriptivo de 296 pacientes (148 niñas; 148 niños) menores de 18 años con DM1 en seguimiento en un hospital terciario de Madrid. Se recogen datos de frecuencia de los genotipos DQ2/DQ8 en un subgrupo de 92 pacientes y del coste adicional de realizar HLA para el cribado de EC. Solo cuando el haplotipo HLA de riesgo (DQ2/DQ8) es negativo no es necesario continuar el estudio serológico seriado para EC.
ResultadosVeintitrés pacientes con DM1 (7,77%) fueron diagnosticados de EC. El 91,3% de los pacientes en los que se estudió el haplotipo HLA presentaron los alelos DQ2 o DQ8. En consecuencia, solo un 8,7% con haplotipo negativo (no DQ2 ni DQ8) se habrían beneficiado del estudio HLA para evitar su seguimiento serológico. En nuestra población el coste adicional de realizar el estudio HLA representa un coste sin beneficio de 105,2 €/paciente en cada paciente positivo para DQ2 o DQ8.
ConclusionesEl estudio HLA no es coste-efectivo como método de cribado de EC en la DM1 dada la frecuente asociación de DM1 con los genotipos de riesgo de desarrollar EC.
Type 1 diabetes mellitus (T1DM) is frequently associated with other autoimmune diseases, including celiac disease (CD), an antibody-mediated disease caused by gluten and related prolamines in genetically predisposed individuals which is characterized by the presence of a variable combination of gluten-dependent clinical manifestations, specific antibodies, HLA-DQ2 and HLA-DQ8 haplotypes, and enteropathy.1
CD is six times more frequent in people with T1DM than in the general population, and prevalence of CD in T1DM has been reported to range from 1.6% and 16.4%.2,3 This difference is related to the ethnic origin of the populations, the different prevalence of CD in the different countries, the age groups studied, performance of an active versus a symptom-based screening, and the different criteria used to diagnose CD.
A strong association of CD with the HLA-DQ2 and DQ8 haplotypes is known.1 More than 90% of patients with CD express the class II molecule HLA-DQ2, and the remaining majority are carriers of HLA-DQ8 (8%–10%). From 20% to 30% of healthy population controls are positive for DQ2, and 40% for DQ2 or DQ8, but only 3% of subjects positive for HLA-DQ2 and/or DQ8 develop CD. Thus, a positive DQ2 and/or DQ8 test has little specificity for diagnosis of CD, though the predictive value of a negative result for this haplotype is very high, because in the absence of DQ2/DQ8, CD is extremely unlikely.
HLA-DR3 and DR4 haplotypes are strongly associated with T1DM, and approximately 30%–50% of patients are DR3/DR4 heterozygotes: in the United States 35%, versus 2.4% of the general population.4
From 60% to 70% of patients with T1DM and CD are asymptomatic or have few symptoms.5,6 Children with DM1 and symptoms of CD tend to be diagnosed with CD at earlier ages (under 5 years of age) than those with no symptoms. The risk of CD is higher in patients diagnosed with diabetes at a younger age and during the first 5 years after diagnosis. T1DM usually develops before than CD, and when the opposite occurs, patients are older when T1DM is diagnosed.
Some authors report poorer metabolic control of T1DM and an increased risk of hypoglycemia and retinopathy in patients with undiagnosed associated CD.7 In addition, CD may be responsible for significant clinical manifestations such as iron-deficient anemia, impaired growth, failure to thrive, osteopenia, and liver impairment, with an increased risk of subsequent complications of ulcerative jejunitis and lymphoma.
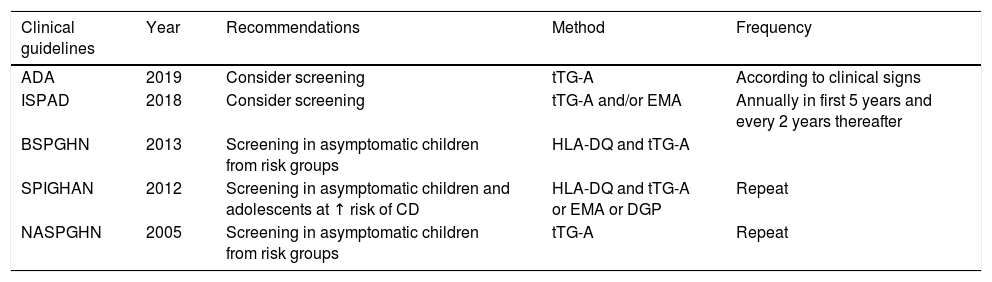
It is recognized that children and adolescents with asymptomatic T1DM should be screened for CD to prevent the adverse effects and associated complications and to maximize growth. Different clinical guidelines or recommendations, not based on adequate evidence, are available for these age groups, and no clinical guidelines are available in adults1,8–11 (Table 1).
Recommendations and clinical guidelines for screening for celiac disease in children and adolescents with T1DM.1,8–11
| Clinical guidelines | Year | Recommendations | Method | Frequency |
|---|---|---|---|---|
| ADA | 2019 | Consider screening | tTG-A | According to clinical signs |
| ISPAD | 2018 | Consider screening | tTG-A and/or EMA | Annually in first 5 years and every 2 years thereafter |
| BSPGHN | 2013 | Screening in asymptomatic children from risk groups | HLA-DQ and tTG-A | |
| SPIGHAN | 2012 | Screening in asymptomatic children and adolescents at ↑ risk of CD | HLA-DQ and tTG-A or EMA or DGP | Repeat |
| NASPGHN | 2005 | Screening in asymptomatic children from risk groups | tTG-A | Repeat |
ADA: American Diabetes Association; BSPGHN: British Society of Pediatric Gastroenterology, Hepatology and Nutrition; DGP: deamidated gladin peptide antibodies; EMA: endomysium antibodies; ESPGHAN: European Society of Pediatric Gastroenterology, Hepatology and Nutrition; ISPAD: International Society for Pediatric and Adolescent Diabetes; NASPGHN: North American Society of Pediatric Gastroenterology, Hepatology and Nutrition; tTG-A: transglutaminase antibodies.
The European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) is the most important society in pediatric gastroenterology. Its guidelines (and those of other societies) propose that HLA typing (human leukocyte antigen histocompatibility system) is performed, provided it is available, in all patients with diseases associated to CD (including T1DM) as the first diagnostic test before starting serological screening.1,10 Study of the HLA haplotype would be helpful to rule out CD, as this is argued to be a cost-effective test, since CD may be ruled out with a 99% certainty in patients with HLA negative for DQ2 or DQ8, and continued serological follow-up would not be required this subgroup. Positive subjects should undergo measurement of serum immunoglobulin IgA and transglutaminase IgA antibodies (tTG-A).
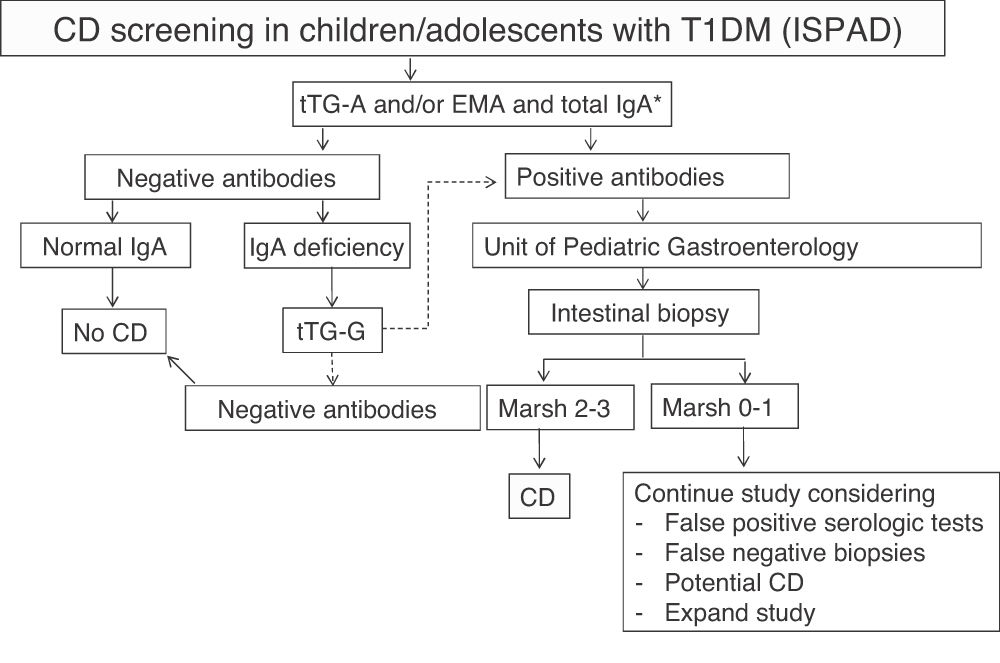
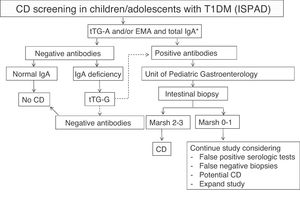
The International Society for Pediatric and Adolescent Diabetes (ISPAD) recommends serological screening for CD in all patients with T1DM at the start of diabetes by measuring tTG-A.9 When IgA deficiency is detected (in the general population it is 1:500, but appears to be more common in T1DM with CD), specific IgG antibodies should be measured. Screening should then be performed annually or every two years, and more frequently if required by the situation or the child has a first-degree relative with CD. According to it, screening should be extended beyond five years after T1DM onset. When antibodies are positive, patients should be referred to the gastroenterologist to confirm diagnosis and discuss diet.
The strategy used for screening has important cost-economic and psychological implications for patients and their families. Absence of the HLA-DQ2 and/or DQ8 haplotypes makes highly unlikely/rules out the diagnosis of CD, and only in those cases continued serological screening is unnecessary. The DR3 and DR4 alleles are however in a strong linkage disequilibrium with DQ2 and DQ8, and most patients with T1DM carry the CD risk haplotypes. Thus, in some series approximately 90% of patients with T1DM express the DQ2 or DQ8 haplotypes, as compared to 40% of the general population.4
The objectives of this study were to ascertain the proportion of patients with non-DQ2 or DQ8 HLA in a sample of children and adolescents with T1DM, and to analyze different strategies for CD screening in T1DM from the clinical and cost-effectiveness viewpoints.
Patients and methodsStudy populationThis was a retrospective, descriptive study that reviewed the clinical histories of 296 patients (148 girls and 148 boys) with T1DM attending the pediatric diabetes unit of Hospital Universitario Ramón y Cajal in Madrid (Spain). The study was carried out with the approval of the ethics committee of our institution.
Inclusion criteriaThe study was limited to children and adolescents with DM1 up to 18 years of age, which is when the transition to adult units is made.
Exclusion criteriaTo date, there are no clinical guidelines for CD screening in adult patients with T1DM, and this population was therefore not included.
Laboratory testsScreening for CD was performed in all cases after screening for serum IgA deficiency and using tTG-A antibodies. The ELISA org A-T-TRANSGLUTAM kit® (Palex Medical S.A.), positive value >16, was used. Identification of the HLA histocompatibility antigens was performed in a subgroup of patients at the immunology laboratory of Hospital Ramón y Cajal using the SSO (oligonucleotide hybridization) typing assay One Lambda® (RSSO 2Q_008_03).
Statistical analysisSPSS 24 software was used. Descriptive data are given as means and standard deviations.
Economic analysisIn our hospital, the cost of HLA study reagents is € 35, while one IgA measurement costs € 1.21 and one tTG-A measurement costs € 2.1. To perform the cost-effectiveness study, the cost per product unit: was considered: IgA € 1.69, tTG-A € 21.97, and HLA-DQ € 114.92.
For the economic evaluation, a cost-effectiveness analysis was performed for the two strategies proposed.
The number of patients performed the HLA study was investigated as an analysis prior to the decision to implement the new clinical guidelines formulated by the ESPGHAN. This study was intended to provide an estimate in line with the current recommendations for CD screening in the pediatric population with T1DM in the first years after diagnosis, and analyzed the costs derived from five years of follow-up.
ResultsAll 296 patients were diagnosed with T1DM between six months and 16 years of age. Mean patient age at diagnosis of T1DM was 6.1 ± 4.1 years, and follow-up time (time since onset) of T1DM to the study conduct was 6.6 ± 4.1 years.
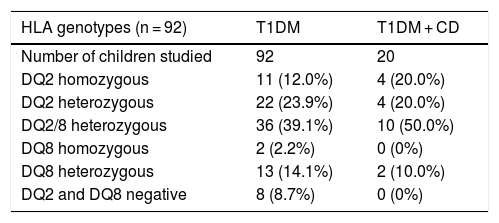
The HLA study was performed in a subgroup of 92 patients, 91.3% of whom had the DQ2 or DQ8 alleles. Therefore, only 8.7% with a negative haplotype (not DQ2 or DQ8) would have benefited from the HLA study to avoid serological follow-up. Table 2 shows the genotypes found.
HLA-DQ genotypes in children and adolescents with T1DM and in patients diagnosed with T1DM and celiac disease (CD).
| HLA genotypes (n = 92) | T1DM | T1DM + CD |
|---|---|---|
| Number of children studied | 92 | 20 |
| DQ2 homozygous | 11 (12.0%) | 4 (20.0%) |
| DQ2 heterozygous | 22 (23.9%) | 4 (20.0%) |
| DQ2/8 heterozygous | 36 (39.1%) | 10 (50.0%) |
| DQ8 homozygous | 2 (2.2%) | 0 (0%) |
| DQ8 heterozygous | 13 (14.1%) | 2 (10.0%) |
| DQ2 and DQ8 negative | 8 (8.7%) | 0 (0%) |
Twenty-three patients (7.77%) were diagnosed with CD, that is, they had both T1DM and CD. Of these, 15 were girls and eight boys (5.41% of boys and 10.14% of girls in our sample). Mean age at diagnosis of DM1 was 4.4 ± 4.4 years, and mean age at diagnosis of CD was 5.1 ± 3.6 years. The HLA study was performed in 20 cases. All patients with CD were positive for DQ2 or DQ8. Mean duration of T1DM at the time of diagnosis of CD was 1.4 ± 2.5 years: 86.9% of children with CD were diagnosed in the first five years after onset of T1DM.
In any case, and although these data are consistent with the scientific literature for the European population, the number of patients included does not allow for extrapolating data from our sample to the population without confirming these conclusions with a larger sample.
Study of costs of reagentPerformance of the HLA study on the sample analyzed (n = 92) represented a cost in reagents for five years of € 3220, and the cost in our overall population would have been € 10,360. The cost of serological follow-up in the 296 patients during the first five years after diagnosis would be € 3466.16 (a single IgA measurement/patient + an annual antibody measurement/patient = € 11.71/patient). IgA deficiency was shown in one patient.
The HLA study would have been useful for avoiding serological follow-up in only 8.7% of the patients (25/296), resulting in a reagent cost of € 875. If studied in all 296 children, the cost would have been € 10,360. Therefore, € 9485 (€ 32 per patient) would have been spent with no benefit.
Cost-effectiveness studyThe data are more robust when direct costs are considered.
In our center, serological screening for CD was performed according to ISPAD recommendations9 (Fig. 1). The cost of serological monitoring in each patient during the first five years after diagnosis was € 111.54/patient: one IgA measurement € 1.69 + tTG-A testing (annually, € 21.97 × 5 years) € 109.85.
ISPAD recommendations for the screening of celiac disease (CD) in children and adolescents with type 1 diabetes mellitus (T1DM).
EMA: endomysium IgA antibodies; tTG-A: transglutaminase IgA antibodies; tTG-G: transglutaminase IgG antibodies.
*In case of selective IgA deficiency, test for IgG class antibodies.
Source: adapted from Mahmud et al.9
The cost of serological monitoring during the first five years after diagnosis in our population of 296 patients was € 33,015.8 (€ 111.54/patient). With an established frequency of patients with negative DQ2 or DQ8 of 8.7% (cases in which the HLA study is useful for avoiding serological monitoring), thus excluding monitoring of 25 cases (€ 33,015.8 ↓ € 2788.5), the total cost of serological screening would have decreased to € 30,227.3.
The cost of an HLA study is € 114.92 per patient. The total cost of the HLA study in the sample analyzed (n = 92) was € 10,572.64. Performance of this test would only have been useful in 8.7% of patients with non-DQ2 or DQ8 HLA, with an extrapolated cost in the sample of 296 patients of € 2873.
Thus, in our population (n = 296), the additional cost of conducting the HLA study would be € 31,143.32 (€ 34,016.32 ↓ € 2873), which represents a cost without benefit of € 105.2/patient in each patient positive for DQ2 or DQ8.
DiscussionIt is currently accepted that screening for CD should be performed in asymptomatic patients with T1DM, but there is no agreement as to which method should be used or when it should be started, or how often should serological monitoring be performed, and what would be the benefit of a gluten-free diet in reducing comorbidities.
In recent years, published studies have reported data suggesting that the HLA test is not cost-effective for CD screening in children and adolescents with T1DM, and that the pediatric gastroenterology guidelines, despite their dissemination, are not being used in clinical practice.12–16
It should be noted that, according to the results published in Dutch, Scottish and Austrian populations, only a small proportion of patients with T1DM could be excluded from continued serological screening for CD after the HLA test. In those studies, 86%, 94%, 92%, and 82.8% of children and adolescents with T1DM, respectively, had an HLA-DQ2 or DQ8 haplotype.12–15
For this reason, before final implementation of the new guidelines for CD screening in T1DM, we investigated the proportion of patients in our population of 296 children and adolescents with T1DM in whom haplotype DQ2 or DQ8 had been studied. HLA typing had been performed in 92 of 296 cases. The DQ2 or DQ8 alleles were found in 91.3% of patients (these are data in the population of Spanish origin, as there were very few cases from other ethnic groups). This result is explained by the linkage disequilibrium between HLA genotypes specific for T1DM and HLA genotypes specific for CD.4 Only 8 out of 92 patients in our sample (8.7%) with a negative HLA-DQ haplotype would have benefited from the test.
In addition, no clinical benefit of HLA testing was found in patients with T1DM, since serological screening would have to be continued in most cases, and performance of HLA testing in the remaining population was questionable.
Cost-effectiveness of CD screening in T1DM in the pediatric age using two diagnostic strategies, serum IgA and tTG-A measurements, on the on the side, and HLA testing, was analyzed in our study. tTG-As were used as marker for serological screening because identification of the tissue transglutaminase enzyme (tTG) as the main autoantigen of endomysium antibodies makes it preferable to use it as screening method, as it can be detected by the enzyme-linked immunoassay (ELISA), which is standardized, cheaper, and more ecological than the indirect immunofluorescence used for detection of endomysium antibodies.
Of course, costs may be different in each country or healthcare system. In our center, the cost of serological monitoring per patient during the first five years after diagnosis is € 111.54, and the cost of the HLA test is € 114.92 per patient. Analysis of the costs of performing in the sample analyzed the HLA study, which would have been useful in only 8.7% of the cases, showed that the result was an increase in the cost without benefit per patient of € 105.2. It was then decided not to continue HLA testing in children and adolescents with T1DM.
Studies in the Netherlands, Scotland, and Austria also suggest that the cost of HLA genotyping is high when compared to serological screening.12–15 In small hospitals, HLA testing is not available and samples have to be sent to a specialized laboratory, which results in a greater increase in costs. The cost could be decreased if a method that only detected the presence of DQ2 and/or DQ8 was used.
In addition, the informed consent of the patient and/or parents is required for genetic testing. The potential added psychological burden of performing a study that will not be useful in most patients should also be considered.
Our study shows that HLA genotyping in patients with T1DM is not sufficient to identify patients at risk of developing CD. The sample includes 23 patients with T1DM and CD, of which 20 had undergone HLA testing and all had the DQ2 or DQ8 risk haplotypes. This shows that HLA testing is useful in patients with symptomatic CD or in those diagnosed by screening, but not as a population-based screening method. In any case, diagnosis of CD in patients with T1DM should be confirmed by intestinal biopsy, and the HLA test does not prevent this procedure.1
It is controversial and debatable for how many years should screening be performed. Since most cases of CD are diagnosed in the first five years of T1DM, screening should be performed at diagnosis of T1DM and 2–5 years later, as proposed by Pham-Short et al.2 in their systematic review, and should be considered at other times if there are symptoms suggestive of CD. However, seroconversion of CD antibodies may occur at any time after diagnosis of T1DM, and for other authors this would make it advisable to continue screening after five years since diagnosis of diabetes.17
In conclusion, it is not cost-effective to test HLA as a screening method for CD in T1DM, given the frequent association of T1DM and CD with CD risk haplotypes, which would make it mandatory to continue serological screening.
FundingThis investigation received no specific financial support from public agencies, the commercial sector or non-profit organizations.
Conflicts of interestThe authors state that they have no conflicts of interest.
To Doctors J.L. Castañer Alabau and G. Roy Ariño, of the Histocompatibility and Autoimmunity Sections of the Department of Immunology, Hospital Universitario Ramón y Cajal, for typing of the HLA haplotype and serological tests in patients with type 1 diabetes.
Please cite this article as: Roldán Martín MB, Márquez Romero C, Guerra Vilches E, Ruiz Usabiaga J, Barrio Castellanos R, Martín Frías M, et al. Cribado de enfermedad celiaca en niños y adolescentes con diabetes mellitus tipo 1: ¿qué estrategia utilizar? Endocrinol Diabetes Nutr. 2021;68:153–158.