Diabetic foot osteomyelitis (DFO) is the most common infection associated to diabetic foot ulcers (DFU). This review is designed to provide an update on the diagnosis and treatment of DFO based on an analysis of MEDLINE through PubMed using as search criterion “Diabetic Foot Osteomyelitis”. Authors have included in this review the most relevant manuscripts regarding diagnosis and treatment of DFO. After review and critical analysis of publications, it may be concluded that diagnosis of DFO is not simple because of its heterogeneous presentation. Clinical inflammatory signs, probe-to-bone test, and plain X-rays are postulated as the basic tests for clinical diagnosis when DFO is suspected. Diagnosis should be supported by laboratory tests, of which ESR (>70mm/h) has been shown to be most precise. MRI is the most accurate imaging test, especially for differential diagnosis with Charcot foot. Pathogen isolation by bone culture is essential when the patient is treated with ATB only. Medical or surgical treatment should be based on the clinical characteristics of the patient and the lesion. Surgery should always be an option if medical treatment fails.
La osteomielitis (OM) es la infección más frecuente asociada a las úlceras de pie diabético (PD). En la presente revisión se pretende ofrecer una actualización sobre el diagnóstico y el tratamiento de la OM de PD tras analizar fundamentalmente la base de datos MEDLINE a través de Pubmed con los criterios de búsqueda «Diabetic Foot Osteomyelitis». Se incluyeron en la presente revisión a criterio del autor los artículos más relevantes en el campo diagnóstico y terapéutico de la OM de PD. Tras la revisión y el análisis crítico de las publicaciones se puede concluir que el diagnóstico de la OM en PD no es sencillo debido a su presentación heterogénea. La recogida de signos clínicos inflamatorios junto al test del Probe-To-Bone y la radiografía simple se postulan como las pruebas de diagnóstico clínico básicas ante la sospecha de OM. La confirmación diagnóstica debería estar respaldada con la evaluación de pruebas de laboratorio, donde la VSG (>70mm/h)ha demostrado ser el valor más preciso. La RMN es la prueba de imagen con mayor precisión diagnóstica y cobra especial valor en el diagnóstico diferencial con el pie de Charcot. El aislamiento del patógeno mediante cultivo óseo es básico sobre todo cuando el paciente se trata exclusivamente con ATB. El tratamiento médico o quirúrgico debe basarse en las características clínicas del paciente y de la lesión, debiendo ser la cirugía siempre una opción posible en caso de fracaso del tratamiento médico.
Diabetic foot (DF) is a complication of diabetes mellitus resulting from foot ulcers caused by external or internal trauma associated with different stages of diabetic neuropathy and peripheral vascular disease.1 The most severe consequence of a diabetic foot ulcer is a major or minor amputation.2 Major amputation has been related to a dramatic reduction in the life expectancy of these patients, whose mortality rates approach and sometimes surpass those of colon, prostate and breast cancers, and Hodgkin's disease.3
Foot or leg amputation mainly results from ischemia- or infection-related events, the latter being the main cause.4 Diabetic foot infection may involve soft tissues or bone. Soft tissue infections are more severe and have a worse prognosis. However, osteomyelitis (OM) is the most common diabetic foot infection, accounting for more than 20% of moderate infections and 50%–60% of severe infections, which are associated with a high amputation rate.5
Osteomyelitis in diabetic foot represents a diagnostic and therapeutic challenge, and many consequences of the condition are clearly associated with late diagnosis, delayed referral, or inadequate treatment.
This literature review is intended to establish recommendations based on both the evidence and the authors’ clinical experience to provide readers with diagnostic criteria and guidance for better therapy.
MethodsThis literature review has been based on a retrospective search up to July 2016 of the main healthcare databases, particularly MEDLINE through PubMed. The search criteria used to select articles were the keywords “diabetic foot osteomyelitis” included in the title or abstract. The review also included current versions of the international consensus guidelines on the evaluation and management of diabetic foot infections published by the Infectious Diseases American Association (2012 IDSA guidelines), and the International Working Group on the Diabetic Foot (2015 IWGDF guidelines).
Article selection was directional and at the authors’ discretion, and focused on diabetic foot osteomyelitis (DFO) diagnosis and treatment. Once the articles had been examined, the review was structured using the following sections: diagnosis of DFO (clinical diagnosis, laboratory tests, microbiological and histological analysis, and imaging tests), and treatment of DFO (medical and surgical).
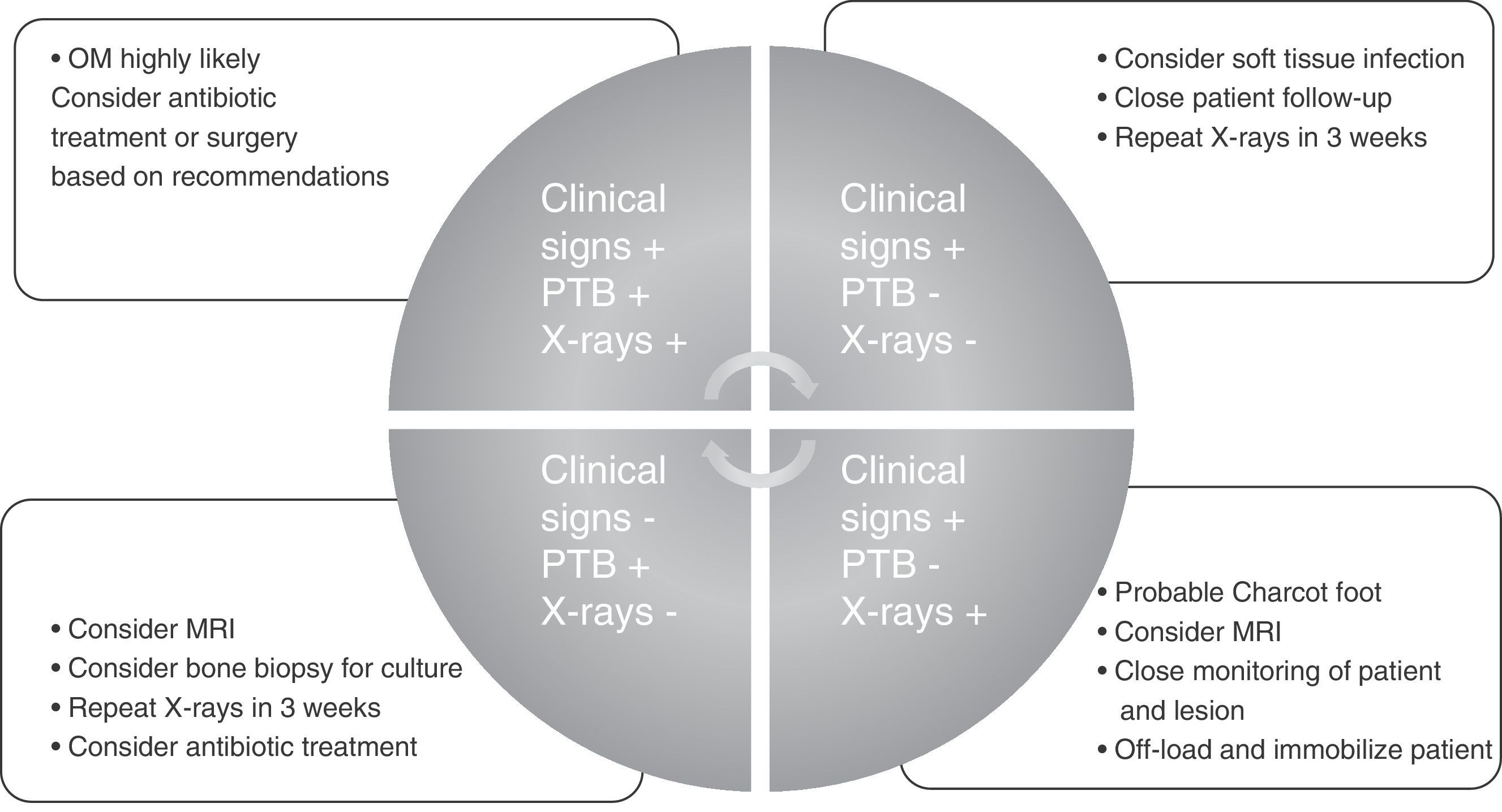
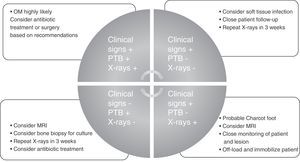
Diagnosis of diabetic foot osteomyelitisDiagnosis of DFO always begins with clinical suspicion of the infection. Suspicion is relatively obvious when OM is associated with soft tissue infection because there is inflammatory response in the tissues. The presence of clinical signs of inflammation in an ulcer near a bony prominence (pain, heat, redness, swelling, and cellulitis) and/or a purulent or synovial discharge from a joint suggest OM. However, according to the literature, the clinical signs associated with bone infection are not very relevant for diagnosis.6
Visible bone through the ulcer, the exposure of capsular structures, granuloma not adhering to the ulcer bed, sausage toe (gross inflammation of a toe that prevents the identification of intra-articular folds) and/or an ulcer that does not progress within 12 weeks in a patient without ischemia and with an adequate off-loading device are all signs directly related to DFO.5
It should be noted that diabetic neuropathy present in most patients with osteomyelitis may mask the clinical signs and symptoms of the infection.5 Similarly, the presence of peripheral vascular disease may minimize clinical signs. Because of this, different clinical presentations of OM, associated with different prognoses, have been reported in the literature.7
However, the main challenge in OM diagnosis is the possibility of bone infection without clinical manifestations, which occurs in approximately 50% of cases of chronic or fibrotic OM.8
When OM is suspected or needs to be ruled out, two clinical tests, which the literature recommends being used together may be performed. The first is the probe-to-bone (PTB) test, which consists of bone palpation through the ulcer with a sterile blunt probe. This procedure has a sensitivity and specificity of 0.87 and 0.91 respectively, a positive predictive value (PPV) of 0.57, and a negative predictive value (NPV) of 0.98.9 One article summarizing the available evidence in the diagnosis of DFO stated that a positive PTB test in an infected wound is highly suggestive of OM, whereas a negative PTB test does not exclude the diagnosis.10 In uninfected ulcers or low risk patients, OM is unlikely if the PTB test is negative and a positive PTB test is poorly specific.10 One article11 reported for PTB tests a 94% efficiency rate, sensitivity and specificity levels of 98% and 78%, a PPV of 95%, and a NPV of 91%, thus suggesting that the test is an effective tool for diagnosing OM.
However, some authors have recently questioned the value of this test, especially for patients for whom greater accuracy is needed,12 stating that a positive PTB test is not synonymous with OM, and suggesting that it should be considered as a screening method rather than as a diagnostic test. They also warned that systematic and indiscriminate use of this test may overestimate the diagnosis, leading to unjustified antibiotic treatment with the resultant risk of generating resistance. Obviously, as with any other diagnostic test, the result of the PTB test is related to the prevalence of the condition.
Based on the foregoing, it is advisable to always interpret the PTB test along with plain X-rays.
One of the main difficulties in the diagnostic interpretation of OM by X-rays is that it takes approximately two weeks for bone loss to become visible.5 X-rays are therefore recommended both at the start and two weeks later. Negative follow-up radiographic findings make the presence of OM unlikely, while the development of bony erosions makes OM very likely.5 A review10 stated that since the reported studies did not serially evaluate X-rays, their accuracy as a diagnostic method is low to moderate, probably because of the delay until radiographic changes become visible.
An additional difficulty is the interpretation of X-rays alone, that is, without knowing the clinical characteristics of the ulcer. This often occurs when a radiologist interprets X-rays without having seen the patient. X-ray interpretation without seeing the patient or knowing his/her history or background results in a low diagnostic association strength, even among experienced clinicians.13 This suggests that X-rays should always be interpreted by the requesting physician, in addition to the reporting radiologist.
Although both the level and magnitude of bone destruction seen in X-rays are clearly important for diagnosis, they have not been associated with any prognostic value when surgery is performed.14
Most authors agree that DFO may be accurately diagnosed when the PTB test is positive and there are radiographic signs of bone destruction (sensitivity of 97%, specificity of 92%, PPV of 97%, and NPV of 93%), but the healthcare providers and their level of specialization in diabetic foot management also have an effect.15,16
Therefore, joint diagnostic interpretation of both the PTB test and plain X-rays is recommended to further diagnostic agreement and decrease variability between the clinicians who interpret them.17
When diagnostic suspicion is supplemented with the abovementioned clinical tests, it is advisable to have laboratory tests providing information related to OM. Infection has been shown to impair metabolic control in patients, making them particularly sensitive to changes in blood glucose levels.4 However, changes in the classical inflammatory markers are not commonly found in OM, and although several studies have searched for an analytical marker to facilitate OM diagnosis, the data are not conclusive yet.
The biomarker which has been shown to be the most important in the diagnosis of OM is the erythrocyte sedimentation rate (ESR). An article by Malabu et al.18 concluded that the ESR was the hematological parameter which best discriminated between OM and cellulitis. A study comparing the clinical and laboratory findings in patients with and without OM19 reported that ESR values ≥65mm/h combined with an ulcer size ≥2cm2 had a sensitivity of 83%, specificity of 77%, PPV of 80%, and NPV of 81% for the diagnosis of OM. There is general agreement that ESR values over 70mm/h increase the likelihood of OM, and ESR continues to be the most useful and widely used parameter for diagnosing DFO.20,21 It should be noted, however, that bone infection may exist even if ESR values are normal; thus, like other inflammatory markers, ESR offers a summation value, but it is not indispensable for the diagnosis of OM.22
C-reactive protein (CRP) has a limited value as compared to the ESR and even procalcitonin (PCT).10 Changes in serum PCT levels have been shown in DF infections, but additional research is required to determine the value of this test.5
Regardless of the diagnostic value of these inflammatory markers, some studies have tried to prove their prognostic value or even their use as OM cure markers. One study combined the ESR and CRP to assess the results of treatment of DFO23 and noted no correlation of these markers with OM remission or cure. Higher ESR values were associated with poor treatment results, while lower white blood cell counts and glomerular filtration rates were related to the remission of bone infection. Another study showed that ESR became normal in treated OM patients at healing, regardless of treatment, with no correlation at all with CRP values.24
Other laboratory markers for monitoring OM have been assessed without much success. Thus, a case–control study25 assessed N-terminal telopeptide and bone alkaline phosphatase as bone turnover markers that might be of use in the diagnosis and follow-up of OM patients. However, no significant differences were found in these levels (they were similar at admission and after 6 weeks of treatment) even when patients with poor outcomes were compared to patients with favorable outcomes. Another study26 analyzed inflammatory markers to diagnose and monitor DFO, and showed PCT to be the best marker for detecting patients with OM at initial diagnosis. Proinflammatory cytokines did not appear to be useful for differentiating bone from soft tissue infections, but interleukin-6 (IL-6), together with CRP, the ESR, and PCT, were decreased in patients with OM as compared to those with soft tissue infection.
Some experimental data suggest that high levels of tumor necrosis factor α may be directly related to histopathological changes of OM, and that the local release of IL-6 is also increased in bacterial OM.27
Although no adequate evidence is available regarding the value of these parameters, the current IDSA4 guidelines suggest that decreases in previously elevated markers may be of help for deciding on the discontinuation of antibiotic therapy. Clinical examination combined with laboratory tests may also be strong indicators of OM, thus obviating the need for more costly imaging procedures.19,22
When a diagnosis of OM cannot be made using the above methods or there are doubts about differential diagnosis, more specialized tests may be performed, including magnetic resonance imaging (MRI). MRI is considered the most accurate of the imaging tests available for the diagnosis of OM (sensitivity 90%, specificity 83%)28 because of its interpretative accuracy; however, its precision in the presence of ischemia is still unclear.29 Studies have shown that MRI has the best diagnostic accuracy and that it is also quite useful for assessing the extension and depth of soft tissue infection. However, its specificity may be affected by the difficulty in differentiating OM from other causes of marrow edema.10 MRI has the advantage that it may be used for diagnosis, treatment, and follow-up.30
Computed tomography (CT) has been used for years combined with different markers for differential diagnosis of other diseases, such as Charcot foot, where the value of 18F-fluordeoxyglucose positron emission tomography has been reported.15 Another study assessed the efficacy for OM diagnosis of 67Ga single photon emission tomography combined with percutaneous biopsy; this combination was found to have a high accuracy and safety for the diagnosis of OM in patients with signs of soft tissue infection.31
Hybrid 18F-FDG PET and PET/CT techniques have become alternative imaging tests for the diagnosis of OM, but the results to date are preliminary, and additional research is needed. In addition, glucose fluctuations may affect the tissue uptake of 18F-FDG.30
White blood cell CT has been used to assess OM remission with values of sensitivity, specificity, PPV, and NPV to predict OM relapses of 100%, 91.5%, 71.5%, and 100% respectively, as compared to 80%, 33%, 20%, and 89% for X-rays and 100%, 12.5%, 15.5%, and 100% for three-phase bone scintigraphy.32 Another study where white blood cell CT was performed before and after OM treatment did not reach conclusive results regarding its prognostic value.33
Bone scans are too non-specific for diagnosing bone infection, except for labeled white blood cell scintigraphy, which has shown a moderate value in the diagnosis of OM. However, its precision regarding the anatomical location of bone infection is not the best.5,30
Three-phase bone scan using techmetium-99 has also been used to diagnose OM, but it has a high rate of false positives because of its low specificity, and may remain positive four months after successful treatment.10 Labeled white blood cell scintigraphy is useful for the diagnosis and follow-up assessment of medically treated OM, but it is an expensive, time-consuming test, and less specific than MRI.10
The isolation of the pathogen causing the infection is of the utmost importance, especially in patients treated with antibiotics only.34 There is no agreement as to how to obtain the bone sample for culture, but it is assumed that only a bone culture is useful for prognosis.
A swab culture is not reliable and carries the risk of isolating microorganisms not implicated in OM, generating bacterial resistance and aggravating the condition. Senneville et al.35 showed that the results obtained from swabs did not reliably identify the bacteria isolated from bone samples as only 17.4% (n=12) of bone culture and swab samples were identical. Elamurugan et al.36 concluded that swab cultures are less accurate than cultures of bone biopsies for the identification of all microorganisms responsible for OM. An additional article by Malone et al.37 concluded that deep ulcer cultures show a good correlation with bone cultures (25 out of 34 cases, 73.5%, p<0.001), and therefore represent a sensitive assessment method that may provide guidance on the probable pathogens causing bone infections, and help in selecting the most adequate antibiotic when bone biopsy is not feasible.
The sampling procedure for bone culture may also influence the result, as there may be false positives due to potential contamination during sampling through the ulcer, or false negatives in patients with prior treatment or erroneous sampling from an uninfected area.5 The latest consensus guidelines published by IWGDF21 define percutaneous bone sampling as the best alternative.35 However, this method requires trained professionals and a fluoroscope to guide the biopsy needle, as well as a surgical environment. Sampling through the ulcer still seems to be a reliable alternative, but should be done after careful wound debridement and after an antibiotic washout period of at least 48–72h.38
Histological examination of the bone sample is still considered the gold standard for the diagnosis of OM. Four different types of OM are distinguished based on the tissue's inflammatory infiltrate: chronic, acute, chronic-acute, or fibrosis.8 This study makes it possible to understand why the clinical presentations of OM are so diverse, and that there are no clinical signs in most cases of chronic and fibrotic OM. The definition of these histological patterns has helped pathologists, who did not previously have a theoretical protocol for the histological analysis of this type of infection, to agree on diagnosis.39 It is also known that histological changes caused by OM are clearly distinguishable from those caused by Charcot foot.40
The study of the microbiome of bacteria causing DFO using gene sequencing techniques has recently become more common.41 Compared to the standard methods, this technique isolates more anaerobic pathogens, more Gram-positive bacilli, and a higher proportion of polymicrobial infections. The use of advanced biological molecular technology is of particular interest in OM, especially when the chronic nature of the infection and bacterial adhesion make pathogen isolation difficult.41
Treatment of diabetic foot osteomyelitisThere are multiple articles in the literature on the treatment of OM, but there is no agreement regarding the best therapeutic option. Standardization of a single treatment option is far from easy because DFO is not homogeneous, and may have several clinical presentations and may or may be not associated with ischemia and soft tissue infection. On the other hand, the medical or surgical specialization of the professional in charge of the patient, and the care environment in which patient management takes place clearly determine the preference for either medical treatment (antibiotics only) or surgery.
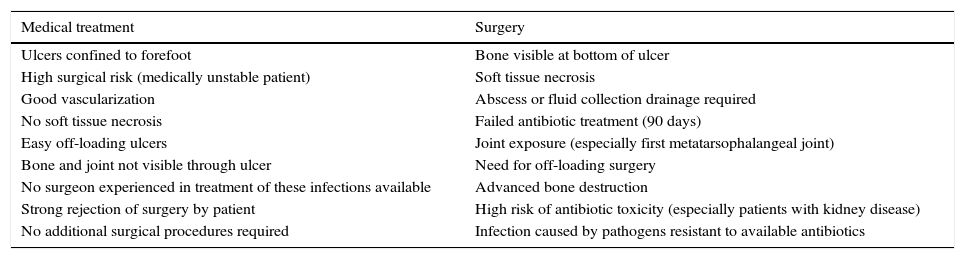
There is some consensus as to when the first treatment option should be medical and when surgical (Table 1).42
Indications of primary treatment according to patient characteristics and the lesion associated with diabetic foot osteomyelitis.
| Medical treatment | Surgery |
|---|---|
| Ulcers confined to forefoot | Bone visible at bottom of ulcer |
| High surgical risk (medically unstable patient) | Soft tissue necrosis |
| Good vascularization | Abscess or fluid collection drainage required |
| No soft tissue necrosis | Failed antibiotic treatment (90 days) |
| Easy off-loading ulcers | Joint exposure (especially first metatarsophalangeal joint) |
| Bone and joint not visible through ulcer | Need for off-loading surgery |
| No surgeon experienced in treatment of these infections available | Advanced bone destruction |
| Strong rejection of surgery by patient | High risk of antibiotic toxicity (especially patients with kidney disease) |
| No additional surgical procedures required | Infection caused by pathogens resistant to available antibiotics |
Modified from Lipsky.42
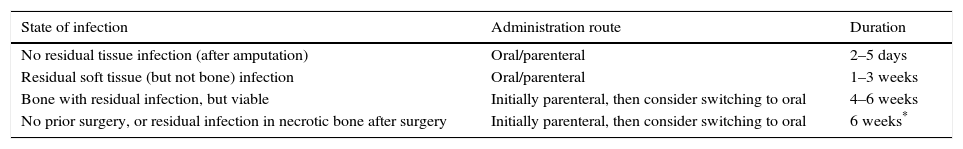
The duration of antibiotic therapy reported in the literature is highly variable: from a few weeks to more than one year. The main aspects which need to be taken into consideration are when to perform bone resection, which antibiotic to use, by which route, and for how long (Table 2).4,43 It is advisable to verify the diagnosis of OM before antibiotics are administered, and to consider a bone culture when antibiotic therapy alone has been decided upon, in order to decrease the possibility of generating resistance.21
Recommended administration route and duration of antibiotic therapy depending on the presence of residual tissue infection and prior surgical debridement.
| State of infection | Administration route | Duration |
|---|---|---|
| No residual tissue infection (after amputation) | Oral/parenteral | 2–5 days |
| Residual soft tissue (but not bone) infection | Oral/parenteral | 1–3 weeks |
| Bone with residual infection, but viable | Initially parenteral, then consider switching to oral | 4–6 weeks |
| No prior surgery, or residual infection in necrotic bone after surgery | Initially parenteral, then consider switching to oral | 6 weeks* |
Modified from IDSA 2012 Guidelines.4
Several articles have reported the efficacy of medical treatment in OM. Embil et al.44 concluded that in most cases OM was well managed with oral treatment, with or without debridement (28% of patients with OM required bone debridement, and 10% required a minor amputation). They also stated that this treatment would be useful at hospitals with limited resources in terms of specialists in infectious diseases and surgery. A retrospective study34 assessed the results of OM treated without surgery, and found that bone culture-based antibiotic therapy was a predictor of success in diabetic patients who underwent no surgery, and was associated with infection remission. A prospective study45 reported healing in 66.9% of patients with antibiotic treatment alone, while 13.9% required amputation, of which 1.5% were major amputations. One study46 used a combination of oral rifampin plus ofloxacin to treat mild to moderate OM for a median of six months. Cure was defined as the disappearance of all signs and symptoms of infection, and the absence of relapse during follow-up. Cure was achieved in 88.2% of patients, and 76.5% of these maintained remission for a mean post-treatment follow-up period of 22 months. Another study47 identified the clinical variables affecting the outcome of nonsurgical treatment of OM and reported that during a 12-month follow-up period, 63.5% of patients treated achieved remission (no clinical or radiological signs of infection). Thus, almost two-thirds of patients with OM were cured without their having to undergo surgical bone resection.
Another treatment option is topical antibiotic therapy, which has the advantages of reducing the duration of oral antibiotic therapy, decreasing hospital costs, using higher antibiotic concentrations in the affected area with fewer pharmacokinetic effects, and more potency against resistant bacteria.48,49 However, the currently available data are insufficient to assess the efficacy of this treatment in DFO.4,21
Although bone cultures and bone biopsy have apparently been shown to be helpful for selecting adequate antibiotic therapy, there are no adequate data regarding the most suitable administration route or optimal treatment duration.50 A recent study51 showed that six weeks of treatment achieve the same results as 12 weeks, but with better gastrointestinal tolerance, and it has therefore been assumed that this duration is sufficient to treat OM.21
The main limitations of treatment of OM with antibiotics alone include: the need for a bone culture to ensure good prognosis, the lack of criteria regarding the administration route to be used depending on infection severity and bacterial resistance, the lack of agreement as to the most adequate treatment duration, higher amputation rates, a higher risk of relapse, antibiotic toxicity, the possibility or inducing bacterial resistance, and the lack of certainty regarding bone infection cure, as most published studies consider the remission of signs of infection as indicative of treatment success.42,52
The advantages of antibiotic treatment are that it avoids potential surgical complications, may be given in any care setting, decreases the costs associated with surgery and avoids the anatomical changes associated with the surgical procedure.42
There are also criteria that require the use of surgery as the first treatment option (Table 1). However, surgery should always be considered if antibiotic therapy fails. Karchmer and Gibbons published the first article on the effectiveness of surgery in DFO in a series of 110 patients with diagnosis verified by bone biopsy in 96% of cases. This study found a major amputation rate of 19% and a cure rate of 50%.53 A subsequent study54 assessing the results of surgery in patients with histopathologically confirmed OM concluded that conservative surgery without minor or major amputation is successful in almost half the patients with DFO.
In patients where surgery partially eradicates bone infection, strategies consisting of the postoperative use of a super-oxidized solution55 have allowed for limb salvage in 100% of the cases, with a mean time to cure of 6.8 weeks.
Conservative surgery (defined as the removal of infected bone only, without amputation) combined with antibiotic treatment is an attractive option because it may reduce the changes in foot biomechanics and minimize the duration of antibiotic therapy.52 It is accepted that the combination of antibiotics with the surgical resection of infected bone may cure most cases of OM52 and is effective for both clinical cure and limb salvage.56
The first prospective, randomized clinical trial24 comparing surgery and medical treatment for OM, published in 2014, concluded that antibiotic therapy and surgery yielded similar results in terms of rates of cure, time to cure, and short-term complications in patients with neuropathic forefoot ulcers without soft tissue necrosis. A retrospective article compared a group of OM patients treated with surgery with one treated with antibiotics and found similar results.57 Because of the high risk of reulceration associated with the resection of a metatarsal head,58 surgical procedures that minimize the chance of reulceration should be considered. The amount of metatarsal bone resected and the length of the first metatarsal bone have been related to the risk of reulceration after surgery.59,60
The advantages of surgery are that it shortens antibiotic therapy, decreases the major amputation rate, allows for bone sampling for microbiological and histological examination, permits the removal of necrotic bone, as well as of bacteria and biofilm, prevents recurrence by removing bony prominences, and provides an opportunity to stabilize the foot.42
The disadvantages of surgery are that it may increase the risk of reulceration, that it is an expensive procedure, and increases the risk of perioperative morbidity, foot instability, and the risk of transfer reulceration.42
To conclude and summarize, we propose a guide for diagnosis and treatment that may be used in any care setting and by any medical or surgical specialist (Fig. 1).
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Lázaro-Martínez JL, Tardáguila-García A, García-Klepzig JL. Actualización diagnóstica y terapéutica en el pie diabético complicado con osteomielitis. Endocrinol Diabetes Nutr. 2017;64:100–108.