Patients with chronic kidney disease (CKD) and diabetes mellitus (DM) have high cardiovascular risk. Both conditions are related to systemic atherosclerosis and vascular calcification. The prevalence and severity of coronary artery calcification (CaC) is higher in patients with DM, regardless of their renal function. Data about the long-term prognostic role of CaC in diabetic patients with CKD are scarce.
Material and methodsWe carried out a prospective longitudinal study enrolling 137 patients with advanced CKD. A non-enhanced multislice coronary computed tomography (CT) was performed at baseline. CaC was assessed using Agatston method. Patients were stratified according to their CaC score: severe calcification group (CaCs≥400HU) and mild-moderate calcification group (CaCs<400HU).
ResultsThe median follow-up time was 87.5 months. DM was found in 28% of subjects. The patients with DM showed more severe CaC, lower albumin and higher C-reactive protein serum levels. Serum albumin was correlated with severe CaC (r=−0.45, p=0.009). Overall mortality rate reached 58%. Patients with DM also tended to have higher mortality compared to non-diabetic subjects (X2 3.51, p=0.061) especially those with severe CaC showed higher mortality than those with severe CaC without DM (93% vs.73%, p=0.04).
ConclusionsPatients with advanced CKD and DM have more severe CaC, increased inflammation-malnutrition data and higher mortality compared to those without DM.
Los pacientes con enfermedad renal crónica (ERC) y diabetes mellitus (DM) tienen un elevado riesgo cardiovascular. Ambas enfermedades se relacionan con el desarrollo de ateroesclerosis sistémica y calcificación vascular. La prevalencia y la severidad de la calcificación arterial coronaria (CaC) es mayor en personas con DM, independientemente de su función renal. Los datos acerca del papel pronóstico a largo plazo de la CaC en pacientes con DM y ERC son escasos.
Material y métodosSe diseñó un estudio prospectivo que incluía a 137 pacientes (85 en hemodiálisis y 52 con ERC avanzada). Se realizó una tomografía computerizada (TC) helicoidal multicorte coronario basal. La CaC se cuantificó mediante el método de Agatston y los pacientes fueron clasificados en CaC leve-moderada (CaC<400UH) y severa (CaC≥400UH).
ResultadosEl tiempo medio de seguimiento fue de 87,5 meses. El 28% eran pacientes con DM; tenían una CaC más severa, menor nivel de albúmina y una proteína C reactiva más elevada. La albúmina se correlacionó con la CaC severa (r=−0,45; p=0,009). La mortalidad fue del 58%. Los casos con DM mostraban una tendencia lineal de mayor mortalidad en comparación con los sujetos sin DM (Chi cuadrado 3,51, p=0,061). Los pacientes con DM y CaC severa tuvieron, además, una mayor mortalidad en comparación con aquellos con CaC severa sin DM (93% vs.73%; p=0,04).
ConclusionesLos pacientes con ERC avanzada y DM presentan una CaC más severa, datos bioquímicos compatibles con una mayor inflamación-malnutrición y una mayor mortalidad en comparación con aquellos sin DM.
Patients with chronic kidney disease (CKD) are at an increased risk of suffering cardiovascular (CV) disease, with up to 40% of all deaths being attributable to it.1 Similarly, cardiovascular disease is the main cause of morbidity and mortality in patients with diabetes mellitus (DM), and the risk of coronary artery disease is up to 5 times higher in patients with type 2 DM (DM2) than in patients without diabetes.2,3
Diabetes mellitus is a risk factor for the development of systemic atherosclerosis and vascular calcification.4 There is a correlation between coronary artery calcification (CaC) and the presence of atherosclerosis. Specifically, the degree of CaC as assessed by computed tomography (CT) is closely correlated to the histological quantification of calcium in such arteries in asymptomatic subjects in all age ranges.5
The mechanisms underlying vascular calcification are multiple and complex, and comprise chronic inflammation, oxidative stress and imbalances in bone-mineral metabolism.6 Vascular calcification and atherosclerotic lesions represent active processes similar to those seen in bone formation. The smooth muscle cells are seen to transform into cells with an osteoblastic phenotype. In vitro studies have shown that hyperglycemia is able to induce this transformation and increase the expression of bone matrix proteins such as BMP-2 (bone morphogenetic protein) and BMP-4 within the middle layer of the arterial wall.7
The prevalence of CaC is higher in patients with DM, regardless of renal function, reaching 70% in some series.8 In turn, CaC is particularly prevalent in patients with CKD, even in the early stages of the disease.1,9–11 Patients with advanced CKD subjected to renal replacement therapy (RRT) present more severe vascular calcification, and the latter progresses faster, particularly in patients with DM.12
Coronary artery calcification has recently been associated with the development of CV events, global mortality and CV mortality in patients with advanced CKD (stages 4–5) and on hemodialysis.13,14 However, few studies have analyzed the influence of DM upon CaC in patients with advanced CKD and its mortality-predicting capacity in such patients.
The present study analyses CaC measured by multislice computed tomography (MSCT) in a population with DM and advanced CKD, under both hemodialysis and in predialysis conditions. The association of CaC with mortality in this population is also examined.
MethodsStudy cohort and data collectionA post hoc analysis was made of the baseline data from a previously described series15 in order to assess the differential characteristics and CaC in patients with type 2 DM versus patients without DM (cohorts), subjected to renal replacement therapy and with advanced CKD under predialysis conditions.
The study included a total of 137 patients subjected to longitudinal prospective follow-up over a 10 year period (from January 2005 to July 2015) at the Nephrology Unit of Hospital Universitario Príncipe de Asturias (Madrid, Spain). The clinical and laboratory test data were compiled from the case histories (both electronic and those contained in the site files).
The inclusion criteria were: patient age over 18 years, stage 4–5 CKD, and enrolment in a chronic hemodialysis program (with a stay of at least 6 months prior to inclusion). The exclusion criteria included: acute renal failure, active glomerular disease, pregnancy, intercurrent diseases associated with a life expectancy of under one year, and an inability to perform MSCT (carriers of mechanical valve implants or coronary stents). Kidney transplant patients were excluded both at study entry and during follow-up.
All patients signed the corresponding informed consent document. The study protocol was approved by the local Ethics Committee.
At the start of follow-up, all patients underwent MSCT without intravenous contrast to assess baseline CaC. A 16-detector CT system was used (Light Speed Plus GE Medical System). Image processing was performed with Advantage Workstation 4.0. Coronary artery calcification was quantified using the Agatston method, which establishes a threshold of 130 Hounsfield units (HU) to determine whether a lesion is calcified or not. A score is subsequently calculated for each individual lesion, multiplying the area by a cofactor dependent upon the maximum HU value of each lesion. Finally, the calcification score is calculated by summing the calcification score of the left main coronary artery, the left anterior descending artery, the circumflex artery and the right coronary artery.16
Coronary artery calcification scores between 100 and 400HU indicate moderate atherosclerosis and a relatively high probability of coronary artery disease, while scores of >400HU indicate severe and extensive atherosclerotic disease, with a high probability of obstructive coronary disease and a high risk of symptomatic myocardial ischemia.17 Based on this premise, we established two calcification categories according to the baseline CaC score (CaCs): mild-moderate CaC (CaCs<400HU) and severe CaC (CaCs≥400HU).
As part of the routine assessment at the Nephrology Unit, a blood sample was collected in the month prior to the MSCT for the measurement of biochemical parameters related to kidney function and lipid and bone-mineral metabolism.
The estimation of urea clearance standardized for total body water (Kt/V) was used as a control of hemodialysis efficacy. This parameter takes into account the urea clearance of the dialyzer, calculated from ionic dialysance (k); the duration of dialysis therapy (t) (in minutes); and the body volume of the distribution of urea (V). The minimum clinical target for reaching an appropriate Kt/V was defined as 1.2–1.3 per session.18
The length of stay on hemodialysis was defined as the time elapsed from the entry of the patient into the hemodialysis program to the day of MSCT.
All-cause mortality was recorded during the follow-up period. Survival was defined as the time from patient enrolment to death due to any cause.
Statistical analysisQuantitative variables exhibiting a normal distribution were expressed as the mean and standard deviation, while those with a non-normal distribution were expressed as the median and interquartile range (IQR). Data distribution was assessed with the Kolmogorov–Smirnov test. The subjects were divided into two groups according to the presence or absence of type 2 DM. The chi-squared test was used to compare the two groups and their standardized residuals, as applicable, while the Student t-test or Kruskal–Wallis test was used for comparing dichotomous and quantitative variables according to their type of distribution. Spearman's correlation test was used to evaluate the degree of association between the CaCs of patients with DM and the remaining numerical variables.
Univariate proportional Cox regression analysis was used to explore the association between CaCs and mortality in the patients with type 2 DM versus those without DM, with a level of significance of p<0.1. The data are presented based on the hazard ratio (HR) and corresponding 95% confidence interval (95%CI).
The Kaplan–Meier test was used to estimate and describe the frequency of mortality events in cases with and without DM, while the Mantel–Cox (log-rank) test was used to compare the survival curves between the defined CaCs groups.
A p-value of <0.05 was considered statistically significant unless a different cut-off point was specified. The SPSS version 20.0 statistical package (Chicago, IL, USA) was used throughout.
ResultsThe study comprised 137 patients, 85 of whom were on hemodialysis, while 52 presented CKD under predialysis conditions. The median follow-up time was 87.5 months (IQR 29.5–111). The median age was 66 years (IQR 51.5–71), and the median time on hemodialysis was 25 months (IQR 10–53). Twenty-eight percent of the subjects had type 2 DM (n=38) and 89% presented arterial hypertension (according to Joint National Committee 7 criteria and/or the prescription of antihypertensive medication).
Of the patients with DM, 60.5% were on renal replacement therapy, while the rest were under predialysis conditions. In the latter group, 40% had stage 4 CKD.
The prevalence of CaC in the total study sample was 87%. The CaCs ranged from 0 to 8798HU, with a median of 600HU (70–1794). Sixty-one percent of the subjects presented severe CaC (CaCs≥400HU).
The overall mean Kt/V of the series was 1.39 (SD 0.29). Based on a cohort analysis, the estimated Kt/V in patients with DM was 1.26 (0.31), while the estimated Kt/V in patients without DM was 1.43 (0.27) (p=0.027). Both groups reached the recommended minimum target (Kt/V≥1.2).
The prevalence of CaC in the subjects with DM was 97%. Of these patients, 84.2% (n=32) belonged to the severe CaC category (CaCs≥400HU) versus 52% (n=51) of the patients without DM (p=0.001). Only 2.6% of the patients with DM failed to show CaC at MSCT (CaCs=0HU), versus 17.3% of the patients without DM (p=0.04). The mean CaCs (2070 [1257.47]) was significantly higher in the patients with long-evolving diabetes (more than 10 years) than in those with a shorter disease duration (580.67 [505.61]) (p=0.023).
Of the patients with DM, 79.3% were receiving insulin therapy. The mean time elapsed from DM onset was 20.63 years (6.89). The estimated mean glycosylated hemoglobin (HbA1c) concentration in this group was 7.07% (1.66).
There were no significant differences in the degree of CaC in patients with DM on hemodialysis versus the predialysis group (1870.3 [95%CI: 1239.75–2500.86] vs. 1515.93 [95%CI: 537.27–2494.6]) (p=0.89).
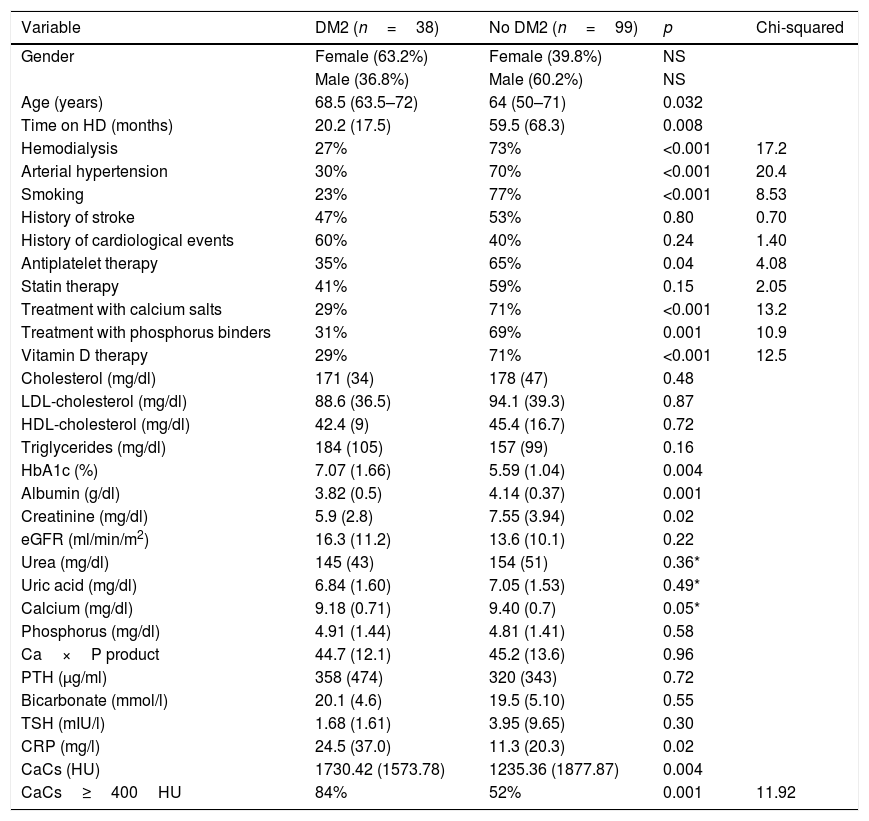
The patients with DM were older (68.5 years [63.5–72] vs. 64 [50–71]) (p=0.032), with a shorter prior time on hemodialysis (20.21 months [17.46] vs. 59.48 [68.28]) (p=0.008), and had more severe CaC (1730.42HU [±1573.78] vs. 1235.36 [±1877.87] (p=0.004), compared with the patients without DM.
With regard to the laboratory test parameters, the patients with DM had lower plasma creatinine (p=0.02), lower albumin (p=0.001), and higher C-reactive protein (CRP) concentrations (p=0.02). Table 1 shows the other differences in the laboratory test values.
Comparison of demographic, clinical and biochemical variables at the start of the study in CKD patients with and without type 2 diabetes mellitus.
| Variable | DM2 (n=38) | No DM2 (n=99) | p | Chi-squared |
|---|---|---|---|---|
| Gender | Female (63.2%) | Female (39.8%) | NS | |
| Male (36.8%) | Male (60.2%) | NS | ||
| Age (years) | 68.5 (63.5–72) | 64 (50–71) | 0.032 | |
| Time on HD (months) | 20.2 (17.5) | 59.5 (68.3) | 0.008 | |
| Hemodialysis | 27% | 73% | <0.001 | 17.2 |
| Arterial hypertension | 30% | 70% | <0.001 | 20.4 |
| Smoking | 23% | 77% | <0.001 | 8.53 |
| History of stroke | 47% | 53% | 0.80 | 0.70 |
| History of cardiological events | 60% | 40% | 0.24 | 1.40 |
| Antiplatelet therapy | 35% | 65% | 0.04 | 4.08 |
| Statin therapy | 41% | 59% | 0.15 | 2.05 |
| Treatment with calcium salts | 29% | 71% | <0.001 | 13.2 |
| Treatment with phosphorus binders | 31% | 69% | 0.001 | 10.9 |
| Vitamin D therapy | 29% | 71% | <0.001 | 12.5 |
| Cholesterol (mg/dl) | 171 (34) | 178 (47) | 0.48 | |
| LDL-cholesterol (mg/dl) | 88.6 (36.5) | 94.1 (39.3) | 0.87 | |
| HDL-cholesterol (mg/dl) | 42.4 (9) | 45.4 (16.7) | 0.72 | |
| Triglycerides (mg/dl) | 184 (105) | 157 (99) | 0.16 | |
| HbA1c (%) | 7.07 (1.66) | 5.59 (1.04) | 0.004 | |
| Albumin (g/dl) | 3.82 (0.5) | 4.14 (0.37) | 0.001 | |
| Creatinine (mg/dl) | 5.9 (2.8) | 7.55 (3.94) | 0.02 | |
| eGFR (ml/min/m2) | 16.3 (11.2) | 13.6 (10.1) | 0.22 | |
| Urea (mg/dl) | 145 (43) | 154 (51) | 0.36* | |
| Uric acid (mg/dl) | 6.84 (1.60) | 7.05 (1.53) | 0.49* | |
| Calcium (mg/dl) | 9.18 (0.71) | 9.40 (0.7) | 0.05* | |
| Phosphorus (mg/dl) | 4.91 (1.44) | 4.81 (1.41) | 0.58 | |
| Ca×P product | 44.7 (12.1) | 45.2 (13.6) | 0.96 | |
| PTH (μg/ml) | 358 (474) | 320 (343) | 0.72 | |
| Bicarbonate (mmol/l) | 20.1 (4.6) | 19.5 (5.10) | 0.55 | |
| TSH (mIU/l) | 1.68 (1.61) | 3.95 (9.65) | 0.30 | |
| CRP (mg/l) | 24.5 (37.0) | 11.3 (20.3) | 0.02 | |
| CaCs (HU) | 1730.42 (1573.78) | 1235.36 (1877.87) | 0.004 | |
| CaCs≥400HU | 84% | 52% | 0.001 | 11.92 |
Data are presented as number of patients and percentage (N [%]); mean and standard deviation (±SD). Chi-squared, Student t-test or Kruskal–Wallis test*, as applicable. Time on HD: time from start of hemodialysis to day of MSCT; history of cardiological events: includes ischemic heart disease (chest pain, acute myocardial infarction) and heart failure.
CaCs (HU): coronary artery calcification score (in Hounsfield units); eGFR: estimated glomerular filtration rate; PTH: parathyroid hormone; TSH: thyroid stimulating hormone.
Significant value p<0.05.
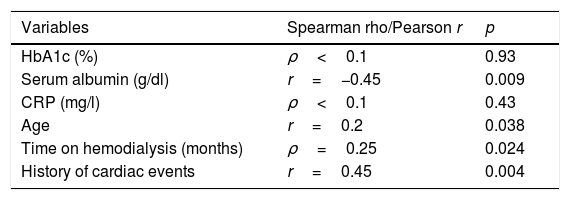
In the patients with DM, severe CaC (CaCs>400HU) was correlated to the serum albumin levels (r=−0.45; p=0.009), age (r=0.2; p=0.038), and the existence of previous cardiological events (r=0.45; p=0.004) (Table 2). No correlation was found between HbA1c (%) and severe CaC. There were no other associations with the remaining studied biochemical variables, the prior time on hemodialysis, or a history of arterial hypertension or stroke.
Principal study variables and their correlation to severe coronary calcification (≥400HU) in the cohort of patients with type 2 diabetes mellitus.
| Variables | Spearman rho/Pearson r | p |
|---|---|---|
| HbA1c (%) | ρ<0.1 | 0.93 |
| Serum albumin (g/dl) | r=−0.45 | 0.009 |
| CRP (mg/l) | ρ<0.1 | 0.43 |
| Age | r=0.2 | 0.038 |
| Time on hemodialysis (months) | ρ=0.25 | 0.024 |
| History of cardiac events | r=0.45 | 0.004 |
During the follow-up period, the total mortality rate of the sample was 58% (n=80). In the group of patients with DM, the mortality rate was 74% (n=28). Of these, 93% belonged to the severe CaC group versus 73% in the case of the deceased patients without DM (chi-squared test 4.41; p=0.04).
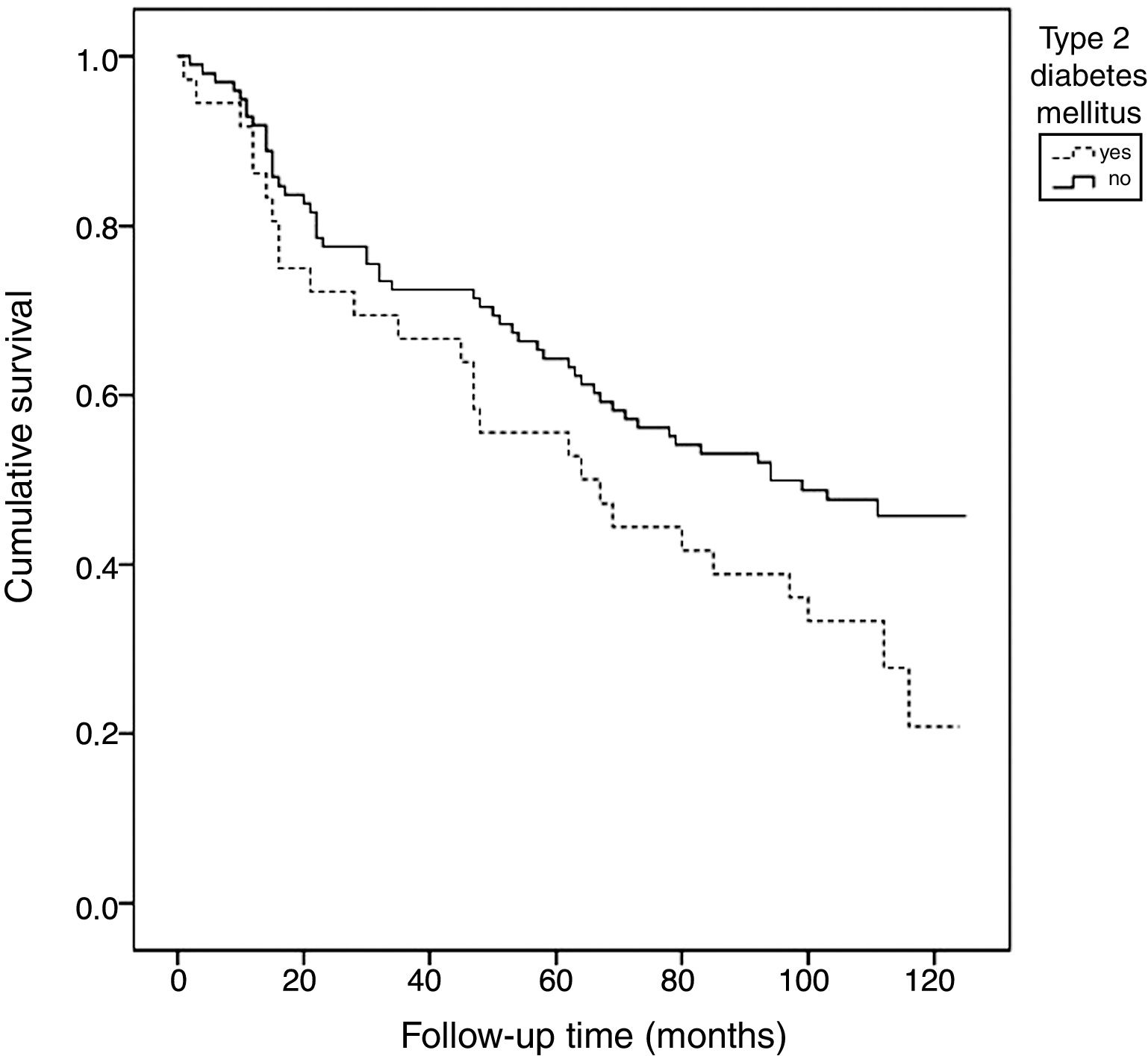
According to the Kaplan–Meier survival analysis (unadjusted) corresponding to total mortality, the patients with DM exhibited a linear trend toward higher mortality over follow-up compared with the patients without DM (67.72 months [95%CI: 53.11–93.34] vs. 81.53 [95%CI: 72.37–90.68], chi-squared test: 3.51) (p=0.061) (Fig. 1).
In the univariate Cox survival analysis, the subjects with DM and CaCs≥400HU had an increased risk of mortality during the follow-up period, though statistical significance was not reached (HR 1.63, 95%CI: 0.9–2.98; p=0.11). This group could not be compared with the patients with DM and CaCs<400HU, due to the small number of mortality events recorded among the latter (2 deaths). Statistical significance was likewise not reached in the case of mortality in patients without DM and with CaCs≥400HU (HR: 1.41, 95%CI: 0.75–2.64; p=0.29). The remaining studied variables were not significantly associated with increased mortality (data not shown) in the DM group.
DiscussionIn our series, the patients with DM had higher CRP and lower albumin levels in serum, which may suggest a greater inflammatory component. The malnutrition-inflammation complex in patients on dialysis is known to be a common problem, and is associated with increased morbidity and mortality.19 There is also an association between the aforementioned complex and hypoalbuminemia and vascular calcification in patients subjected to renal replacement therapy.20 In turn, a relationship has been described between nutritional status and bone metabolism. In this regard, hypoalbuminemia appears to increase the synthesis of proinflammatory cytokines, and is related to adynamic bone disease.21,22 In our study, a negative correlation was found between albumin levels and severe vascular calcification in patients with DM, this correlation being weaker in patients without DM (r=−0.25; p=0.03). Thus, a decrease in plasma albumin could constitute a marker of increased CaC in patients with DM and advanced CKD.
The diabetic subjects on dialysis had a lower Kt/V than the patients without DM. It is well documented that intradialysis hemodynamic events are more common in patients with DM, and that these individuals moreover are at an increased risk of vascular access dysfunction. These factors may interfere with the quality of dialysis.23 In addition, the limitations of Kt/V as a dialysis efficacy parameter need to be taken into account, since different factors such as patient gender, body size, muscle mass and age need to be considered for its adequate interpretation.24 Despite the above, the estimated mean Kt/V in patients with DM was not lower than the minimum target of 1.2.
In our study, the patients with DM and CKD had a higher prevalence of CaC and a higher CaCs as compared to the group without DM. Hyperglycemia is known to affect vascular calcification through multiple mechanisms, including oxidative stress, advanced glycation end products, and endothelial dysfunction. These factors lead to an increase in reactive oxygen species (ROS) that induces a shift in the cell phenotype from vascular smooth muscle to bone cells.7 Insulin resistance and leptin levels are also implicated in atherosclerosis and vascular calcification.25 On the other hand, no correlation was found between HbA1c (%) and CaCs. This finding could be explained by the fact that HbA1c is not a good metabolic control parameter in this population. In effect, in patients with advanced CKD and on hemodialysis, HbA1c may underestimate glycemic control. Erythrocyte survival is shortened, and moreover treatment with erythropoietin stimulating agents increases the proportion of young red cell precursors, giving rise to a false decrease in HbA1c concentration.26
Previous studies have confirmed that there is a greater prevalence of CaC in the population with DM, the figure reaching as high as 80% depending on the series.3,27,28 In patients with diabetic nephropathy, this prevalence is even higher.28 In our series, 84% of the patients with DM had severe CaCs, this percentage being higher than that seen in other DM populations without kidney disease.3,28,29 This may be due in part to the additional influence of advanced CKD upon bone-mineral metabolism.1,11 In addition, patients with DM and CKD present a greater risk of CaC progression and show a greater quantitative increase in its progression than those without DM.9,10 Kronmal et al., after analysing a series of subjects with no known cardiovascular disease, concluded that DM is the strongest predictive risk factor for CaC progression, with disease duration remaining as the only risk factor for the progression of vascular calcification.30
Although CaC is associated with the presence of albuminuria and diabetic nephropathy in subjects with DM, it is not clear whether there are differences in the severity of CaC according to the CKD stage in this population.31 We found no significant difference in the degree of CaC in patients with DM and on hemodialysis as compared to patients with DM under predialysis conditions.
Patients with DM have a shorter life expectancy, mainly as a consequence of cardiovascular disease.2 In our series, the Kaplan–Meier analysis identified greater mortality among the patients with DM during the follow-up period. Furthermore, higher mortality was seen among the diabetic patients with severe CaC than in those less severe degrees of calcification.
In patients with advanced CKD and on hemodialysis, the CaCs plays a predictive role in terms of patient mortality, regardless of other traditional cardiovascular risk factors.1,13,14 In turn, previous studies have shown that, in the case of patients with DM, those with greater CaC suffer greater mortality and are at an increased risk of cardiovascular events.3,29,32 In a recently published meta-analysis, patients with DM and CaC≥10HU presented a relative risk of mortality and cardiovascular events of 5.47 (95%CI: 2.59–11.53; p<0.001).33
The univariate Cox regression analysis revealed no significant association between the CaCs and mortality among the patients with DM. Few studies to date have analyzed the capacity of CaC to predict cardiovascular risk and mortality in individuals with DM and CKD.10 This population is characterized by an increased prevalence of CaC, more severe CaC, and greater mortality than those without DM. The usefulness of the CaCs as a prognostic tool in this subgroup of patients with DM therefore needs to be established. It is important to clarify whether the early treatment of some of the etiopathogenic factors implicated in vascular calcification is able to modify its course.
The present study constitutes a post hoc sub-analysis and involves a small sample size; the results obtained therefore must be regarded as preliminary findings. The relatively low prevalence of patients with DM may have affected the power of the statistical analysis, preventing us from adjusting for other traditional cardiovascular risk factors. Furthermore, there were no control MSCT scans to assess the progression of CaC during follow-up; we therefore do not know whether the presence of diabetes, treatment or its modifications might influence the evolution of CaC.
Proteinuria was not recorded, and so it is not possible to discard its possible influence upon the observed differences in serum albumin. However, it should be noted that these were mostly found in patients on dialysis with virtually no residual renal function. Changes in serum albumin are largely conditioned by a lack of protein intake and/or increased protein catabolism. We found the most common cause of CKD in patients with DM to be diabetic nephropathy, which does not typically manifest with nephrotic syndrome. Furthermore, nephrotic syndrome usually presents in the context of active glomerular disease, and the prevalence of glomerulonephritis in this population was <2%.
Lastly, it can be concluded that the prevalence and severity of CaC is high in patients with advanced CKD and subjected to hemodialysis, and greater in patients with associated DM. Patients with DM and CKD have lower albumin levels and higher CRP concentrations than those without DM. Serum albumin is negatively correlated to severe CaC, and this correlation is stronger in patients with DM.
Our analysis showed diabetic patients with advanced CKD and severe CaC (≥400HU) to suffer greater mortality than those with severe CaC in the absence of DM.
There was no statistically significant evidence to suggest that a CaCs≥400HU was predictive of mortality in this population. Studies with larger sample sizes are needed to determine the potential predictive role of CaC in relation to global and cardiovascular mortality in patients with DM and advanced CKD, and its implications for individualizing patient management with a view to reducing morbidity and mortality.
Conflicts of interestNone.
Please cite this article as: Cano-Megías M, Bouarich H, Guisado-Vasco P, Pérez Fernández M, de Arriba-de la Fuente G, Álvarez-Sanz C, et al. Calcificación arterial coronaria en pacientes con diabetes mellitus y enfermedad renal crónica avanzada. Endocrinol Diabetes Nutr. 2019;666:297–304.