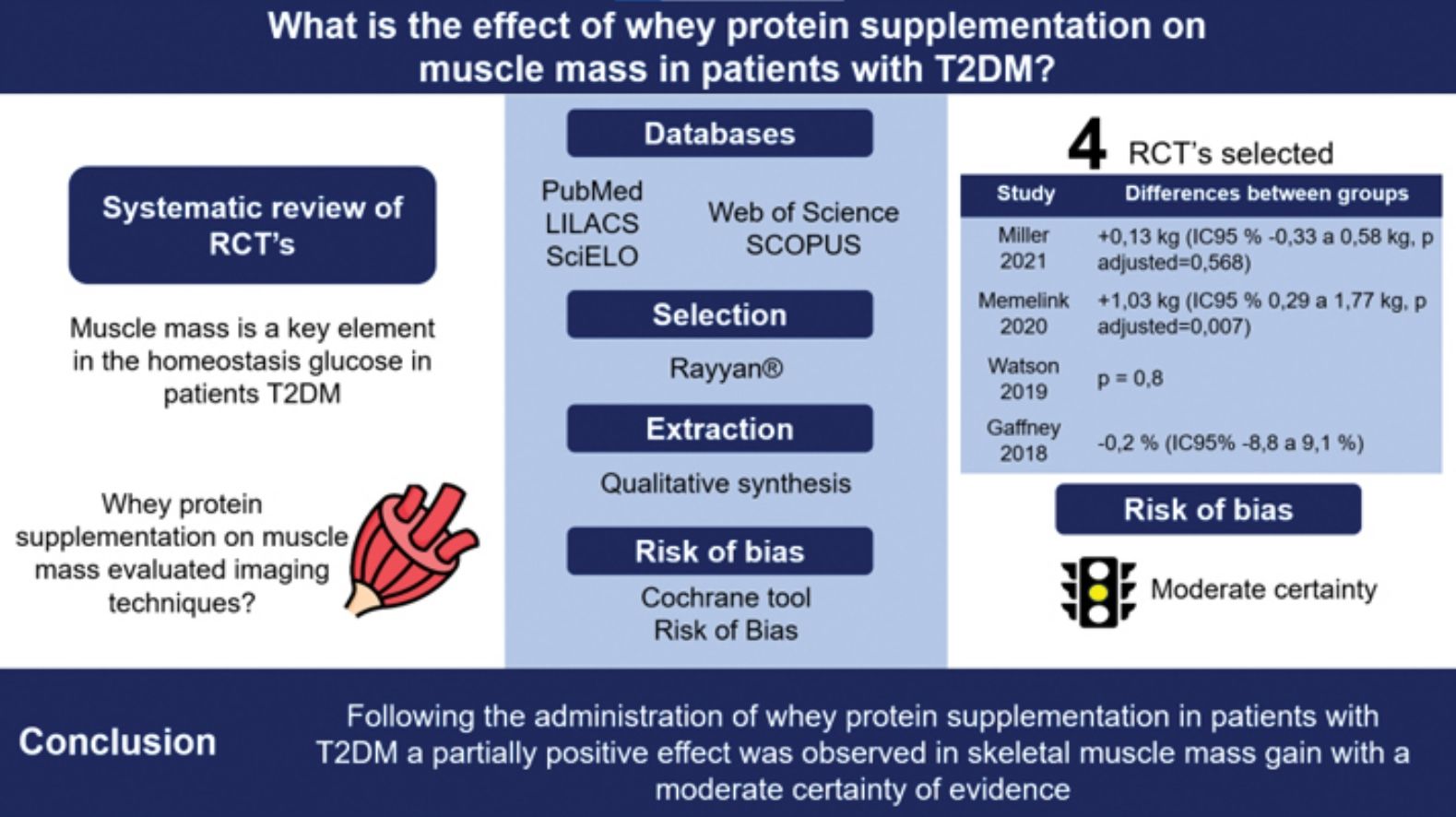
To investigate the overall effect of whey protein supplementation on skeletal muscle mass in adults with type 2 diabetes mellitus (T2DM).
MethodsSystematic review of reports on corporal muscle mass from clinical trials that assessed the use of whey protein supplementation by means of validated techniques in patients with T2DM. PubMed, SCOPUS, Web of Science, LILACS, and SciELO databases were searched up to April 2022. Risk of bias was assessed by the Cochrane Collaboration Risk of Bias tool. We conducted a qualitative synthesis of information.
ResultsFour studies (424 participants) that met the selection criteria were identified out of 1,787 records. Of these, 3 studies assessed the total muscle mass using dual-energy X-ray absorptiometry (DXA), and 1 reported changes to the transverse diameter of the vastus lateralis muscle with ultrasound imaging. In the intervention groups, DXA assessments demonstrated an increase in total muscle mass in 3 studies and in the appendicular muscle mass in 2. Changes to the proportion of muscle mass were not seen in the DXA studies and only a discrete difference was seen in the comparative groups studied by ultrasound imaging.
ConclusionFollowing the administration of whey protein supplementation in patients with T2DM, a partially positive effect was seen in skeletal muscle mass gain with a moderate certainty of evidence.
Investigar el efecto general de la suplementación con proteína de suero sobre la masa del músculo esquelético en adultos con diabetes mellitus tipo 2 (DM2).
MétodosRevisión sistemática de informes relacionados con la masa muscular corporal de ensayos clínicos que evaluaron el uso de suplementación con proteína de suero mediante técnicas validadas en pacientes con DM2. Se realizaron búsquedas en las bases de datos PubMed, SCOPUS, Web of Science, LILACS y SciELO hasta abril de 2022. El riesgo de sesgo se evaluó mediante la herramienta Cochrane Collaboration Risk of Bias. Se realizó una síntesis cualitativa de la información.
ResultadosSe identificaron cuatro estudios (424 participantes) que cumplieron con los criterios de selección a partir de 1.787 registros. De estos, tres estudios evaluaron la masa muscular total mediante absorciometría de rayos X de energía dual (DXA) y uno informó cambios en el diámetro transversal del músculo vasto lateral con imágenes de ultrasonido. En los grupos de intervención, la evaluación mediante DXA demostró aumento de la masa muscular total en tres estudios y de la masa muscular apendicular en dos. No se observaron cambios en la proporción de masa muscular en los estudios de DXA y sólo se observó una discreta diferencia en los grupos comparativos estudiados mediante ecografía.
ConclusiónTras la administración de suplementos de proteína de suero en pacientes con DM2, se observó un efecto parcialmente positivo en la ganancia de masa del músculo esquelético con una certeza de evidencia moderada.
Over the past few decades noncommunicable chronic diseases such as type 2 diabetes mellitus (T2DM) have gained widespread interest due to the pressure they put on public health. Prevention, early diagnosis, and intervention to avoid or delay chronic complications must be addressed in full compliance with a resolution passed by the United Nations.1 Data published by the International Diabetes Federation (IDF) calculated that 1 in every 10 adults between the ages of 20 and 79 years have T2DM worldwide. Of these, 3 out of 4 live in low or middle-income countries and treatment takes up to 9% of the world health care budget. It has been estimated that by 2045, approximately 700 million adults will be affected by this disease.2 T2DM is a public health problem that generates tremendous spending for health care systems as cost-effective interventions are required.
The pathological characteristics of T2DM vary in their natural history, clinical presentation, and progression. Physiopathological features are also variable.3 Insulin resistance and the inflammatory process associated with ageing result in loss of strength and function which are determining factors of sarcopenia.4 This tends to be overlooked within the diagnosis of obesity in patients with T2DM which, along with a sedentary lifestyle, increases deterioration of muscle mass thereby generating decreased glucose capture followed by hyperglycaemia and an impaired cellular metabolism. Sarcopenia is a rarely identified complication of diabetes5 that increases the risk of metabolic dysfunction.6 Therefore, assessment, diagnosis, and treatment are necessary to limit its impact and complications.
Nutritional management for T2DM should be individualized to control glycemia through medical nutritional therapy. An adequate intake of high biological value protein has gained acceptance over the past few years due to the positive effects of amino acids, such as leucine, that actively participates in protein synthesis by activation of the mTOR pathway.7
In this signalling pathway, leucine plays a key role in generating an anabolic stimulus, which leads to the increase and regeneration of muscle fiber while counteracting the catabolic effects of inflammation due to the hyperglycemic status of T2DM patients.8,9 Leucine is a bioactive component of whey protein that has insulinotropic properties,8,10–12 acts as an antioxidant with an anti-inflammatory effect inhibiting the production of free radicals, contributes to glycemic regulation through the incretin effect by increasing satiety thus leading to less energy intake in obese patient.13–16
Nutritional care is a key component of patient management, especially medical nutritional therapy such as supplementation with high biological quality proteins. Whey protein can improve metabolism and reduce the inflammatory response promoting euglycemia, avoiding the incidence of sarcopenia, or contributing to its management and, in general, improving the patient's overall health status.
Most systematic reviews that have evaluated the use of whey protein supplementation in diabetic patients refer to the glycemic control associated with its insulinotropic effect and delay in gastric emptying. We are not aware of any publications that explore the evidence describing its effect on muscle mass in patients with T2DM. The objective of this study is to examine that relationship.
Materials and methodsResearch question: What effect does the whey protein supplementation have on the muscle mass of adult patients diagnosis of T2DM?
Literature searchThe evidence-based PICO (Population, Intervention, Comparator, Outcome) model was used to ask the question and facilitate the literature search as follows:
P=adults with a diagnosis of T2DM.
I=whey protein supplementation, alone, or mixed with other nutrients.
C=regular dietary consumption of other types of proteins (casein, soy, or protein mixtures) or placebo.
O=changes in the amount of the skeletal muscle mass.
Selection criteriaRandomized, controlled clinical trials, published in English or Spanish, that assessed muscle mass by various validated means such as dual-energy X-ray absorptiometry (DXA), CAT scan, bioelectrical impedance analysis (BIA), or ultrasound studies were admitted.
DatabasesThe following five databases were searched up to April 2022: PubMed, SCOPUS, Web of Science, Literatura Latinoamericana y del Caribe en Ciencias de la Salud (LILACS) via Biblioteca Virtual de la Salud (BVS), and Scientific Electronic Library Online (SciELO). References from the selected studies were also searched for additional relevant titles. Search terms can be viewed in Table 1S (Supplementary information). The supplementary information shows the search strategy used for the various information sources.
Screening processThe early screening of abstracts and titles was conducted using the Rayyan® app (Cambridge, MA. United States of America; https://rayyan.qcri.org/welcome) a web application designed to help with organizing and conducting literature reviews.17
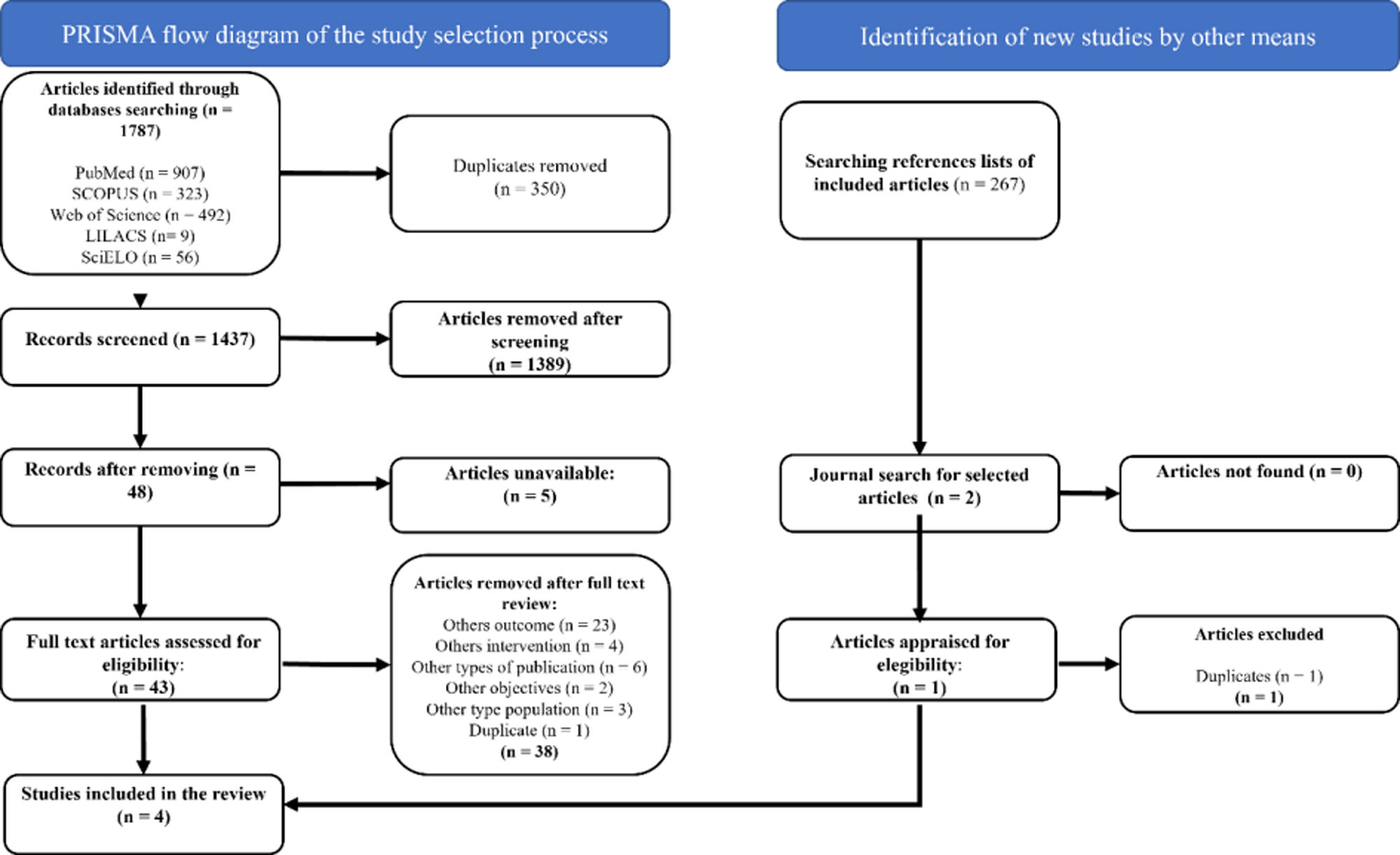
Study selectionTwo authors independently assessed the relevance of titles and abstracts based on eligibility criteria. After eliminating duplicates, full text articles were obtained to revalidate inclusion. The authors’ selections were compared, and all differences of opinion were resolved by consensus. The study selection process is outlined in a PRISMA flow diagram.18
Data extractionThe following data items were collected from each of the selected studies: type of study, sociodemographic and clinical characteristics of participants, the intervention and comparators, and the outcomes. Tables of these data items were created.
Outcome measurementChanges in muscle mass following the use of whey protein supplementation alone or mixed with other nutrients were assessed by means of CAT, DXA, BIA or ultrasound, either in terms of proportional changes or in kilograms.
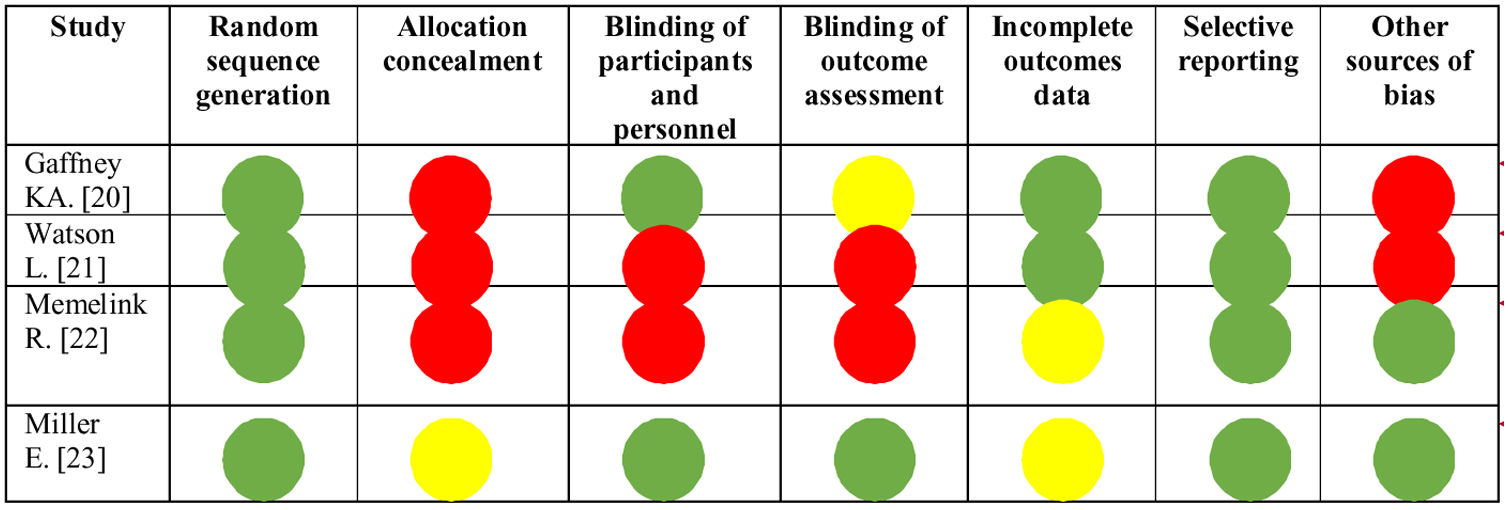
Risk of biasEvaluation of risk of bias was performed by two independent reviewers; when differences were found a third reviewer was consulted to come to a final decision. The “Risk of bias” tool from the Cochrane collaboration was used for this purpose.
Bias was assessed as a judgment (high, low, or unclear) for individual elements from seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcomes, selective reporting, and “other” sources of bias.19
Protocol registrationThis systematic revision protocol was registered on the PROSPERO platform with code CRD42022332118.
ResultsIn the five databases searched a total of 1,787 records were retrieved, 43 of which were selected after evaluation of titles and abstracts. Exclusion criteria consisted of incorrect outcomes (n=23), a different intervention (n=4), wrong publication (n=6), unrelated to the objective of the study (n=2), wrong population (n=3), and duplicated reference (n=1). Finally, 4 randomized controlled trials (RCTs) with a total of 424 participants (209 in the intervention group and 215 controls) were selected for this revision.20–23 The selection process can be seen in the PRISMA 2020 flow chart (Figure 1).
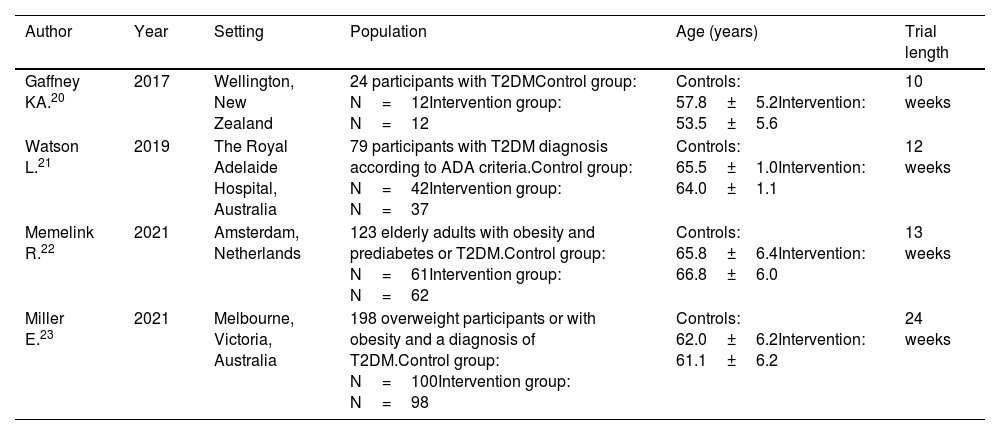
All the selected publications were RCTs.20–23 The trials were conducted in the Netherlands,22 New Zealand,20 and Australia21,23 and population characteristics were similar (Table 1). Three studies included populations between 40 and 75 years old.20,22,23 One study included a broader age range of 18-75 years old.21 Three studies included overweight patients20,21,23 and one study included obese patients.22 One study included patients with prediabetes (n=11) who were excluded from the analysis.22 The duration of the intervention was 10 to 13 weeks in three of the studies20–22 and 24 weeks in one.23
Baseline characteristics.
| Author | Year | Setting | Population | Age (years) | Trial length |
|---|---|---|---|---|---|
| Gaffney KA.20 | 2017 | Wellington, New Zealand | 24 participants with T2DMControl group: N=12Intervention group: N=12 | Controls: 57.8±5.2Intervention: 53.5±5.6 | 10 weeks |
| Watson L.21 | 2019 | The Royal Adelaide Hospital, Australia | 79 participants with T2DM diagnosis according to ADA criteria.Control group: N=42Intervention group: N=37 | Controls: 65.5±1.0Intervention: 64.0±1.1 | 12 weeks |
| Memelink R.22 | 2021 | Amsterdam, Netherlands | 123 elderly adults with obesity and prediabetes or T2DM.Control group: N=61Intervention group: N=62 | Controls: 65.8±6.4Intervention: 66.8±6.0 | 13 weeks |
| Miller E.23 | 2021 | Melbourne, Victoria, Australia | 198 overweight participants or with obesity and a diagnosis of T2DM.Control group: N=100Intervention group: N=98 | Controls: 62.0±6.2Intervention: 61.1±6.2 | 24 weeks |
ADA: American Diabetes Association; T2DM: type 2 diabetes mellitus.
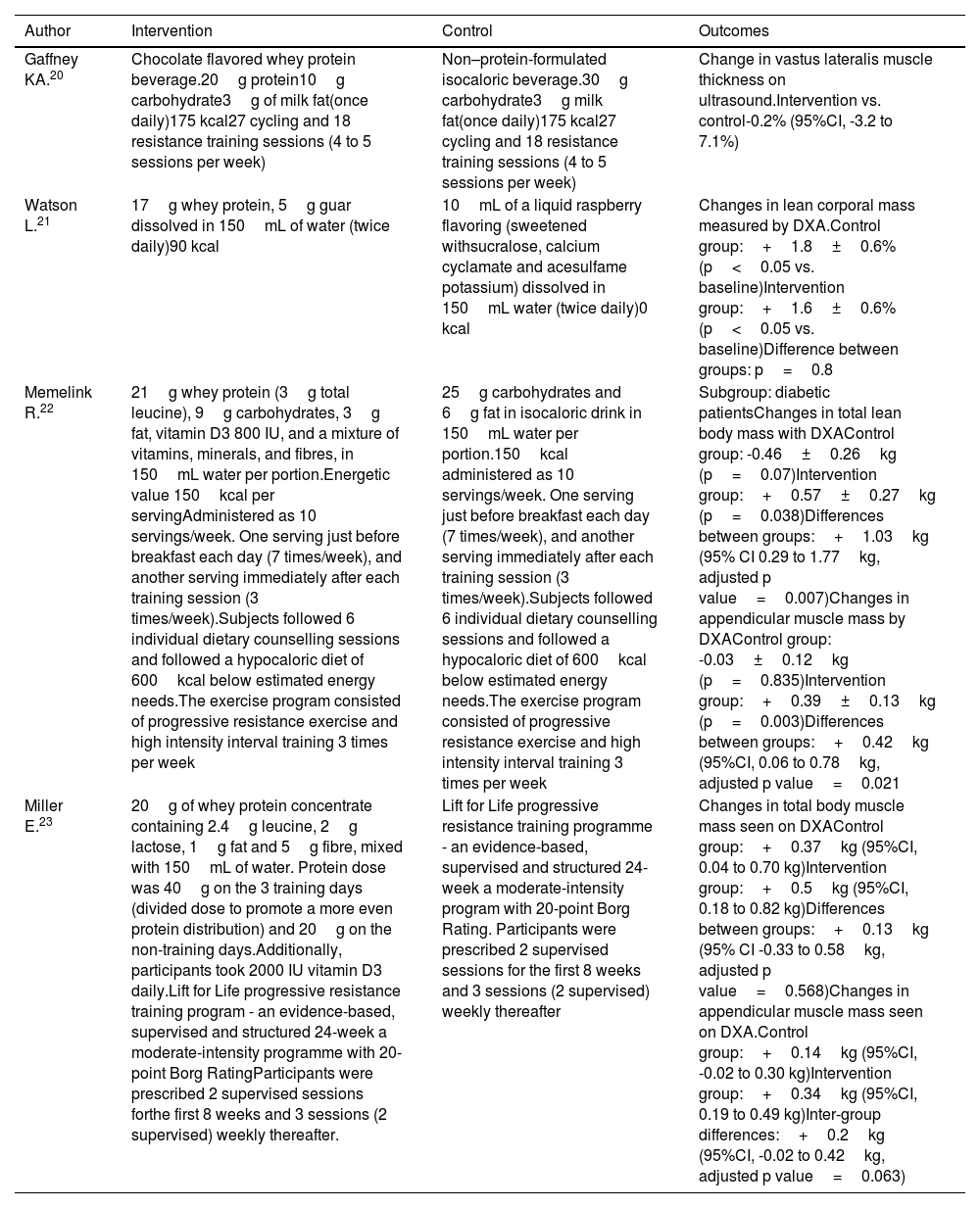
The comparators of the selected studies are described in Table 2. The included studies used 17g/d,21 20g/d,20 21g/d,22 and 40g/d23 whey protein supplementation. Aside from the administration of whey protein supplementation, additional interventions such as exercise were reported in three of the four studies,20,22,23 the use of 5g of guar gum was reported in one,21 vitamin D supplements in varying doses from 800 IU23 up to 2,000 IU in one23 and supplementation with leucine in doses from 2.4g23 up to 3g22 in two of the studies. Of those studies that used exercise as a co-intervention, one of the publications indicated strength and aerobic exercises23, two studies used resistance exercise training,20,22 and one study did not indicate exercise at all.21
Characteristics of the interventions and outcomes.
| Author | Intervention | Control | Outcomes |
|---|---|---|---|
| Gaffney KA.20 | Chocolate flavored whey protein beverage.20g protein10g carbohydrate3g of milk fat(once daily)175 kcal27 cycling and 18 resistance training sessions (4 to 5 sessions per week) | Non–protein-formulated isocaloric beverage.30g carbohydrate3g milk fat(once daily)175 kcal27 cycling and 18 resistance training sessions (4 to 5 sessions per week) | Change in vastus lateralis muscle thickness on ultrasound.Intervention vs. control-0.2% (95%CI, -3.2 to 7.1%) |
| Watson L.21 | 17g whey protein, 5g guar dissolved in 150mL of water (twice daily)90 kcal | 10mL of a liquid raspberry flavoring (sweetened withsucralose, calcium cyclamate and acesulfame potassium) dissolved in 150mL water (twice daily)0 kcal | Changes in lean corporal mass measured by DXA.Control group:+1.8±0.6% (p<0.05 vs. baseline)Intervention group:+1.6±0.6% (p<0.05 vs. baseline)Difference between groups: p=0.8 |
| Memelink R.22 | 21g whey protein (3g total leucine), 9g carbohydrates, 3g fat, vitamin D3 800 IU, and a mixture of vitamins, minerals, and fibres, in 150mL water per portion.Energetic value 150kcal per servingAdministered as 10 servings/week. One serving just before breakfast each day (7 times/week), and another serving immediately after each training session (3 times/week).Subjects followed 6 individual dietary counselling sessions and followed a hypocaloric diet of 600kcal below estimated energy needs.The exercise program consisted of progressive resistance exercise and high intensity interval training 3 times per week | 25g carbohydrates and 6g fat in isocaloric drink in 150mL water per portion.150kcal administered as 10 servings/week. One serving just before breakfast each day (7 times/week), and another serving immediately after each training session (3 times/week).Subjects followed 6 individual dietary counselling sessions and followed a hypocaloric diet of 600kcal below estimated energy needs.The exercise program consisted of progressive resistance exercise and high intensity interval training 3 times per week | Subgroup: diabetic patientsChanges in total lean body mass with DXAControl group: -0.46±0.26kg (p=0.07)Intervention group:+0.57±0.27kg (p=0.038)Differences between groups:+1.03kg (95% CI 0.29 to 1.77kg, adjusted p value=0.007)Changes in appendicular muscle mass by DXAControl group: -0.03±0.12kg (p=0.835)Intervention group:+0.39±0.13kg (p=0.003)Differences between groups:+0.42kg (95%CI, 0.06 to 0.78kg, adjusted p value=0.021 |
| Miller E.23 | 20g of whey protein concentrate containing 2.4g leucine, 2g lactose, 1g fat and 5g fibre, mixed with 150mL of water. Protein dose was 40g on the 3 training days (divided dose to promote a more even protein distribution) and 20g on the non-training days.Additionally, participants took 2000 IU vitamin D3 daily.Lift for Life progressive resistance training program - an evidence-based, supervised and structured 24-week a moderate-intensity programme with 20-point Borg RatingParticipants were prescribed 2 supervised sessions forthe first 8 weeks and 3 sessions (2 supervised) weekly thereafter. | Lift for Life progressive resistance training programme - an evidence-based, supervised and structured 24-week a moderate-intensity program with 20-point Borg Rating. Participants were prescribed 2 supervised sessions for the first 8 weeks and 3 sessions (2 supervised) weekly thereafter | Changes in total body muscle mass seen on DXAControl group:+0.37kg (95%CI, 0.04 to 0.70 kg)Intervention group:+0.5kg (95%CI, 0.18 to 0.82 kg)Differences between groups:+0.13kg (95% CI -0.33 to 0.58kg, adjusted p value=0.568)Changes in appendicular muscle mass seen on DXA.Control group:+0.14kg (95%CI, -0.02 to 0.30 kg)Intervention group:+0.34kg (95%CI, 0.19 to 0.49 kg)Inter-group differences:+0.2kg (95%CI, -0.02 to 0.42kg, adjusted p value=0.063) |
DXA: dual-energy X-ray absorptiometry.
The nutritional intervention included the use of whey protein supplementation dissolved in flavoured drinks and water in all studies,20–23 some were mixed with other nutrients such as carbohydrates, fats, or micronutrients. The energy intake ranged between 80 and 175kcal.20–23 The intervention of interest in this systematic review was a secondary outcome in the four clinical studies included.20–23
Effect of whey protein supplementation on muscle massThree of the studies evaluated muscle mass by means of DXA while 1 study determined changes in the transverse diameter of the vastus lateralis muscle using ultrasound imaging20 (Table 2). Furthermore, two of the studies reported data in terms of proportional changes in the muscle mass20,21 while the other two expressed results in kilograms.22,23
Variations in the effect of whey protein supplementation on muscle mass were observed in T2DM patients. Gaffney et al.20 evaluated the change in vastus lateralis muscle thickness as seen on ultrasound, but did not identify changes when the proportional muscular mass between groups was compared (-0.2%). Increase in total muscle mass was seen in the three studies that used DXA for evaluation purposes.21–23 Watson et al. identified statistically significant gains in the total body mass in both the intervention and control groups.21 However, statistically significant differences between the groups were not observed (p=0.8). A similar observation was made by Miller et al. (adjusted p value=0.568).23 On the other hand, the study conducted by Memelink et al.22 found changes favorable to the intervention cohort (+1.03kg; 95%CI, 0.29 up to 1.77kg; adjusted p value=0.007). Two studies observed appendicular muscle mass volume gain with DXA. Memelink et al.22 observed favorable changes in the intervention group (+0.42kg; 95%CI, 0.06 up to 0.78kg; adjusted p value=0.021), while Miller et al. did not see any statistically significant changes (+0.2kg; 95%CI, -0.02 up to 0.42kg, adjusted p value=0.063) (Table 2).
Risk of biasThe evaluation of risk of bias in the selected articles is shown in Figure 2. In general, allocation concealment was the domain that presented most risk of bias, followed by blinding of outcome assessment. One study presented less risk of bias vs. the others.23
DiscussionThe main findings from this systematic review are based on the analysis of four interventional studies that sought to determine the effect of oral whey protein supplementation on muscle mass in patients with T2DM. These patients undergo an inflammatory process that can inhibit anabolism and lead to muscular catabolism combined with the ageing process.24 Therefore, most studies were conducted in elderly subjects20,22,23 who showed changes to muscle mass synthesis25 due to anabolic resistance that commonly presents after the age of 40.26 Only one study examined the effect of whey protein supplementation in a wider age range (from 18 up to 75 years) which may differ a little from that of elderly population.21 In general, nutritional interventions reported in clinical trials have a 10-12 week-follow-up period for observation of the outcomes. In the trials selected for this review the intervention follow up period was 10 to 13 weeks.20–22 In one study, follow up spanned throughout 24 weeks,23 but no difference was observed between the groups. None of the clinical studies reviewed included patients with sarcopenia.20–23
It is possible that the beneficial effect of whey protein supplementation had a synergistic effect with the other interventions such as resistance exercise.27,28 These types of combined interventions assist in the synthesis of muscle mass29 and prevent wasting due to systemic inflammation and insulin resistance seen in T2DM.30 For example, in three studies20,22,23 resistance exercise training was a co-intervention. Despite of this, in two studies20,23 the muscle mass gain was clinically, yet not statistically, significant.
The neutral effect of the intervention with whey protein supplementation on muscle mass identified in this review may be explained in several ways. For example, three of the studies described the participants’ uncontrolled dietary intake of proteins which per se might be enough to favour the synthesis of muscle mass and be a confounding factor when analyzing outcomes.
Miller et al.23 showed that the intake of protein increased weight from 1.21 up to 1.31g/kg/day in the intervention group on training days and up to 1.50g/kg/day on non-training days. Memelink et al.22 and Watson et al.21 both found differences in dietary intake between the intervention cohort and controls. Gaffney et al.20 did not document energy or protein intake and as such the absence of this information may have impacted the outcomes. The same study established that the control group had a dietary intake of 60g of carbohydrate on training days whereas the intervention group used only 20g. We should remember that an adequate synthesis of muscular mass depends on a sufficient energy supply required for anabolic function.31
The participants in three of the four trials20,22,23 was overweight or obese by the body mass index definition and thus at risk of insulin resistance.32 This favors a decreased anabolic effect on muscle synthesis,33 which could be explained by the hypocaloric nutrition plan used in one of the selected studies22 that would have affected muscle mass synthesis.34,35
A further explanation for the contradictory findings could be the use of various presentations and mixtures of the whey protein supplementation used in the trials. Whey protein supplementation is available in three forms: concentrate, isolate, and hydrolysate. Whey protein concentrate contains 35% to 80% protein, whey protein isolate contains 85% to 90% protein, and whey protein hydrolysate consists of proteins that have undergone proteolytic enzymatic hydrolysis.36,37 The doses and frequency of whey protein supplementation described in the trials can be considered as sufficient quantities to help satisfy the normal protein requirements of participants who often report low consumption of protein food sources.,38 together with the side effects of drugs such a metformin and GLP-1 analogues due to their anorexigenic effect.39,40
No reports in the medical literature describing the effect of other types of proteins in patients with T2DM have been identified. Two systematic reviews indicated that supplementation with different types of protein (whey protein or soy) or amino acids including leucine, can improve skeletal muscle mass in frail, elderly, community dwelling adults with sarcopenia, with or without the addition of exercise.41,42 A meta-analysis showed that soy protein supplementation has similar effects as whey protein supplementation in the formation of lean muscle mass in response to resistance exercise in healthy subjects.43 However, the effect of either one of these proteins could depend on the presence of a catabolic illness. Despite these results, the use of multimodal therapies to treat sarcopenia in populations at risk or those already affected by the disease, have been proposed.
Regarding the certainty of the available evidence, we should mention that Watson et al.21 identified an increased muscle mass in both comparison groups, but also a high risk of bias, while Memelink et al.22 presented a moderate risk of bias due to allocation concealment and blinding of participants, evaluators and outcome assessment.
Limitations of this systematic review include restricted access to databases such as EMBASE (Excerpta Medica dataBASE) and CENTRAL (Cochrane Central Register of Controlled Trials). Due to the clinical heterogeneity of the studies included it was impossible to perform quantitative analysis of the data to measure the effect of the intervention on muscular mass. Therefore, further evidence is required regarding the ideal moment for the use of whey protein supplementation to exert its anabolic effect in patients with chronic diseases such as diabetes.
ConclusionsThe maintenance or improvement of skeletal muscle in T2DM patients is highly relevant as it is a tissue with high potential for glucose uptake due to abundant availability of cellular receptors. Moreover, it stores glucose as glycogen, an energy source, thus contributing to improved glycemic control and consequently overall disease control.44–46 The findings of this systematic revision demonstrate a partial positive effect regarding gain of muscle mass in those participants treated with whey protein supplementation with a moderate certainty of evidence. It is possible that these changes were not sufficiently documented as the evaluation of muscle mass was not a primary outcome in the studies and the effect of additional interventions, such as physical exercise and the intake of other proteins may have confounded the results.
Further studies are required to evaluate the advantages of the use of whey protein supplementation as part of a medical nutritional and metabolic therapy in patients with T2DM.
Authors’ contributionsLopez D.: conceptualization, methodology, investigation, formal analysis, original drafting. Lopez Ucrós N.: conceptualization, methodology, investigation, formal analysis, original drafting. Posada Álvarez C.: writing – original draft, review, and editing. Savino P.: original drafting, review, editing, and supervision.
FundingThis research did not receive any specific grants from funding agencies from the public, private, or nonprofit sectors. The study was conducted with resources from the participating centers.
Conflicts of interestNone declared.