Obesity and diabetes are two closely related disorders. Lifestyle changes and drug treatment do not achieve successful diabetes remission. A treatment option for these patients is bariatric surgery (BS). The partial and complete remission rates vary, depending on the type of technique used (restrictive or malabsorptive), with malabsorptive surgery being more effective in terms of both weight reduction and diabetes remission (DR). Different scales (DiaRem, Ad-DiaRem or 5y-Ad-DiaRem) predict the probability of DR after BS, particularly after gastric bypass surgery. Some studies report higher DR rates in surgery with a greater malabsorptive component. Our aim was to study the benefits of BS at one year and 5 years in terms of the weight and blood glucose profile in patients with obesity and type 2 diabetes mellitus; assess percentage DR according to ADA criteria; determine the DR predictive capacity of different scores; and examine which variables predict DR at one and five years after biliopancreatic diversion (BPD). Percentage overweight reduction and the decrease in both blood glucose and HbA1c were greater with BPD. Complete diabetes remission was approximately 80% at one and 5 years after BS. In general, the scores that determine the probability of DR show poor discriminative capacity in malabsorptive surgery. Presurgery HbA1c predicts DR at one and 5 years after BPD. The type of surgery performed should be individualized, based on the severity of diabetes and the specific characteristics of each patient.
Obesidad y diabetes son dos entidades muy relacionadas. Las modificaciones en los hábitos de vida y el tratamiento farmacológico no consiguen una remisión exitosa de la diabetes. Una opción terapéutica para estos pacientes es la cirugía bariátrica (CB). En función del tipo de técnica empleada (restrictiva o malabsortiva), las tasas de remisión parcial y completa varían, siendo más efectivas las malabsortivas, tanto en reducción de peso como en remisión de diabetes (RD). Existen diferentes escalas (DiaRem, Ad-DiaRem o 5y-Ad-DiaRem) que predicen la probabilidad de RD tras CB, sobre todo tras bypass gástrico. Algunos estudios muestran tasas de RD más elevadas, en cirugías con mayor componente malabsortivo. Nuestro objetivo fue estudiar los beneficios de la CB al año y 5 años, en cuanto a peso y perfil glucémico en pacientes con obesidad y DM2, evaluar el porcentaje de RD según criterios ADA, determinar la capacidad predictiva de RD de distintos scores y examinar que variables predicen RD al año y a cinco años de la derivación biliopancreática (DBP). La DBP presenta mayor porcentaje de sobrepeso perdido (PSP) y una mayor reducción tanto en glucemia como en HbA1c. La remisión completa de la diabetes oscila en un 80% aproximadamente a 1 y 5 años de la CB. En general los scores que determinan probabilidad de RD tienen poco poder discriminativo en cirugías malabsortivas. La HbA1c precirugía predice RD a 1 y 5 años tras DBP. Se debe individualizar el tipo de cirugía realizada, en función de la gravedad de la DM2 y las características específicas de cada paciente.
Obesity and diabetes mellitus place a health and economic burden on our society.1 The aim of diabetes management is not only strict control of blood glucose and maintaining an ideal weight, but also preventing or reducing the incidence of associated macrovascular and microvascular complications.2 Lifestyle changes (diet and physical exercise programmes) and drug treatment achieve poor and only transient weight reduction, without successful diabetes remission (DR) or metabolic control. Bariatric surgery (BS) has been shown to significantly reduce weight, achieve DR in the short term in approximately 60%–90% of patients3 and maintain weight loss in the long term, reducing associated cardiovascular complications.4 According to the 2020 American Diabetes Association (ADA) guidelines, surgery should be considered in adults with type 2 diabetes mellitus (DM2) and body mass index (BMI) from 30.0 to 34.9 kg/m2 if weight loss or improvement of comorbidities is not achieved with non-surgical methods. It should also be the recommended treatment in patients with DM2 and BMI from 35.0 to 39.9 kg/m2 when hyperglycaemia is not controlled with lifestyle changes and optimal medical treatment, and suggested in individuals with BMI ≥ 40 kg/m2, regardless of degree of blood glucose control.5 The consensus document of the Spanish Society for the Study of Obesity (SEEDO) establishes a series of criteria, which must be met before undergoing BS. The most important of these include: ruling out another endocrine disorder; maintaining psychological stability; and understanding the procedure and associated responsibilities.6 There are different BS procedures: restrictive techniques (decrease in calorie intake by reducing stomach capacity); malabsorptive (decrease in nutrient and therefore calorie absorption, by performing an intestinal bypass); and mixed (combination of the two previous components). DR can be measured by an improvement in the blood glucose profile (blood glucose plus HbA1c) and no longer needing antidiabetic drugs. There are preoperative variables that determine post-surgery DR (age, gender, BMI, duration of DM, HbA1c, antidiabetic treatment, C-peptide and plasma insulin). There are also several validated scores (DiaRem,7 Ad-DiaRem8 and 5y-Ad-DiaRem9), which predict DR after gastric bypass (GB), but their utility has not been demonstrated in surgery with a greater malabsorptive component, such as biliopancreatic diversion (BPD), in which the DR rate is higher. DiaRem is a tool that identifies which patients with obesity and DM2 will have diabetes remission after BS. The predictive power of this score is based on different variables such as age, HbA1c, use of insulin and the use of other blood glucose-lowering agents. Ad-DiaRem adds two new variables to the original DiaRem, achieving a better predictive capacity of DR. 5y-Ad-DiaRem includes baseline values and one-year post-BS follow-up parameters, and identifies patients at risk of relapse with good precision.
The objectives of our study were: to describe the changes in blood glucose and weight parameters at one year and five years after BS, according to surgical technique; to assess percentage DR according to ADA criteria at one and five years after BPD; to determine the DR predictive capacity of different scores after BPD; and to examine which variables predict DR at one and five years after BPD.
MethodsWe conducted a retrospective observational study of patients with high-risk obesity, under follow-up by the Nutrition Unit of the Complejo Asistencial Universitario de León [University Hospital of León] who had undergone BS in the period from January 1999 to February 2017 (377 patients). Patients were selected who had undergone BPD or sleeve gastrectomy (SG), who had diabetes before the BS as an associated comorbidity (132 patients) and who had a minimum follow-up of one year after the procedure. Fifteen patients who had BPD were excluded as there was no record of anthropometric or baseline blood glucose data. Ultimately 117 patients were included, 105 operated on by BPD and 12 by SG. The weight and blood glucose response was assessed in all cases at one year post-BS and at five years in 66 patients who underwent BPD (31 patients were lost to follow-up and in eight patients, five years since the procedure had not yet elapsed). None of the patients who underwent SG could be assessed at five years, as two were lost to follow-up and in ten cases, five years since the procedure had not yet elapsed. The following variables were collected for the study: sociodemographic (age, gender, blood glucose-lowering therapy and associated comorbidities); and anthropometric (height, current weight, ideal weight, baseline BMI at one year and five years after BS). To define ideal weight, the Metropolitan Life Insurance Company tables were used.10 The percentage of weight loss (%WL) and percentage of excess weight loss (%EWL) were calculated at one year and five years after the procedure [(%WL = initial weight - current weight/initial weight × 100), (%EWL = initial weight - current weight/initial weight - ideal weight × 100)]. We also analysed analytical variables (blood glucose and HbA1c at baseline and at one and five years post-BS, blood glucose variation and variation in HbA1c at one year and five years post-BS) and type of surgical procedure performed (SG or BPD). SG involves performing a longitudinal gastrectomy, preserving the pylorus and forming a tube in the stomach along the lesser curvature. It restricts food intake by reducing stomach capacity, leading to significant weight loss, not only due to the reduced intake, but also due to the decrease in ghrelin levels, responsible for increasing appetite.11 BPD is a technique that combines moderate gastric restriction and severe intestinal malabsorption; it also has an anorexic effect caused by distension of the small intestine and can lead to Dumping syndrome as a result of accelerated gastric emptying.12
Considering the small number of patients with SG in our series and their shorter follow-up time, we only studied the possibility of DR in the group of patients with BPD so the sample would be homogeneous. For each patient with BPD, the likelihood of remission was classified as high or low, based on the DiaRem, Ad-DiaRem and 5y-AdDiaRem score. The ADA criteria13 were used to determine remission status in two categories: remission (partial or complete) and no remission. Partial remission (PR) was defined as HbA1c <6.5% and fasting basal glucose <125 mg/dl without antidiabetic drugs for at least 12 months. Complete remission (CR) was defined as HbA1c <6.0% and fasting basal glucose <100 mg/dl without antidiabetic drugs for at least 12 months. No remission was defined as failure to meet any of the above criteria.
The study design was evaluated and approved by the Ethics Committee of the Complejo Asistencial Universitario de León on 28 April 2015. Statistical analysis was performed using IBM SPSS Statistics 15®, available at the Research Unit of the Complejo Asistencial Universitario de León. The normality of the distribution of the quantitative variables was determined with the Kolmogorov-Smirnov test. A descriptive study of the main variables and anthropometric and blood glucose parameters was carried out according to the type of BS. Qualitative variables are expressed as percentages and absolute frequencies. Quantitative variables with normal distribution are expressed as mean and standard deviation (SD) and non-normal variables as median and interquartile range (IQR). To assess the predictive capacity of the different scores, we calculated the positive predictive value (PPV), and to determine which of the scores had the best discriminative power for predicting diabetes remission, we performed an analysis using ROC curves and area under the curve (AUC). A logistic regression was used to predict which variables influenced DR the most at one year and five years after BPD.
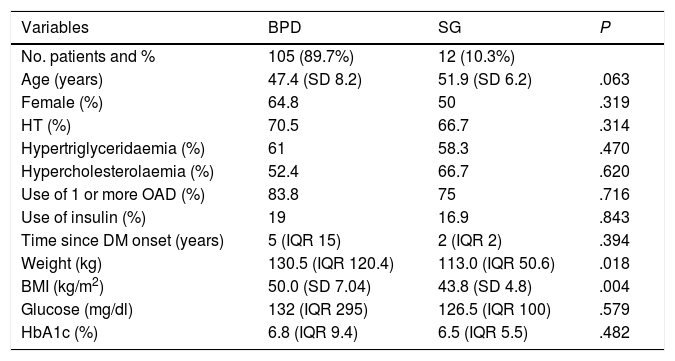
ResultsSample selection and general dataUltimately, 117 patients were included in the study: 63.2% were female, with a mean age of 47.8 years (SD 8.1); 70.1% had associated hypertension (HT), 60.7% hypertriglyceridaemia and 33.8% hypercholesterolaemia. All patients had a minimum follow-up of one year. At five years, only 62.9% of the patients with BPD (n = 66) and none of those operated on by SG had follow-up. The median time since onset of diabetes was five years (IQR 15); 82.9% were on treatment with one or more oral antidiabetic drugs and 18.8% were on insulin therapy. Baseline blood glucose and HbA1c were 132 mg/dl (IQR 295) and 6.8% (IQR 9.4), respectively. The pre-surgery weight and BMI were 129.7 kg (IQR 120.4) and 49.4 kg/m2 (SD 7.1), respectively. The procedure performed was BPD in 89.7% (n = 105) and SG in 10.3% (n = 12). Differences in baseline parameters between BS techniques are shown in Table 1.
Differences in baseline parameters between BS techniques.
| Variables | BPD | SG | P |
|---|---|---|---|
| No. patients and % | 105 (89.7%) | 12 (10.3%) | |
| Age (years) | 47.4 (SD 8.2) | 51.9 (SD 6.2) | .063 |
| Female (%) | 64.8 | 50 | .319 |
| HT (%) | 70.5 | 66.7 | .314 |
| Hypertriglyceridaemia (%) | 61 | 58.3 | .470 |
| Hypercholesterolaemia (%) | 52.4 | 66.7 | .620 |
| Use of 1 or more OAD (%) | 83.8 | 75 | .716 |
| Use of insulin (%) | 19 | 16.9 | .843 |
| Time since DM onset (years) | 5 (IQR 15) | 2 (IQR 2) | .394 |
| Weight (kg) | 130.5 (IQR 120.4) | 113.0 (IQR 50.6) | .018 |
| BMI (kg/m2) | 50.0 (SD 7.04) | 43.8 (SD 4.8) | .004 |
| Glucose (mg/dl) | 132 (IQR 295) | 126.5 (IQR 100) | .579 |
| HbA1c (%) | 6.8 (IQR 9.4) | 6.5 (IQR 5.5) | .482 |
BPD: biliopancreatic diversion; SG: sleeve gastrectomy; HT: hypertension; OAD: oral antidiabetic agents; DM: diabetes mellitus; BMI: body mass index.
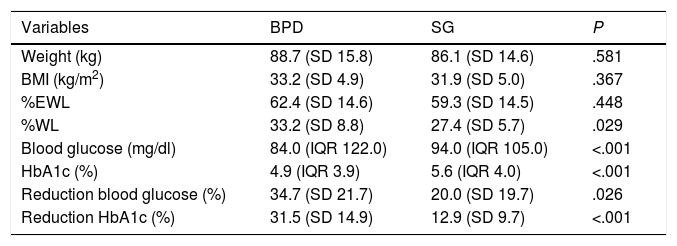
The overall weight parameters at one year post-BS were: weight 88.5 kg (SD 15.7), BMI 33.1 kg/m2 (SD 5.0), %EWL 62.0% (SD 14.5), and %WL 32.5% (SD 8.7). Five years post-BS, the weight data were: weight 87.5 kg (SD 20.0), BMI 33.7 kg/m2 (SD 6.0), %EWL 61.5% (SD 22.1), and %WL 34.2% (SD 13.7). In our series, the patients were operated on using two different surgical techniques. The descriptive analysis of the weight parameters stratified by technique is shown in Table 2. There are no weight data at five years for SG due to lack of follow-up of these patients, so the anthropometric variables for year five post-surgery all correspond to the BPD procedure.
Weight and blood glucose parameters at one year post-BS, according to surgical technique.
| Variables | BPD | SG | P |
|---|---|---|---|
| Weight (kg) | 88.7 (SD 15.8) | 86.1 (SD 14.6) | .581 |
| BMI (kg/m2) | 33.2 (SD 4.9) | 31.9 (SD 5.0) | .367 |
| %EWL | 62.4 (SD 14.6) | 59.3 (SD 14.5) | .448 |
| %WL | 33.2 (SD 8.8) | 27.4 (SD 5.7) | .029 |
| Blood glucose (mg/dl) | 84.0 (IQR 122.0) | 94.0 (IQR 105.0) | <.001 |
| HbA1c (%) | 4.9 (IQR 3.9) | 5.6 (IQR 4.0) | <.001 |
| Reduction blood glucose (%) | 34.7 (SD 21.7) | 20.0 (SD 19.7) | .026 |
| Reduction HbA1c (%) | 31.5 (SD 14.9) | 12.9 (SD 9.7) | <.001 |
BPD: biliopancreatic diversion; SG: sleeve gastrectomy; BMI: body mass index; %EWL: percentage of excess weight loss; %WL: percentage of weight loss.
The overall blood glucose parameters at one year post-BS were: blood glucose 84.5 mg/dl (IQR 122.0), HbA1c 5.0% (IQR 6.8), reduction in blood glucose and HbA1c versus the baseline value; 33.2% (SD 21.8) and 29.5% (SD 15.5), respectively. 9.4% of the patients were on treatment with one or two oral antidiabetic agents and none was on insulin treatment. The overall blood glucose data at five years post-BS were: blood glucose 85.0 mg/dl (IQR 183.0), HbA1c 4.6% (IQR 4.4), reduction in blood glucose and HbA1c versus the baseline value: 32.6% (SD 21.1) and 31.1% (SD 15.6), respectively. Only 6% of the patients continued on blood glucose-lowering therapy with one or two oral antidiabetic agents. The analysis of the blood glucose parameters stratified by surgical technique is shown in Table 2. There are no data at five years for SG due to lack of follow-up of these patients, so the blood glucose variables for year five post-surgery all correspond to the BPD procedure.
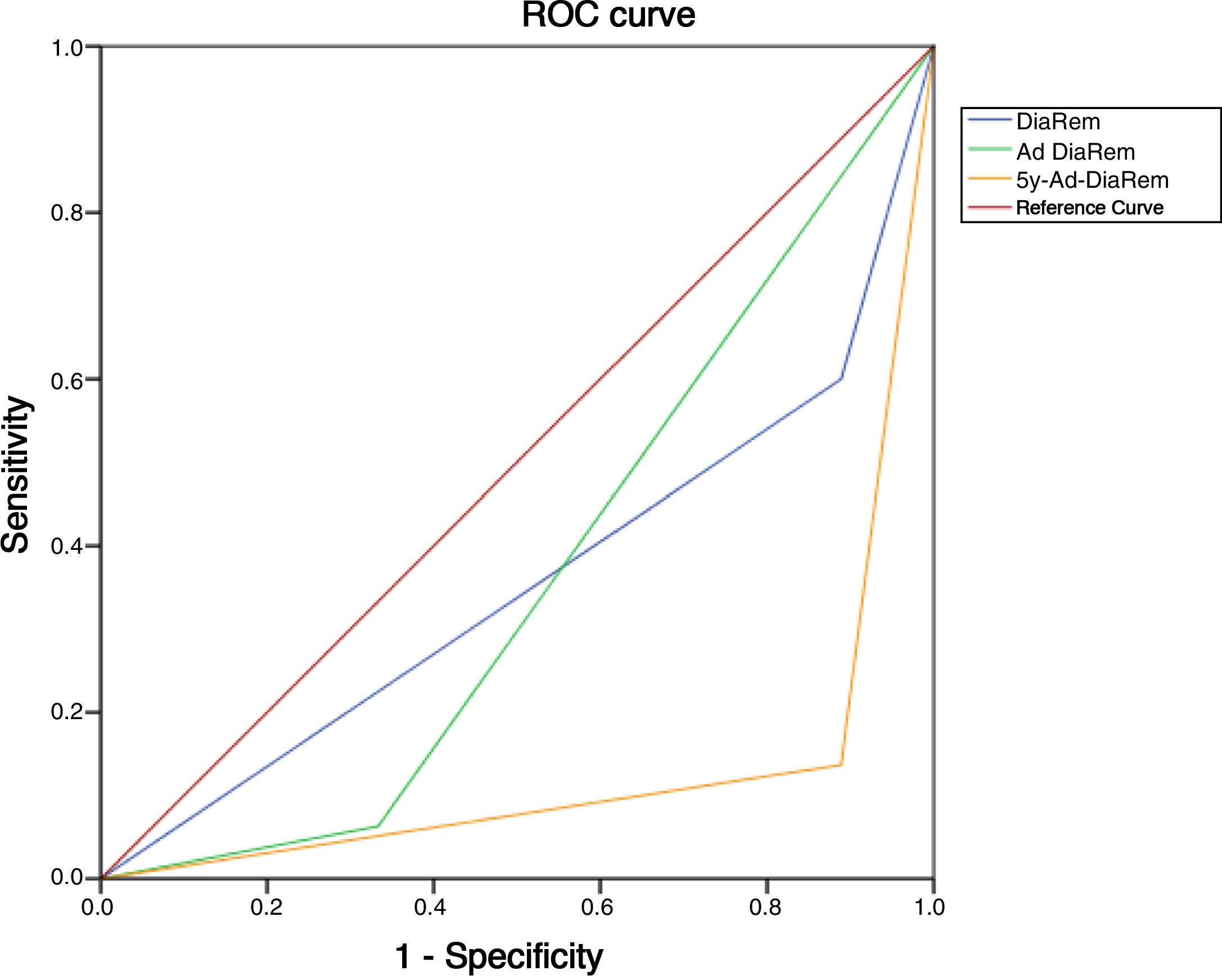
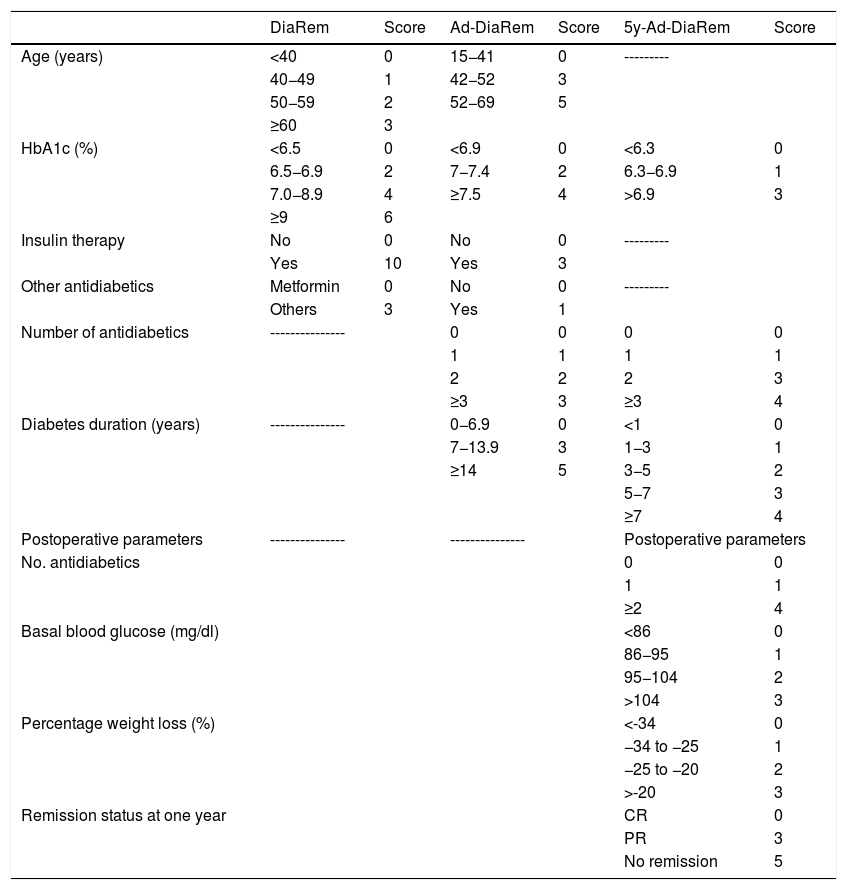
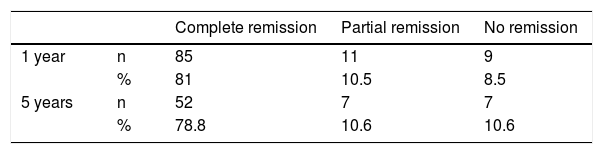
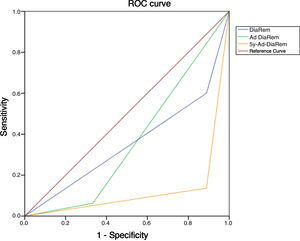
Diabetes remission in BPDThe percentages obtained for DR one year and five years after BPD using the ADA criteria are shown in Table 4. Using other scores that predict the likelihood of DR, we found high likelihood of DR after BPD in our sample of 37.1%, 91.4% and 80% predicted with DiaRem, Ad-DiaRem and 5y-Ad-DiaRem, respectively. The variables that make up the different scores are listed in Table 3. The PPV of DiaRem, Ad-DiaRem and 5y-Ad-DiaRem to assess the predictive capacity of DR after BPD were 97.7%, 93.8% and 98.8%, respectively. The false negative rates (FNR) for DiaRem, Ad-DiaRem and 5y-Ad-DiaRem were 60.42%, 6.25% and 13.5%, respectively. The analysis using ROC curves (Fig. 1) and AUC to assess the discriminative power to predict diabetes remission between scores produced the following results: 0.358 (CI 0.193−0.522) in DiaRem; 0.365 (CI 0.148−0.581) in Ad-DiaRem; and 0.123 (CI 0.00−0.249) in 5y-Ad-DiaRem.
Variables of the different DR scores.
| DiaRem | Score | Ad-DiaRem | Score | 5y-Ad-DiaRem | Score | |
|---|---|---|---|---|---|---|
| Age (years) | <40 | 0 | 15−41 | 0 | --------- | |
| 40−49 | 1 | 42−52 | 3 | |||
| 50−59 | 2 | 52−69 | 5 | |||
| ≥60 | 3 | |||||
| HbA1c (%) | <6.5 | 0 | <6.9 | 0 | <6.3 | 0 |
| 6.5−6.9 | 2 | 7−7.4 | 2 | 6.3−6.9 | 1 | |
| 7.0−8.9 | 4 | ≥7.5 | 4 | >6.9 | 3 | |
| ≥9 | 6 | |||||
| Insulin therapy | No | 0 | No | 0 | --------- | |
| Yes | 10 | Yes | 3 | |||
| Other antidiabetics | Metformin | 0 | No | 0 | --------- | |
| Others | 3 | Yes | 1 | |||
| Number of antidiabetics | --------------- | 0 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | |||
| 2 | 2 | 2 | 3 | |||
| ≥3 | 3 | ≥3 | 4 | |||
| Diabetes duration (years) | --------------- | 0−6.9 | 0 | <1 | 0 | |
| 7−13.9 | 3 | 1−3 | 1 | |||
| ≥14 | 5 | 3−5 | 2 | |||
| 5−7 | 3 | |||||
| ≥7 | 4 | |||||
| Postoperative parameters | --------------- | --------------- | Postoperative parameters | |||
| No. antidiabetics | 0 | 0 | ||||
| 1 | 1 | |||||
| ≥2 | 4 | |||||
| Basal blood glucose (mg/dl) | <86 | 0 | ||||
| 86−95 | 1 | |||||
| 95−104 | 2 | |||||
| >104 | 3 | |||||
| Percentage weight loss (%) | <-34 | 0 | ||||
| −34 to −25 | 1 | |||||
| −25 to −20 | 2 | |||||
| >-20 | 3 | |||||
| Remission status at one year | CR | 0 | ||||
| PR | 3 | |||||
| No remission | 5 | |||||
CR: Complete remission; PR: Partial remission; NR: No remission.
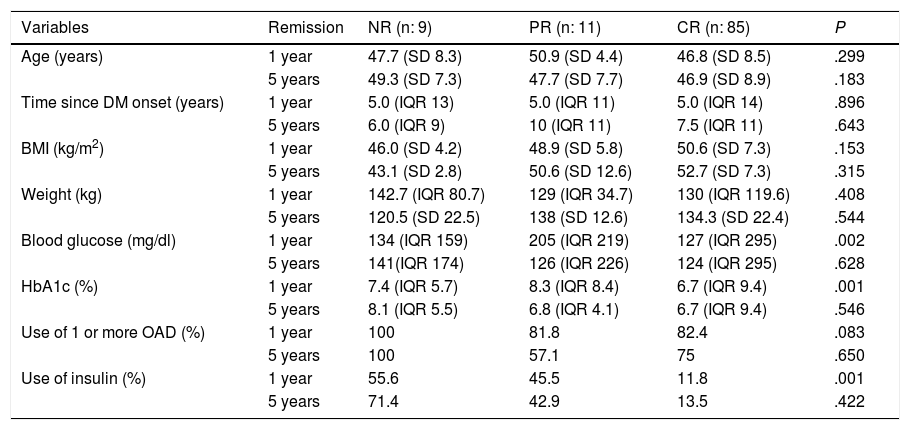
To determine the influence of the variables on DR, it is important to first know the pre-surgery clinical characteristics in patients with DR and patients with no remission according to the ADA, at one and five years after BS (Table 5). In the logistic regression to determine which variables predict DR at one year, 94.3% of the cases were correctly classified according to probability with an adequate Hosmer-Lemeshow test (0.69) and R squared of 0.142. The only variable that predicts remission at one year post-BS is baseline HbA1c [P .002 and OR 2.39 (CI 0.24−0.73)]. For remission at five years, 93.9% of the cases were correctly classified according to probability with an adequate Hosmer-Lemeshow test (0.91) and R squared of 0.324. The variables that predict remission at five years post-BS are baseline BMI [p 0.036 and OR 2.28 (CI 1.1–4.9)] and baseline HbA1c [P .040 and OR 2.47 (CI 0.170−0.96)].
Pre-surgery clinical characteristics in patients with DR and no remission at one and five years after BS.
| Variables | Remission | NR (n: 9) | PR (n: 11) | CR (n: 85) | P |
|---|---|---|---|---|---|
| Age (years) | 1 year | 47.7 (SD 8.3) | 50.9 (SD 4.4) | 46.8 (SD 8.5) | .299 |
| 5 years | 49.3 (SD 7.3) | 47.7 (SD 7.7) | 46.9 (SD 8.9) | .183 | |
| Time since DM onset (years) | 1 year | 5.0 (IQR 13) | 5.0 (IQR 11) | 5.0 (IQR 14) | .896 |
| 5 years | 6.0 (IQR 9) | 10 (IQR 11) | 7.5 (IQR 11) | .643 | |
| BMI (kg/m2) | 1 year | 46.0 (SD 4.2) | 48.9 (SD 5.8) | 50.6 (SD 7.3) | .153 |
| 5 years | 43.1 (SD 2.8) | 50.6 (SD 12.6) | 52.7 (SD 7.3) | .315 | |
| Weight (kg) | 1 year | 142.7 (IQR 80.7) | 129 (IQR 34.7) | 130 (IQR 119.6) | .408 |
| 5 years | 120.5 (SD 22.5) | 138 (SD 12.6) | 134.3 (SD 22.4) | .544 | |
| Blood glucose (mg/dl) | 1 year | 134 (IQR 159) | 205 (IQR 219) | 127 (IQR 295) | .002 |
| 5 years | 141(IQR 174) | 126 (IQR 226) | 124 (IQR 295) | .628 | |
| HbA1c (%) | 1 year | 7.4 (IQR 5.7) | 8.3 (IQR 8.4) | 6.7 (IQR 9.4) | .001 |
| 5 years | 8.1 (IQR 5.5) | 6.8 (IQR 4.1) | 6.7 (IQR 9.4) | .546 | |
| Use of 1 or more OAD (%) | 1 year | 100 | 81.8 | 82.4 | .083 |
| 5 years | 100 | 57.1 | 75 | .650 | |
| Use of insulin (%) | 1 year | 55.6 | 45.5 | 11.8 | .001 |
| 5 years | 71.4 | 42.9 | 13.5 | .422 |
CR: Complete remission; PR: Partial remission; NR: No remission; DM: Diabetes mellitus; BMI: Body mass index; OAD: Oral antidiabetics.
We found an acceptable response in terms of weight loss after BS that remained stable throughout the five years of follow-up. The percentage of patients who achieved complete diabetes remission (according to ADA criteria) both at one year and at five years was high (approximately 80%). Other scores such as DiaRem, Ad-DiaRem and 5y-Ad-DiaRem had little power for predicting DR in BPD. HbA1c values prior to surgery predict DR at one and five years.
Weight change is one of the most important factors for determining the effectiveness of BS and the resolution of associated comorbidities, such as diabetes. %WL and %EWL are two widely used and accepted parameters for measuring the success of BS. Baltasar et al. recommend post-surgery assessment of %EWL and BMI, classifying the results as excellent (BMI < 30 and %EWL > 65%), acceptable (BMI 30–35 and %EWL 50%–65%) and failure (BMI > 35 and %EWL < 50%).14 In our series, pre-surgery weight and BMI were 129.7 kg (IQR 120.4) and 49.4 kg/m2 (SD 7.1), respectively, and %WL and %EWL were used to measure the outcome of the BS. Overall, the greatest reduction in weight and BMI occurred at one year post-BS, with a slight increase in both at five years. The BMI dropped from 49.4 kg/m2 to 33.1 kg/m2 with a %EWL of 62.0% at one year post-BS, and 33.7 kg/m2 with a %EWL of 61.5% at five years post-BS, indicating an acceptable response to BS that remained stable over time. In our sample, the patients underwent two different surgical techniques; restrictive (SG) and malabsorptive (BPD). The patients with BPD had a %EWL of 62.4% and 61.5% at one year and five years after surgery. The patients who had SG had a %EWL of 59.3% at one year. These values are in line with those reported in the literature: 55%–63% in restrictive techniques15,16; and 60%–68% in malabsorptive techniques.17,18 The fact that the subjects operated on by BPD had greater degrees of initial obesity, HT, and hypertriglyceridaemia should be taken into account, as that may have influenced the use of a more effective technique in terms of weight loss in this group.
The BS showed a significant increase in DR compared to conservative treatment, even up to five years after surgery. In the study of Swedish subjects with obesity, the rate of DR shortly after BS was 72% compared to 21% in subjects treated conservatively.19 In our sample, the median time since onset of diabetes was five years (IQR 15); 82.9% were on treatment with one or more oral antidiabetic drugs and 18.8% were on insulin therapy prior to the BS. We found 33.2% and 32.6% reductions in blood glucose levels at one and five years after surgery, with reductions in HbA1c of 29.5% and 31.1%, respectively. Also reduced was the percentage of patients who remained on oral blood glucose-lowering treatment (9.4% and 6% at one and five years, respectively) and suspension of insulin therapy. In the five-year follow-up study by Mingrone et al., GB or BPD were compared with conventional medical treatment in obese, poorly controlled patients with long-standing DM2. Of the patients treated surgically, 50% maintained a PR at five years, while 0% achieved CR at five years. Recurrence of DM2 was observed in half of the patients two years after GB and in one third of the patients two years after BPD, irrespective of the amount of weight loss. This was one of the first studies to clearly indicate that continuous monitoring of blood glucose control is justified after BS, in spite of initial remission of DM2, due to the real risk of recurrence of hyperglycaemia.20
The restrictive and/or malabsorptive component of the different techniques limits the intake or absorption of nutrients, respectively. The neurohormonal component also has an influence; there is an increase in peptide YY (anorexic) and a decrease in ghrelin (orexigenic). The surgery causes an incretin effect, increasing GLP1 and GIP, decreasing glucagon and increasing insulin, inducing a feeling of early satiety at the level of the central nervous system (CNS).3 In morbidly obese patients, malabsorptive procedures seem to have a better antidiabetic effect and superior weight loss outcomes compared to exclusively restrictive procedures, including gastric bands or SG.21 In our patients, one year after BPD, the reduction in blood glucose and HbA1c was greater than in those who had undergone SG (34.7% reduction in blood glucose in BPD vs 20.0% in SG; 31.5% reduction in HbA1c in BPD vs 12.9% in SG). As with weight and BMI, patients who underwent BPD had higher pre-surgery blood glucose and HbA1c values than those who underwent SG.
In the literature, the percentages of DR after BS are highly variable, due to the great diversity in the criteria used to define DR. The ADA published criteria in 2009, taking into account HbA1c and blood glucose levels, without antidiabetic treatment.13 Buchwald et al. found greater DR after BPD (95.1%), followed by GB (80.3%), gastroplasty (79.7%) and gastric band (56.7%), continuing to maintain similar figures two years after the surgery. The percentage of remission was significantly correlated with weight loss.15 Adami et al. achieved DR in 51% at one year post-BPD and 27% at five years, and determined that the initial BMI predicts DR at five years post-BPD.22 In our series, the results in terms of DR (PR + CR) according to the ADA criteria were 91.5% at one year post-BPD and 89.4% at five years, and these data are in line with those reported in the literature.
There are several validated scores that predict DR with restrictive techniques: DiaRem, Ad-DiaRem and 5y-Ad-DiaRem. DiaRem is validated in two cohorts for the prediction of DR after GB. Its main variables are age, use of antidiabetic drugs and HbA1c. Its creators reported a CR or PR rate of 63%,7 using 259 clinical variables to identify independent predictive factors and develop a prediction model. The need for insulin pre-BS was the strongest indicator for predicting remission: patients who required insulin for blood glucose control before surgery were 7.25 times less likely to achieve CR or PR after surgery. The likelihood of DR is established based on the score ranging from 0 to 22, obtained by weighting each variable. The higher the score, the greater the likelihood of remission.21 One criticism of the DiaRem score is that it does not take into account the duration of diabetes. Duration is considered a strong predictor in diabetes remission as it can be an indicator of pancreatic function.23 Ad-DiaRem adds two clinical parameters to the original DiaRem; duration of diabetes and number of antidiabetic drugs. Compared to DiaRem, it has greater accuracy in predicting DR at one year post-GB; it correctly classified 180/213 patients compared to DiaRem, which correctly classified 164/213. This is probably due to the fact that DiaRem included the patient's age, an indirect marker of the duration of diabetes, especially in obese patients in whom the onset of this disease is premature. Diabetes duration is considered a consistent indicator of disease progression, and the best predictor of post-BS DR.8 Scoring methods for predicting DR outcomes one year after surgery cannot accurately predict DR at five years. The 5y-Ad-DiaRem score includes baseline data (duration of diabetes, number of antidiabetic treatments and HbA1c) and parameters after one year of follow-up (blood glucose, number of antidiabetic treatments, remission status, weight loss in the first year). It was more accurate than DiaRem and Ad-DiaRem in predicting DR at five years, reclassifying 13 patients with 90% precision compared to DiaRem (79% precision) and 12 patients with 90% precision compared to Ad-DiaRem (78% precision).9
The percentages of high probability of DR after BPD in our sample were 37.1%, 91.4% and 80% with DiaRem, Ad-DiaRem and 5y-Ad-DiaRem, respectively. The score with the highest predictive power in DR is 5y-Ad-DiaRem (PPV 98.8% with a false negative rate of 13.5%) and then DiaRem (PPV 97.7% but with a higher false negative rate; 60.42%). The discriminative ability to assess DR with the different scores (DiaRem, Ad-DiaRem or 5y-Ad-DiaRem) is low, with AUC less than 0.5. The model does not have the discriminatory power to distinguish between remission and no remission.
An association has been observed in different publications between pre-surgery parameters (laboratory and clinical) and a higher likelihood of DR. Shorter duration of diabetes, lower pre-surgery fasting blood glucose, and techniques such as GB and BPD independently predict higher rates of remission, with better outcomes in DR if surgical intervention is performed early and with better blood glucose control, regardless of the BMI.9 An association has also been found between pre-surgery diabetes treatment and remission rates.24 Age, duration of DM2, degree of control and previous therapeutic regimen were predictive of remission and relapse of DM2.25 It has recently been discovered that circulating succinate levels are reduced after BS (GB, sleeve gastrectomy and sleeve gastroplasty with plication), with a predictive value for DR independent of pre-surgery factors and improving the currently available scores for predicting remission.26 In the future, this measurement could be used to improve DR prediction on new scales. In our study, the patients who achieved CR had a higher BMI and blood glucose, were older and had lower HbA1c than those who did not achieve remission, and they also had a lower percentage of oral antidiabetic agent (OAD) and insulin use. The only variable that predicts remission at one year post-BS is baseline HbA1c. If the rest of the variables remain constant, for each unit decrease in HbA1c, DR is 2.39 times more likely than NR. The variables that predict remission at five years post-BS are baseline BMI and baseline HbA1c. If the rest of the variables remain constant, for each unit increase in BMI, DR is 2.28 times more likely than NR. If the rest of the variables remain constant, for each unit decrease in HbA1c, DR is 2.47 times more likely than NR.
In conclusion, in our study we found an acceptable weight-loss response after BS, which was maintained throughout the follow-up period. The rates for complete diabetes remission according to the ADA criteria were close to 80% at one year and five years post-BPD. Malabsorptive surgery techniques may therefore play an important role when one of the aims of the BS is long-term DR. HbA1c values prior to surgery predict diabetes remission at one and five years post-surgery. DiaRem, Ad-DiaRem and 5y-Ad-DiaRem have little power for predicting diabetes remission in BPD.
We are aware of a number of limitations in the way our study was carried out, including the fact that it was single-centre and retrospective. The selection of the surgical technique was not random, but at the discretion of the team responsible for the patient and based on the patient's comorbidities and characteristics. That meant that the number of patients operated on by SG was not very representative for making comparisons with those operated on by BPD. There are also limitations in the collection of some data due to lack of patient follow-up and the absence of post-surgery data at five years for SG. There is no consensus concerning the criteria to define diabetes remission after bariatric surgery. Randomised clinical trials would be necessary to obtain conclusions with a higher level of evidence than we have presented here.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: González Arnáiz E, Ballesteros Pomar MD, Pintor de la Maza B, González Roza L, Ramos Bachiller B, Barajas Galindo D, et al. Remisión de diabetes tras cirugía bariátrica malabsortiva. Endocrinol Diabetes Nutr. 2021;68:218–226.