In 2010, the Working Group on Consensus and Clinical Guidelines of the Spanish Society of Diabetes (SED) published a consensus on drug treatment in type 2 diabetes mellitus (T2DM) which was approved by different scientific bodies.1 The Working Group thought that it would be useful to publish a consensus document specifically addressed to insulin therapy in T2DM.
This document is an executive summary of a more comprehensive document approved by the boards of the SED and the Spanish Society of Endocrinology and Nutrition. The complete version may be found on the websites of both societies (http://www.seen.es;http://www.sediabetes.org) and on those of all the other scientific bodies which adhere to the consensus.
Criteria for insulin therapy start and withdrawalWhen and how should insulin therapy be givenStart of insulin therapyInsulin therapy may be started at diagnosis or during follow-up.2
- –
At the start of the disease, if there is weight loss, severe ketonuria, or cardinal symptoms of diabetes, especially with HbA1c>9%.
- –
During follow-up, transient or permanent insulin therapy may be required.
There are several options for starting insulin therapy:
- –
Basal insulin. One or two doses of NPH insulin, or one dose of a basal analogue: glargine, detemir, and degludec.
- –
Prandial insulin. Three doses of rapid-acting insulin or an ultra-rapid analogue before meals.
- –
Insulin mixtures. Two or more doses of fixed mixtures of rapid-acting or ultra-rapid insulin with intermediate-acting insulin.
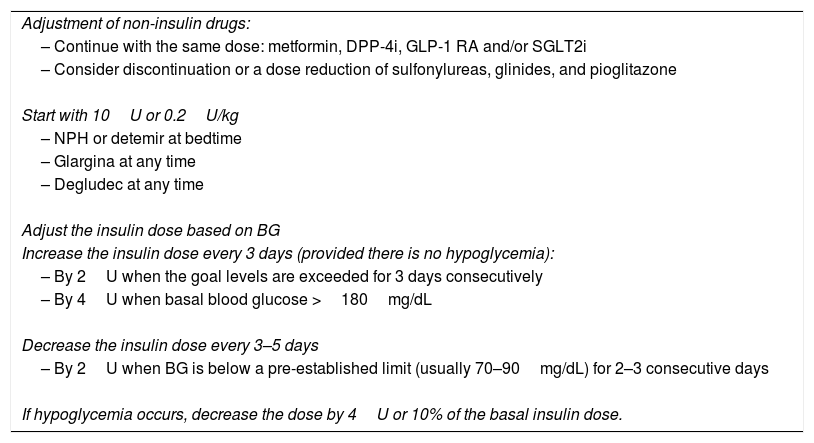
The first option is currently the regimen of choice.3–5 In asymptomatic patients, a dose of 10U or 0.2U/kg in obese patients of basal insulin at bedtime may be used (Table 1). The dose is adjusted based on basal blood glucose level every three days (Table 1). Duly trained patients may also perform titration.
Basal insulin therapy.
| Adjustment of non-insulin drugs: |
| – Continue with the same dose: metformin, DPP-4i, GLP-1 RA and/or SGLT2i |
| – Consider discontinuation or a dose reduction of sulfonylureas, glinides, and pioglitazone |
| Start with 10U or 0.2U/kg |
| – NPH or detemir at bedtime |
| – Glargina at any time |
| – Degludec at any time |
| Adjust the insulin dose based on BG |
| Increase the insulin dose every 3 days (provided there is no hypoglycemia): |
| – By 2U when the goal levels are exceeded for 3 days consecutively |
| – By 4U when basal blood glucose >180mg/dL |
| Decrease the insulin dose every 3–5 days |
| – By 2U when BG is below a pre-established limit (usually 70–90mg/dL) for 2–3 consecutive days |
| If hypoglycemia occurs, decrease the dose by 4U or 10% of the basal insulin dose. |
BG: basal blood glucose level.
If glucose goals are not achieved with basal insulins at doses higher than 0.5U/kg in 3–4months, treatment intensification should be considered.
In highly symptomatic patients with marked basal hyperglycemia (>280–300mg/dL) or ketonuria, two doses of basal insulin, a biphasic regimen, or basal/bolus treatment may be required.
Discontinuation of insulin therapy in patients with T2DMIn some patients, insulin may be discontinued and/or replaced by other antidiabetic drugs. Some variables may predict the efficacy of replacing insulin by other antidiabetic drugs: a) insulin therapy during hospital admission or concomitant disease with adequate prior blood glucose control with antidiabetic drugs other than insulin; b) insulin therapy from the start with sustained adequate control; c) adequate control with not too high doses (<0.5U/kg/day) and diabetes duration <10years; and d) patients undergoing bariatric surgery.
Reduction should be gradual, e.g. 4U per week. Finally, the decision should be agreed with the patient. If hyperglycemic decompensation occurs, insulin should be restarted. Insulin may be required again in the event of concomitant disease.
Insulin therapy regimensBasal insulinTable 2 shows the insulins available. Basal insulins try to simulate the basal pattern to maintain patients close to normal fasting blood glucose levels.
Classification and presentation of insulins in Spain (December 2017).
| Product | Trade name | How supplied |
|---|---|---|
| Ultra-rapid-acting insulins (onset of action: <15min; usual duration: 3–5h) | ||
| Lispro | Humalog® KwikPen® | Five 3-mL prefilled pens |
| Humalog® vial | 10-mL vial | |
| Humalog® 200 KwikPen® | Five 3-mL prefilled pens | |
| Aspart | NovoRapid® FlexPen | Five 3-mL prefilled pens |
| NovoRapid® vial | 10-mLvial | |
| NovoRapid® Penfill® | Five 3-mL cartridges | |
| NovoRapid® PumpCart® | Five 1.6-mL cartridges | |
| Glulisine | Apidra® SoloStar® | Five 3-mL prefilled pens |
| Apidra JuniorStar cartridge® | Five 3-mL cartridges | |
| Apidra® solution for injection | 10-mL vial | |
| Rapid-acting insulins (onset of action: 30min; usual duration: 6–8h) | ||
| Regular | Actrapid® InnoLet® | Five 3-mL prefilled pens |
| Actrapid® vial | 10-mL vial | |
| Humulin regular® | 10-mL vial | |
| Intermediate-action insulins (onset of action: 1h; usual duration 12–18h) | ||
| NPH | Insulatard® FlexPen® | Five 3-mL prefilled pens |
| Insulatard® vial | 10-mL vial | |
| Humulina® NPH KwikPen® | Six 3-mL prefilled pens | |
| Humulina® NPH vial | 10-mL vial | |
| Insulin detemir | Levemir® FlexPen® | Five 3-mL prefilled pens |
| Levemir® InnoLet® | Five 3-mL prefilled pens | |
| Long-acting insulins (onset of action: 1h; usual duration ≥22–26h) | ||
| Glargine | Lantus® SoloStar® | Five 3-mL prefilled pens |
| Lantus® JuniorStar cartridges® | Five 3-mL prefilled pens | |
| Lantus® vial | 10-mL vial | |
| Glargine U-300 | Toujeo® SoloStar® | Three 1.5-mL prefilled pens |
| Glargine (biosimilar) | Abasaglar® KwikPen | Five 3-mL prefilled pens |
| Degludec | Tresiba® Flex Touch® | Five 3-mL prefilled pens |
| All insulins are supplied at concentrations of 100U/mL except for Humalog KiwkPen 200U, with a concentration of 200U/mL, and Toujeo Solostar, with a concentration of 300U/mL. Standard insulin syringes are prepared for concentrations of 100U/mL. Thus, drawing insulin from these pens would multiply the dose by three or by two, with the resultant risk of hypoglycemia. | ||
| Insulin Lantus 100U/mL and Abasaglar (biosimilar) are not exchangeable. They should be prescribed by brand name. | ||
| Premixed combinations | ||
| Regular 30%+NPH 70% | Humulina® 30:70 KwikPen® | Six 3-mL prefilled pens |
| Mixtard® 30 InnoLet® | Five 3-mL prefilled pens | |
| Humulin® 30:70 vial | 10-mL vial | |
| Mixtard® 30 vial | 10-mL vial | |
| Lispro 25%+ | ||
| Lispro protamine 75% | Humalog® Mix 25™ KiwkPen® | Five 3-mL pens |
| Lispro 50%+ | ||
| Lispro protamine 50% | Humalog® Mix 50™ KwikPen® | Five 3-mL prefilled pens |
| Aspart 30%+ | ||
| Aspart-protamine 70% | NovoMix® 30 FlexPen® | Five 3-mL prefilled pens |
| Aspart 50%+ | ||
| Aspart-protamine 50% | NovoMix® 50 FlexPen® | Five 3-mL prefilled pens |
| Aspart 70%+ | ||
| Aspart-protamine 30% | NovoMix® 70 FlexPen® | Five 3-mL prefilled pens |
- –
NPH insulin. The peak action of NPH insulin occurs at 4–6h, and its effective duration of action is 12h. It may be administered as one or two doses, combined with oral drugs. It may be used in pregnancy.
- –
Insulin detemir is a soluble insulin analogue. The duration of action is dose-dependent: 12h for doses of 0.2U/kg and 20h for doses of 0.4U/kg. In one third of patients, two doses are required to cover the 24h period.
- –
Insulin glargine U-100 is an analogue with a slower onset of action than NPH and a smoother action profile, with no peaks and action lasting up to 18–24h. It should be administered once daily, at the same time every day.
- –
Biosimilar insulin glargine. The summaries of the product characteristics of glargine U-100 and biosimilar glargine 100U/mL are almost superimposable.
- –
Insulin glargine U-300. Glargine formulation with a concentration of 300U/mL. It has a flatter and longer pharmacodynamic and pharmacokinetic profile than glargine U-100 and is associated with less risk of hypoglycemia6–8 and similar HbA1c reductions in patients with T2DM.9 In clinical trials, basal doses 10–18% higher were needed on average as compared to glargine U-300.
- –
Insulin degludec is an analogue with a duration of action longer than 42h with an intrapatient variability four times lower as compared to glargine U-100, with the same efficacy but less nocturnal hypoglycemia. Insulin degludec should be administered daily and allows for highly flexible administration, with dosing intervals ranging from 8 to 40h. It is not reimbursed in Spain as a starting insulin.
All basal insulins have the same efficacy,10,11 but there are differences regarding the risk of hypoglycemia, especially nocturnal hypoglycemia. Any choice must be based on the patient profile in terms of safety and the cost of the treatment.
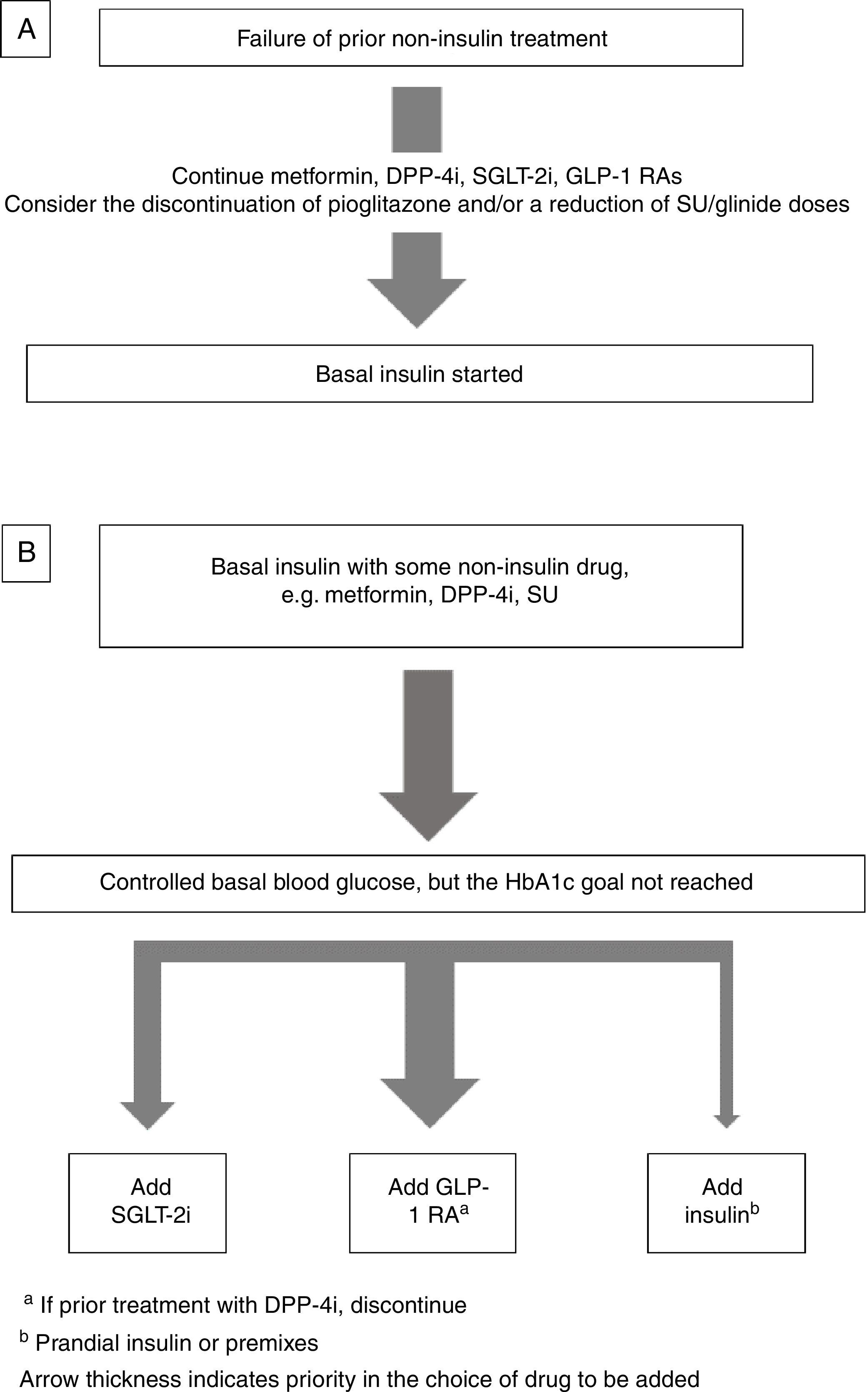
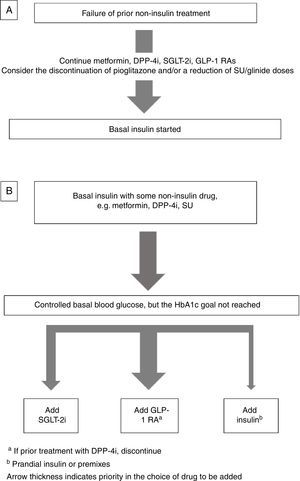
Insulin combined with other drugsBasal insulin therapy is usually the treatment of choice after drugs other than insulin have failed.4,3 As regards drugs that may be used in combination with basal insulin, there are two options (Fig. 1).
If the patient is receiving metformin, DPP-4 inhibitors (DPP-4i), GLP-1 receptor agonists GLP-1 RAs), and/or SGLT-2 inhibitors (SGLT-2i), these should be continued, and the discontinuation of pioglitazone should be considered. If sulfonylureas (SUs) are discontinued, an initial blood glucose impairment may occur. If SUs are continued, a reduction in the dose is advised because of the risk of hypoglycemia3 (Fig. 1A).
Intensification of basal insulin. Which non-insulin drugs should be used?Usually, patients are already receiving an oral antidiabetic, generally metformin with or without SUs or DPP-4i. The most recent guidelines4,3 recommend the addition of a GLP-1 RA or SGLT-2i as an alternative to the addition of new insulin injections.
Basal-plus insulin regimenThis consists of the progressive addition of doses of prandial insulin (or analogue), starting with the meal with the greatest impact on postprandial blood glucose, while maintaining basal insulin. Non-insulin drugs may be continued, but SU discontinuation is preferred because of the increased risk of hypoglycemia.12–14
Before prandial insulin is started, the basal glucose goal should have been achieved. Subsequently, if HbA1c is elevated, rapid-acting insulin (or analogue) should be added.
As regards the starting dose, Appendix A, Table 3 (Supplementary material) shows four options.12–14 The doses should subsequently be adjusted (usually every one or two weeks) until an individualized postprandial blood glucose goal is achieved. Appendix A, Table 4 (Supplementary material) shows several possibilities.
Once the postprandial goal is achieved, if the goal HbA1c has not been reached, an additional dose of prandial insulin should be given at another meal.
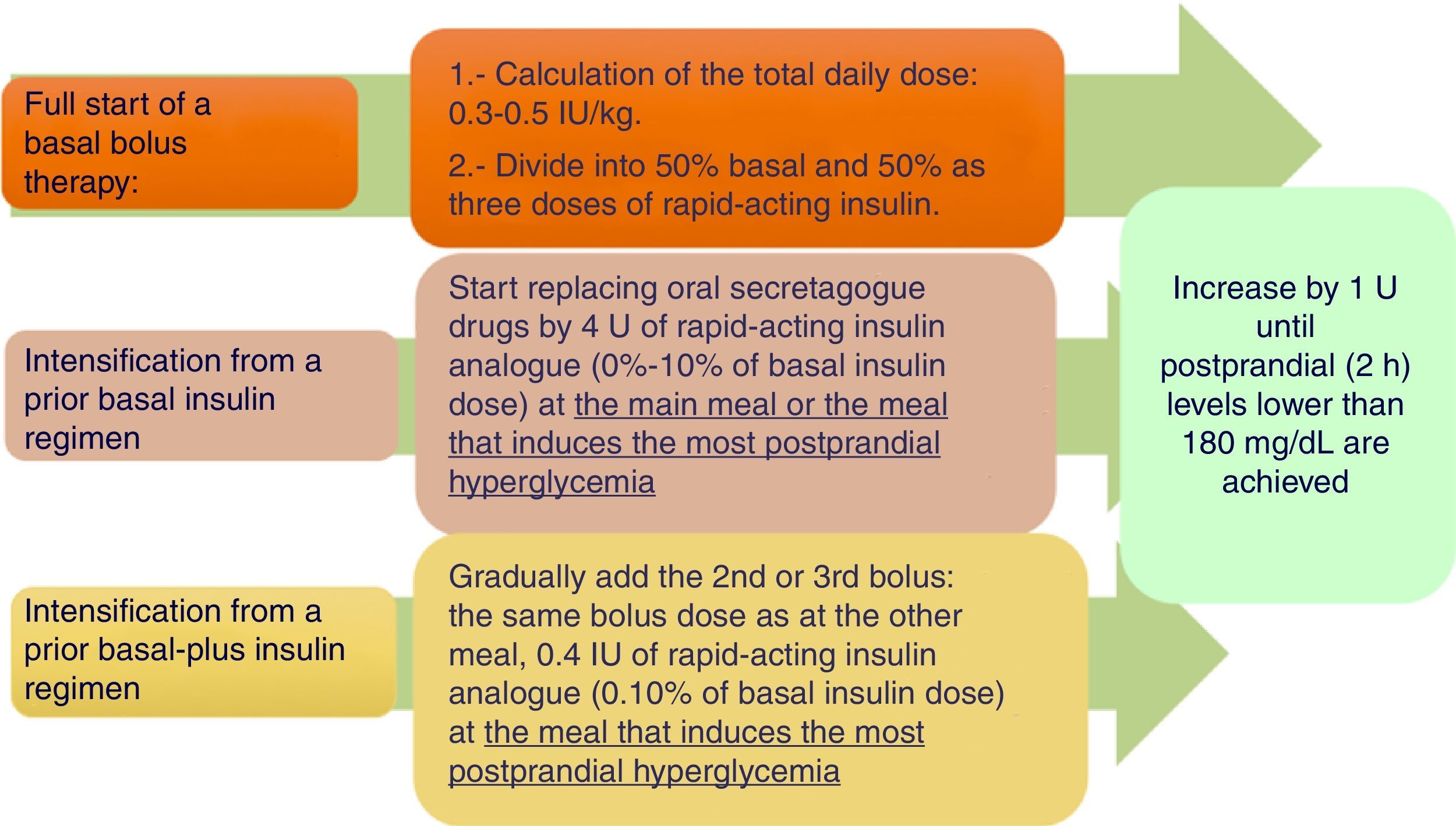
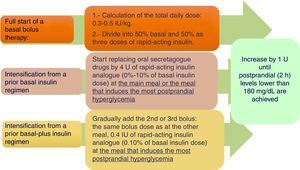
Basal-bolus insulin regimenWhen treatment with basal insulin or premixed insulins does not achieve the control goal in T2DM, the clinical practice guidelines recommend the addition of rapid-acting insulin before meals.4 Meta-analyses have not confirmed a greater hypoglycemic potency, but have reported a lower hypoglycemia rate as compared to other regimens.15–17Fig. 2 shows a practical algorithm for regimen start and adjustment.
Practical recommendations:
Bolus dose calculation. Different systems have been proposed (Appendix A, Table 3 of Supplementary material). Our working group considers the first two to be the most prudent options.15
Start in patients using basal/NPH insulin. A switch to a basal analogue and a 20% reduction of the previous dose of slow-acting insulin are advised if control was close to the goal or a high risk of hypoglycemia exists.18
Start in patients using premixed insulin. Administer 50% as a slow-acting insulin and the other 50% as three injections of rapid-acting insulin before the main meals. Depending on prior control, a 20% reduction of the total dose may be advisable.18
Recommendations for insulin adjustment. Attempts should be made to control preprandial blood glucose with basal/slow-acting insulins first, and then to control post-prandial blood glucose with rapid-acting insulin. Different systems have been proposed (Appendix A, Table 4 of Supplementary material).14
Nutritional plan at insulin start. If a basal-plus or basal-bolus insulin regimen is used, diet may be more flexible in terms of times. The time of bolus injection may be adapted to the actual time of the start of intake, and the rapid-acting insulin dose may also be adapted to the composition of each meal.15
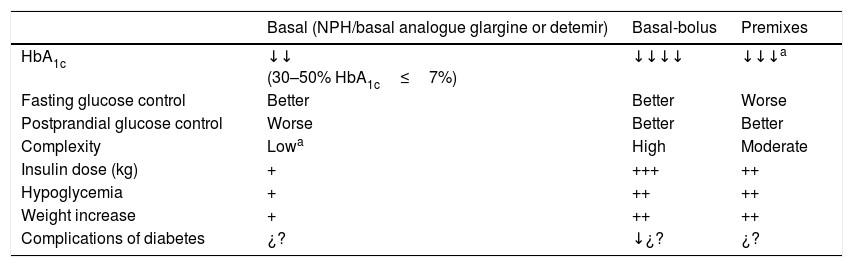
Premixed insulinsIntroductionPremixed insulins are a fixed combination of an intermediate-acting insulin with regular insulin or with a rapid-acting analogue.19,20 They are usually administered twice daily, before breakfast and supper, but may be administered at the three main meals. They may be considered in patients with high previous HbA1c levels (≥9%) or in those whose basal insulin dose is already optimized and prandial control is required.21,22Table 5 shows the characteristics of premixed insulins as compared to basal insulin alone and to the basal/bolus regimen.
Characteristics of insulin therapy using premixes as compared to basal insulin alone and basal-bolus insulin regimens.
| Basal (NPH/basal analogue glargine or detemir) | Basal-bolus | Premixes | |
|---|---|---|---|
| HbA1c | ↓↓ (30–50% HbA1c≤7%) | ↓↓↓↓ | ↓↓↓a |
| Fasting glucose control | Better | Better | Worse |
| Postprandial glucose control | Worse | Better | Better |
| Complexity | Lowa | High | Moderate |
| Insulin dose (kg) | + | +++ | ++ |
| Hypoglycemia | + | ++ | ++ |
| Weight increase | + | ++ | ++ |
| Complications of diabetes | ¿? | ↓¿? | ¿? |
One of the most commonly used strategies for starting insulinization with premixed insulins is the use of the premix at 25% or 30%, administered before breakfast and supper. A 10U dose may initially be given after breakfast and supper, or a dose calculated on the basis of weight (0.3U/kg/day) may be given as divided doses (50–60% before breakfast and 40–50% before supper.
At least three daily measurements of capillary blood glucose are recommended. Profiles are also recommended at six random time points. Appendix A, Table 6 (Supplementary material) shows a dose adjustment procedure.21
Change in insulin regimenDose change between basal insulinsFrom NPH to glargine (original or biosimilar).
If daily injections are given, 100% of the NPH dose should be replaced and then adjusted.
If changing from two NPH injections to one dose of glargine, there should be a reduction of 20–30%.23–25
From NPH to detemir.
The same dose should be maintained.
Insulins or basal analogues to degludec.
When basal insulin is administered once daily, it may be done unit by unit. If changing from basal insulin administered twice daily, a 20% dose reduction is necessary.26
Insulin glargine 100U/mL (original or biosimilar) to glargine 300U/mL and vice versa.
We recommend doing this unit by unit, but 10–18% higher doses of glargine 300U/mL may subsequently become necessary.25 When changing from glargine 300U/mL to glargine 100U/mL, the dose should be reduced by approximately 20%.
Change from other basal insulins to glargine 300U/mL.
This should be done unit by unit if the basal insulin to be replaced by glargine 300U/mL was previously administered as a daily dose. If the basal insulin was administered as two or more doses, the initial dose of glargine 300U/mL should be 80% of the prior basal insulin dose.
Change from a dose of basal insulin to two doses of premixed insulinsThe total dose will usually be maintained. Two thirds of the dose should be given at the previous injection time, and the other third at 12h. The dose should gradually be uptitrated based on the results of self-testing. If hypoglycemia is suspected, the total insulin dose should be reduced by 10–20%.
Change from two doses of premix to a basal/bolus regimen with glargine, detemir or degludecThe previous total dose of the NPH fraction of the mixture, which will be administered as a slow-acting analogue, should be reduced by 20–30%. The dose of rapid-acting or ultra-rapid-acting insulin should be divided between the three meals, and subsequently adjusted.
Change from basal insulin to a basal-bolus insulin regimenThe basal-plus regimen is usually started first, or prandial insulin is added in all three main meals.25,26
Specific clinical situationsInsulin therapy in patients with T2DM and chronic kidney diseaseThe risk of severe hypoglycemia is two times higher in chronic kidney disease.27–31 Less intensive blood glucose monitoring is usually recommended, unless drugs with no risk of hypoglycemia are used.22
The insulin therapy regimen should be adapted to control goals. The use of basal and rapid-acting analogues is generally recommended.
Insulin therapy in glucocorticoid-induced hyperglycemiaThe characteristic blood glucose profile shows a predominance of postprandial over preprandial glucose and of evening over morning glucose (especially with steroids of intermediate half-life given at a single dose with breakfast).32–35
Insulin is usually the treatment of choice for reasons of efficacy and safety. In patients on single morning doses of intermediate-acting glucocorticoids and with no prior insulin treatment, NPH insulin or a biphasic regimen before breakfast may be started, while maintaining non-insulin antidiabetic drugs (Appendix A, Table 7 of Supplementary material).32
Insulin reductions proportional to corticosteroid dose reduction are generally recommended. Appendix A, Table 8 of the supplementary material summarizes the recommendations for patients previously treated with insulin.
Diabetes in patients with terminal cancerOne of the priority objectives in these patients is to avoid hypoglycemia. If insulin therapy is decided upon, single doses of basal insulin, preferably a long-acting analogue, are recommended. If corticosteroids are used, basal insulin may not be sufficient.
Barriers to insulin therapy in T2DMBarriers may be found in patients or their relatives, in health care professionals, or within the health care system, itself.36 They should be addressed and actions taken to overcome them or mitigate their effects.
Therapeutic education associated with insulin therapyThe goals of therapeutic education in diabetes (TED) are as follows4:
- a.
To be aware of any fears regarding treatment.
- b.
To decide upon the appropriate time to start training.
- c.
To know the type of insulin to be administered.
- d.
To agree on the blood glucose control goals.
- e.
To learn the injection technique, administration site and absorption, rotation, injection materials, insulin storage, etc.
- f.
To educate in the prevention and treatment of mild and severe hypoglycemia.
- g.
To manage the ratio of insulin to carbohydrates, physical activity, profiles, special days, and concomitant diseases.
Educational levels should be taken into consideration: survival, basic, and advanced. Education needs to be individualized:
- •
If basal insulin or premixed insulin twice daily is used, a survival educational programme may be sufficient, but if a greater demand is expected, basic TED may be needed.
- •
If a basal-plus insulin regimen is used, a survival programme will be necessary, and the benefits of basic TED should be taken into consideration.
- •
If a basal insulin plus rapid-acting insulin regimen is used, a survival programme will be required, the benefits of basic TED should be taken into consideration, and if a greater demand is expected, advanced TED may be needed.
Please cite this article as: Girbés Borrás J, Escalada San Martín J, Mata Cases M, Gomez-Peralta F, Artola Menéndez S, Fernández García D, et al. Consenso sobre tratamiento con insulina en la diabetes tipo 2. Endocrinol Diabetes Nutr. 2018;65:1–8.
The complete Consensus Document is available at: http://www.seen.es and http://www.sediabetes.org, and as additional material in the Journal web page.