Hypoglycaemia is one of the main determinants of the quality of life of patients with diabetes mellitus (DM), in addition to having negative consequences on their morbidity and mortality.1 Being aware of its possible causes is essential to prevent and optimise its therapeutic management. Linezolid is a widely used antibiotic that has been related to hypoglycaemia as a rare adverse event (AE) in patients with DM, as stated in the FDA technical information sheet.2
The present clinical case describes the presence of sustained hypoglycaemia in a patient with type 1 DM (DM1) while treated with linezolid, an AE that is not always recognised associated with its use. Hypoglycaemia was documented in detail using flash interstitial glucose monitoring (FreeStyle Libre 2®, Abbott).
This was a 70-year-old man, with DM1 diagnosed at age 3, with poor chronic metabolic control, and multiple associated micro- and macrovascular complications. For the last 11 years, he had been followed up by the diabetic foot unit due to a history of neuroischemic ulcers.
The patient developed an infected ulcer on the 3rd metatarsal head of the left foot with evidence of osteomyelitis. The torpid evolution required a wedge amputation of the 3rd toe and empirical antibiotic therapy with linezolid 600mg/every 12h, initially intravenously and later orally. In the microbiological analysis, Staphylococcus aureus and Enterococcus faecalis were isolated, both sensitive to linezolid. Antibiotic treatment was continued for 23 days.
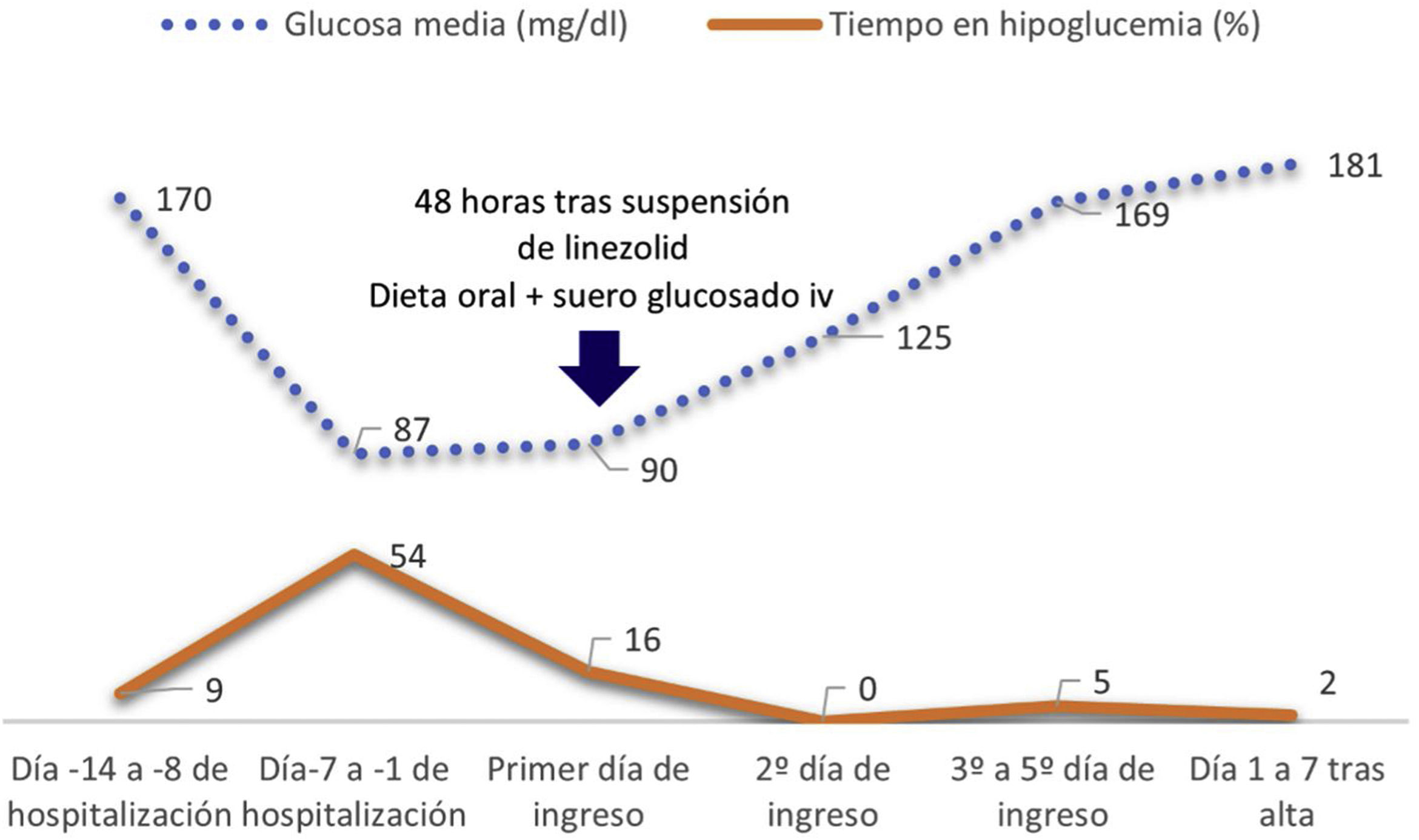
In the first consultation after hospital discharge, the patient reported a very significant reduction in the total insulin dose (DTI) starting on the nineteenth day of treatment with linezolid. Prior to admission, the daily DTI was 35IU (degludec 17U and glulisine 18U distributed with the intakes), reducing insulin degludec by 70% of the total, up to 10IU, maintaining hypoglycaemia (52−66mg/dl) despite the downward adjustment of insulin doses and reduction/suppression of rapid insulin with intakes. Glucose monitoring sensor data from the last 14 days showed a glucose management indicator (GMI) of 6.1%, with time below range (<70mg/dl) of 31%, at the expense of a long time maintained in hypoglycemia (22 episodes × 311min). Comparatively, the monitoring records in the weeks prior to the acute process showed an average glucose of 184mg/dl, with IMT of 7.7% and time in insufficient range (50%) at the expense of high time in hyperglycaemia (47%).
The patient was hospitalized for clinical monitoring and the hypoglycaemia resolved within 48h after discontinuation of linezolid, with increasing insulin requirements upon discharge until his previous DTI. On the day of admission, degludec was no longer administered and serum therapy with glucose was added to his oral diet, with glycemic corrections with small doses of rapid insulin according to controls. Insulin degludec (10IU) could be restarted 24h after hospital admission, as well as insulin aspart with meals, at the same time that glucose serums were suspended. Fig. 1 shows the evolution of interstitial glucose during hospitalisation.
Although the development of hypoglycaemia in patients with DM associated with the administration of linezolid was described for the first time in 2011,3 other factors that could be involved were ruled out: modification of the intake pattern, weight loss, adrenal insufficiency, acute renal failure, pharmacotherapy. interfering and/or monitoring sensor errors.
Linezolid is an antibiotic from the oxazolidinone group with a broad-spectrum bacteriostatic effect against gram-positive bacteria.4 The most commonly described AEs are gastrointestinal symptoms or headache, the most serious being reversible myelosuppression and irreversible peripheral neuropathy.4,5 There are also cases of serotonin syndrome, with the association of serotonin reuptake inhibitor drugs, norepinephrine and tricyclic antidepressants, among others.4
One of the possible mechanisms inducing hypoglycaemia is the increase in peripheral sensitivity to insulin due to the inhibitory effect of linezolid on the activity of the enzyme monoamine oxidase.5 Linezolid belongs to the group of iMAOs, which act on the metabolism of neurotransmitters such as norepinephrine, dopamine and serotonin. The increase at the presynaptic level of amines such as serotonin reduces insulin resistance and improves carbohydrate homeostasis.6 The Naranjo AD scale classifies the association between linezolid and hypoglycaemia in patients with DM as probable.7 The temporal criterion is one of the factors that support the hypoglycaemic association in the different reported cases.4,5,7
The FDA updated and warned in 2012 about the hypoglycaemic effect of linezolid in patients with DM.2 However, this AE, reported after the drug was marketed,8 is not included in the EMA's technical sheet and, therefore, in Spain. This side effect has also been reported, although less frequently, in patients without DM.9
The development of hypoglycaemia with antibiotics such as cefditoren, clarithromycin, tigecycline or fluoroquinolones has been previously reported, in people with or without diabetes. Linezolid was reported in the FDA case series, with the appearance of hypoglycaemia in patients with DM when associated with oral hypoglycaemic treatment.10
In a review of 12 cases of hypoglycaemia in people with DM associated with the use of linezolid, 2 had insulin treatment, 9 had oral antidiabetics, and in one case no regular medication was reported.5 In our case, sustained hypoglycaemia occurred from day 19 after starting treatment with linezolid. The cases described have reported reductions in insulin requirements and hypoglycaemia, with a median on day 7 after starting the antibiotic, range 2–30 days, maintaining the tendency towards hypoglycaemia until 24−36h after cessation of the antibiotic, and therefore our case presented a clinical evolution that coincides with what is described in the literature.7
In clinical practice, doctors must assess the risks/benefits of the use of antimicrobials, as well as take into account possible interactions with the patients' usual treatment. In the case of the use of linezolid, in patients with DM treated with drugs with a risk of hypoglycaemia, we must be alert to this possible association, intervening and warning the patient, to take the appropriate measures, such as adjusting the treatment and contacting their medical team.
Ethical responsibilitiesThe patient gave his informed consent for the communication of anonymized data from his medical history, as well as its publication. The Clinical Research Ethics Committee raised no objections to this publication.