This study was intended to assess the efficacy and safety of a rosehip seed oil (RHO) extract in the prevention and treatment of skin lesions in the hands of patients with type 1 diabetes mellitus (T1DM) caused by finger prick blood glucose monitoring.
Patients and methodA prospective, randomized, controlled, open-label, rater-blinded trial in patients aged 6–17 years with T1DM and intensive blood glucose control (≥7 finger pricks daily) for 12 days. Three main variables (erythema, skin thickening, and loss of skin integrity) were assessed using a scale ranging from 0 (absent) to 3 (severe involvement). The study was approved by the ethics committee of the hospital.
ResultsSixty-eight children, and thus 136 hands, were included; 80 hands received rosehip seed oil and 56 hands acted as controls. Baseline characteristics of both groups were similar, with 76.3% and 78.6% of the hands respectively showing skin lesions at study start. Median final global assessment was 0.10 (0.03; 0.30) in the group that received rosehip seed oil and 0.06 (0.00; 0.23) in the control group. A statistically significant improvement in global assessment was found in the control group (p=0.049). No significant differences were found when the medians of the other main variables were compared. No adverse effects were recorded.
ConclusionA high prevalence of skin lesions secondary to finger prick glucose monitoring, most of them mild lesions, was found at study start. Treatment with rosehip seed oil was safe and was not effective for improving skin lesions.
Evaluar la seguridad y eficacia de un extracto de aceite de rosa mosqueta en la prevención y tratamiento de las lesiones cutáneas en las manos de los pacientes con diabetes mellitus tipo 1 (DMT1) secundarias a las punciones digitales para el control glucémico.
Pacientes y métodosEstudio prospectivo, aleatorizado, controlado, abierto, con evaluadores ciegos e intervencionista en pacientes de edades entre 6 y 17 años con DMT1 y control intensivo de la glucemia con ≥7 punciones capilares diarias durante 12 días. Se evaluaron 3 variables principales (eritema, engrosamiento cutáneo, pérdida de la integridad cutánea) de la siguiente forma: 0: ausente, 1: leve, 2: moderado, 3: intenso. El estudio fue aprobado por el Comité Ético del hospital.
ResultadosSe incluyó a 68 niños, por tanto, 136 manos: 80 recibieron aceite de rosa mosqueta y 56 fueron controles. Las características basales de los 2 grupos fueron similares. El 76,3% y el 78,6% presentaban alguna lesión dermatológica inicial, respectivamente. La mediana de valoración global final fue de 0,10 (0,03; 0,30) y de 0,06 (0,00; 0,23), en el grupo de aceite de rosa mosqueta y grupo control, respectivamente. Se encontró una mejoría estadísticamente significativa de la valoración global solo en el grupo control (p=0,049). No se encontraron diferencias estadísticamente significativas para la comparación de medianas del resto de las variables principales. No se registraron efectos adversos.
ConclusiónSe encontró una alta frecuencia de lesiones dermatológicas secundarias a punciones capilares digitales, la mayoría de las cuales fueron lesiones leves. La aplicación de aceite de rosa mosqueta fue segura y no supuso una mejoría en las lesiones dermatológicas.
The measurement of blood glucose by finger prick testing is essential for metabolic control and therapeutic adjustment in patients with type 1 diabetes mellitus (T1DM). The different clinical guides recommend a variable frequency of at least four to more than 10 daily glycemia measurements in children and adolescents with T1DM receiving intensive treatment or continuous infusion of insulin. In this regard, the American Diabetes Association recommends monitoring before each meal, before bedtime, before physical exercise, when hypoglycemia is suspected, after treatment of hypoglycemia, and occasionally after meals. It concludes that most patients will require between 6 and 10 daily controls.1 On the other hand, the International Diabetes Federation/International Society for Pediatric and Adolescent Diabetes recommends blood glucose monitoring 4–6 times daily, because the frequency of monitoring is correlated to metabolic control.2
These repeated punctures at the same sites represent chronic aggression to the skin barrier of the hands of these patients. In this regard, different secondary lesions may develop, such as lichenification, bruising, fissures, wounds or infections3,4 that are often painful and affect the quality of life of diabetic patients.5 The glycemia self-monitoring technique includes the following recommendations: hand washing with soap and water, non-reuse of lancets, choosing the minimum sufficient depth of the puncture device, selecting a different finger each time testing is made, and performing puncture on the side of the finger, gently squeezing and massaging until a drop of blood is obtained.6
However, it is very common in diabetes clinics to find that patients do not comply with these recommendations. In effect, diabetic patients often reuse lancets, do not choose the recommended area of the finger for puncture, and tend to over-puncture one of the hands (generally the non-dominant hand), and one or two fingers of that hand. Some studies suggest that this may be due to convenience, fear of pain, previous skin problems, or patient experience in obtaining a drop of blood of sufficient volume.7,8 Of note is the lack of published studies on the cutaneous repercussions of repeated finger pricks. Although some authors recommend the application of pure vaseline to protect the hands of these patients,9 no studies have compared the effectiveness of different emollient products in the prevention and treatment of secondary skin lesions to the hands of patients with T1DM.
Rosehip seed oil (RHO) is a vegetable oil containing elements mainly beneficial to the healing process. This oil is extracted from the seeds of a wild bush of the rose family (Rosa moschata or Rosa rubiginosa), and is rich in essential fatty acids, particularly linoleic acid (41%) and linolenic acid (39%). Moreover, it also contains 16% oleic acid, vitamin C and retinoic acid. Some case series and small exploratory studies have evaluated the efficacy and safety of RHO in the prevention and treatment of various clinical conditions such as radiotherapy-induced epithelitis, hidradenitis, venous ulcers, postoperative hypertrophic or torpidly evolving scars, and scars secondary to traffic accidents or cryosurgery or electrosurgery, with positive results.10–13
The present study was carried out to evaluate the safety and efficacy of an RHO extract (Repavar®) in the prevention and treatment of skin lesions to the hands of patients with T1DM secondary to finger prick tests for glycemic control. To this end, a randomized, prospective, controlled, open-label interventional study was designed in patients with T1DM and intensive glycemic control with capillary punctures.
Patients and methodsType of studyA randomized, controlled experimental study was carried out.
Randomization and blindingSimple computer-based randomization of the patients was performed. Following randomization to an intervention group, each hand of the patient was considered a subject of study for the purposes of statistical analysis. This was an open-label study (product application being known to the subjects and field staff) with blinded evaluators (dermatologists).
Study populationThe study involved patients between 6 and 17 years of age and diagnosed with T1DM attending a 12 day summer camp in August 2017.
Inclusion criteriaWe recruited patients of between 6 and 17 years of age, diagnosed with T1DM, who underwent finger prick tests for capillary blood glucose measurement ≥7 times daily before entry to the study. Their legal representatives signed the corresponding informed consent document.
Exclusion criteriaPatients who either did not wish to participate or for whom informed consent from the legal representatives was not obtained were excluded, along with patients with a known allergy to the study product.
Study timelinesThe investigators screened and randomized the patients before summer camp and collected clinical data, information from surveys and photographic images of the hands of the patients on the first day and last day of their stay at a summer camp, which lasted 12 days. Three blinded dermatologists subsequently assessed the photographic images.
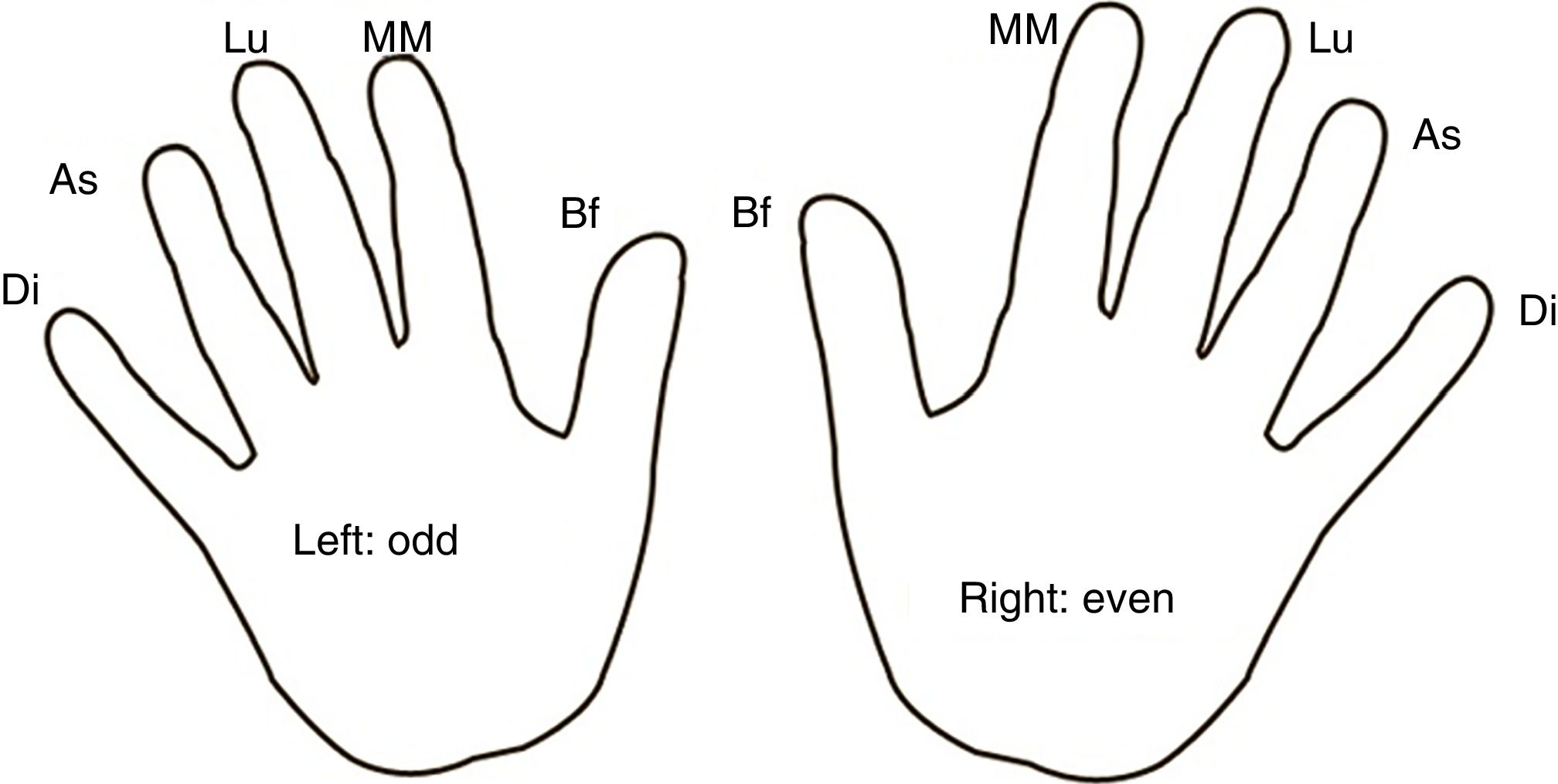
Study protocolA total of ≥7 daily blood glucose tests were performed following a finger and hand rotation map in all subjects (Fig. 1). Single-use lancets with the same depth were employed for all punctures. In the experimental group, RHO was applied to the fingers for 5min twice daily, morning and evening. No substance was applied in the control group.
Follow-upPhotographic controls were performed on day 1 and day 12 in all children (control group and experimental group) (t0, t1). Based on these images, three dermatologists performed a blinded assessment of each finger. Observed adverse effects were recorded. At the second visit (t1), each patient answered a question about the cosmetic properties of the product and a question about perceived treatment usefulness. The application of RHO was supervised by the research staff.
MaterialsThese consisted of the following: a case report form (one for each hand of the patient); a Sony Cyber-shot DSX-HX90V® camera; RHO Repavar® (Laboratorios Ferrer); material for capillary blood glucose testing: sterile single-use lancets 21G (Medlance Plus®; Laboratorios HLT-Strefa S.A.) with a depth of 2.4mm; One Touch Select Plus® glucometers (Laboratorios Lifescan).
Study variablesWe recorded patient age and gender, the time since the onset of T1DM, medication, the intervention group, the use of continuous glucose monitoring before or during summer camp, and any side effects. The information was recorded independently for each hand of each patient; each hand was therefore taken to be a subject of study. Measurements of the three principal variables (erythema, skin thickening, loss of skin integrity) were made using a Likert scale (discrete quantitative scores from 0 to 3) for each finger at the two timepoints (t0, t1), with the arithmetic means for each hand being calculated. The variables were scored as follows: 0: none, 1: mild, 2: moderate, 3: severe. The two satisfaction variables (cosmetic properties and perceived product usefulness) were recorded using two face scales (discrete quantitative scores from 0 to 10).
The primary endpoint was the clinical improvement of the mean fingers of each hand after 12 days of RHO application. The secondary endpoints were: (1) to describe the dermatological lesions at the start of the study; (2) to evaluate patient satisfaction with the cosmetic properties of the product; (3) to evaluate perceived product usefulness; and (4) to assess product safety.
Statistical analysisQualitative variables were reported with their frequency distribution. Quantitative variables were presented as the mean and standard deviation (SD) in the presence of a normal distribution, or as the median and 25th and 75th percentiles, in the presence of a non-normal distribution. The chi-squared test was used for qualitative variables. The Student t-test and Wilcoxon signed-rank test were used to compare measures of central tendency in the presence of a parametric and non-parametric distribution of the variable, respectively. Statistical significance was considered for p<0.05. The SPSS version 19 statistical package was used throughout.
Calculation of sample sizeAssuming an α risk of 5% and a β risk of 20% in two-tailed testing, 76 hands were required in each group to detect a difference equal to or greater than 0.5 units on the Likert scale (scale from 0 to 3). The common standard deviation was assumed to be 1, with a correlation coefficient between the initial and final measurement of 0.4.
Ethical aspectsThe study was carried out in compliance with the basic ethical principles and standards arising from the current revision (revised version of Seoul, 2008) of the Declaration of Helsinki approved by the World Medical Assembly, the Oviedo Convention, and the regulatory specifications contemplated by Spanish legislation. The participating subjects or their legal representatives signed the informed consent prior to inclusion in the study. Before starting the study, the Clinical Research Ethics Committee of Hospital Universitario Gregorio Marañón approved the study protocol (minutes 14/2017). In the case of an adverse event, the subject would be withdrawn from the study, and the event and its circumstances would be duly recorded. There were no funding entities or grants.
Economic aspectsThe investigators received no financial compensation. Laboratorios Ferrer supplied RHO Repavar®. The sterile single-use lancets (Medlance Plus®; Laboratorios HLT-Strefa S.A.) were supplied by Laboratorios Menarini. The One Touch Select Plus® glucometers were provided by Lifescan Laboratories (Fig. 2). There were no funding entities or grants.
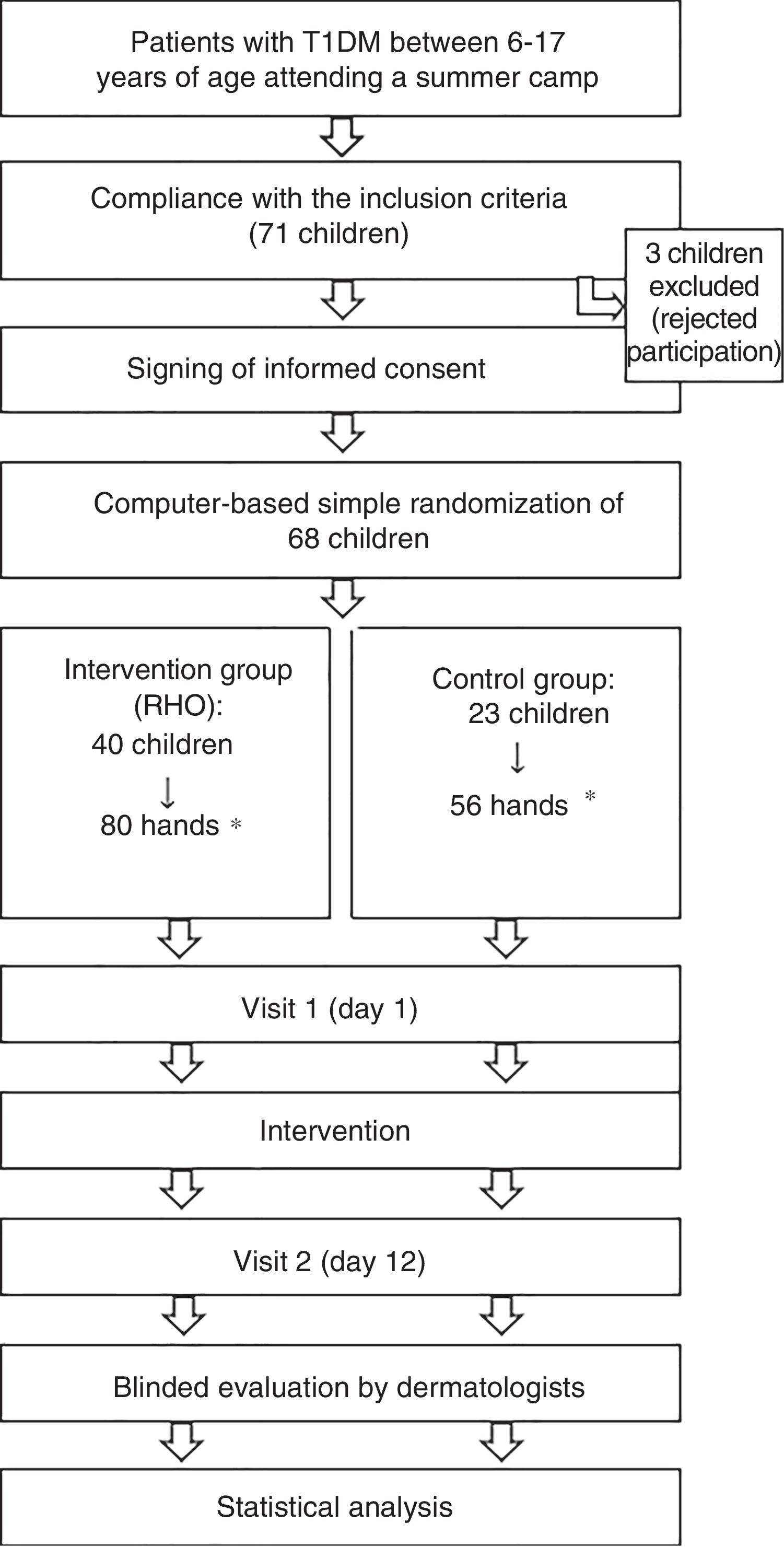
Patient flow chart of the clinical trial phases.
RHO: rosehip seed oil; T1DM: type 1 diabetes mellitus.
*Simple randomization was performed (n=68 children). Following randomization to an intervention group, each hand of the patient was considered a subject of study for the purposes of statistical analysis.
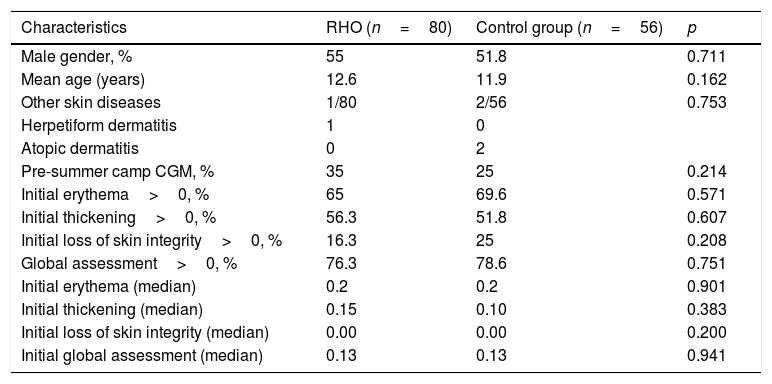
Of the initial sample of 71 children, three declined to participate in the study and were excluded. A total of 68 children were included (136 hands) and randomized to the RHO group (80 hands) or the control group (56 hands). The baseline characteristics of the two groups were similar. A total of 76.3% of the hands receiving RHO and 78.6% of the control hands presented some initial dermatological lesion (global assessment>0) (Table 1).
Baseline characteristics of the study sample.
| Characteristics | RHO (n=80) | Control group (n=56) | p |
|---|---|---|---|
| Male gender, % | 55 | 51.8 | 0.711 |
| Mean age (years) | 12.6 | 11.9 | 0.162 |
| Other skin diseases | 1/80 | 2/56 | 0.753 |
| Herpetiform dermatitis | 1 | 0 | |
| Atopic dermatitis | 0 | 2 | |
| Pre-summer camp CGM, % | 35 | 25 | 0.214 |
| Initial erythema>0, % | 65 | 69.6 | 0.571 |
| Initial thickening>0, % | 56.3 | 51.8 | 0.607 |
| Initial loss of skin integrity>0, % | 16.3 | 25 | 0.208 |
| Global assessment>0, % | 76.3 | 78.6 | 0.751 |
| Initial erythema (median) | 0.2 | 0.2 | 0.901 |
| Initial thickening (median) | 0.15 | 0.10 | 0.383 |
| Initial loss of skin integrity (median) | 0.00 | 0.00 | 0.200 |
| Initial global assessment (median) | 0.13 | 0.13 | 0.941 |
RHO: rosehip seed oil; CGM: continuous glucose monitoring.
Scoring of variables: 0: none; 1: mild; 2: moderate; 3: severe.
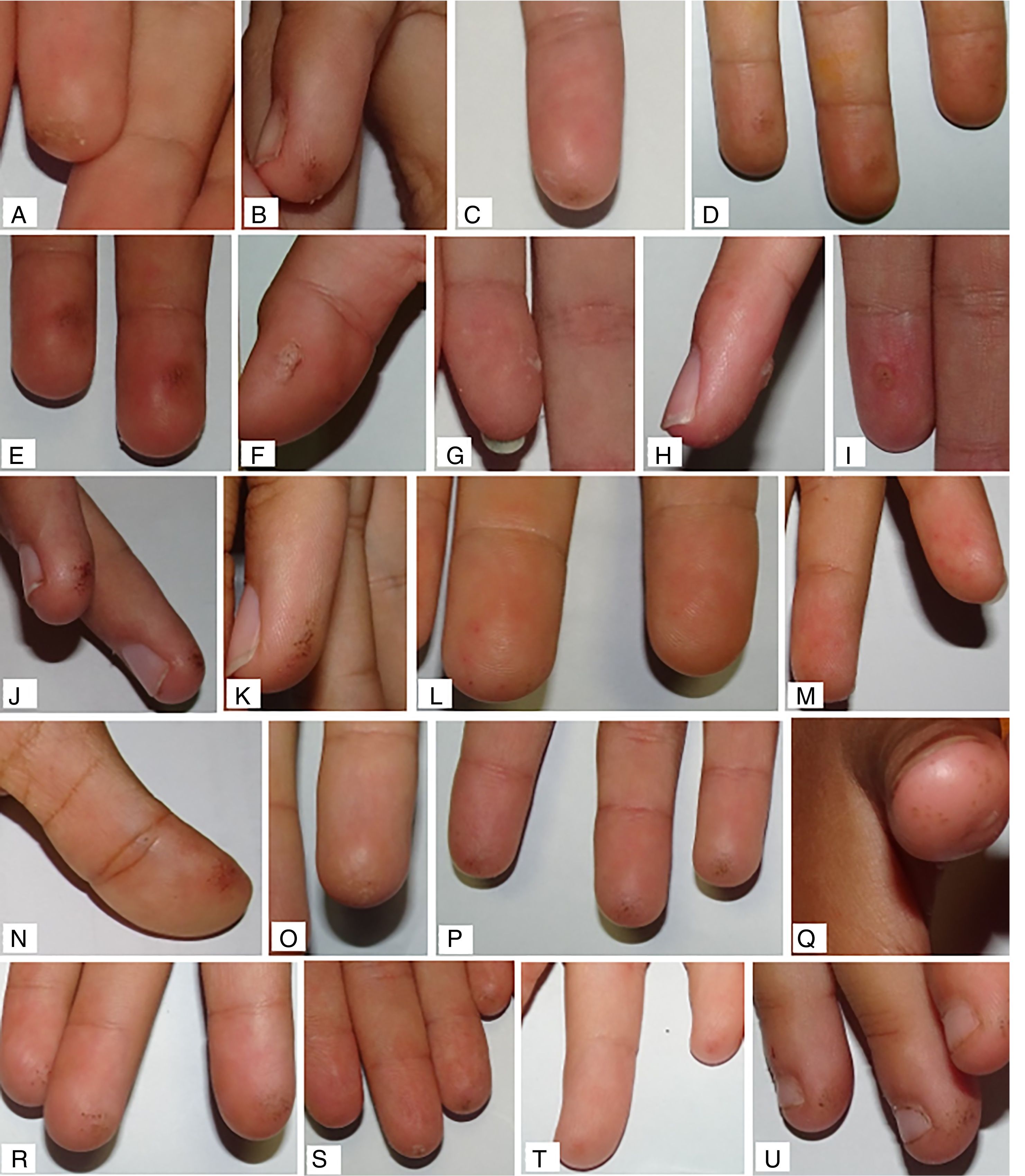
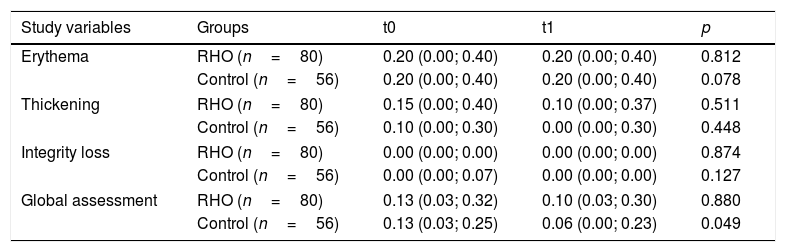
The final median global assessment was 0.10 (0.03; 0.30) and 0.06 (0.00; 0.23) in the RHO group and control group, respectively. When compared to the initial assessment of each group, improvement was observed, but with statistically significant differences only in the control group (p=0.049 in the control group; p=0.880 in the RHO group). No statistically significant differences were found in the comparison of the median values of the other principal variables (Table 2). In the analysis stratified by subgroups, continuous glucose monitoring (CGM) prior to the study was not found to act as a confounder. Examples of the observed skin lesions are shown in Fig. 3.
Comparison of efficacy outcomes.
| Study variables | Groups | t0 | t1 | p |
|---|---|---|---|---|
| Erythema | RHO (n=80) | 0.20 (0.00; 0.40) | 0.20 (0.00; 0.40) | 0.812 |
| Control (n=56) | 0.20 (0.00; 0.40) | 0.20 (0.00; 0.40) | 0.078 | |
| Thickening | RHO (n=80) | 0.15 (0.00; 0.40) | 0.10 (0.00; 0.37) | 0.511 |
| Control (n=56) | 0.10 (0.00; 0.30) | 0.00 (0.00; 0.30) | 0.448 | |
| Integrity loss | RHO (n=80) | 0.00 (0.00; 0.00) | 0.00 (0.00; 0.00) | 0.874 |
| Control (n=56) | 0.00 (0.00; 0.07) | 0.00 (0.00; 0.00) | 0.127 | |
| Global assessment | RHO (n=80) | 0.13 (0.03; 0.32) | 0.10 (0.03; 0.30) | 0.880 |
| Control (n=56) | 0.13 (0.03; 0.25) | 0.06 (0.00; 0.23) | 0.049 | |
RHO: rosehip seed oil.
Results expressed as median and quartiles 25 and 75.
Scoring of variables: 0: none; 1: mild; 2: moderate; 3: severe.
No adverse effects were recorded with the application of RHO.
Other resultsThe mean subjective improvement score was 3.30 (±1.98) for the RHO group (range 0=very positive to 10=very negative). The mean cosmetic satisfaction score was 3.90 (±2.77) for the RHO group (range 0=very positive to 10=very negative).
DiscussionThere is no doubt that frequent capillary puncture tests are the cornerstone of treatment for patients with T1DM. Different studies have demonstrated a direct relationship between the frequency of capillary puncture testing and lower HbA1c levels, and have identified fear of pain as a cause of lower puncture frequency, along with socioeconomic considerations, among other factors.5,8,14–16
One third of all patients with diabetes mellitus have skin manifestations. However, few publications have examined the dermatological impact of capillary puncture tests on patients with T1DM.17,18 Likewise, recommendations for hand care in these patients are far from specific and are based on very little evidence.9 The present study for the first time describes dermatological lesions secondary to capillary puncture testing in a sample of children and adolescents with T1DM and subjected to intensive treatment. Most of the sample (76.3% of the hands receiving RHO and 78.6% of the control hands) had some skin lesions at the start of the study, the most common being erythema (65% and 69.6%, respectively), followed by skin thickening (56.3% and 51.8%) and loss of skin integrity (16.3% and 25%). Most of the patients had mild lesions, with a mean score for all the primary variables of <1.
The study has the following strengths: (1) the prevention through randomization of any differences between the groups which did not derive from the use of RHO and (2) the use of blinded evaluators to prevent assessment bias.
Rosehip seed oil is rich in unsaturated fatty acids, linoleic and linolenic acid, which participate in prostaglandin synthesis, membrane generation, defense mechanisms, growth and other biological processes related to cell regeneration. This may contribute to the stimulation of epithelization.13 Some case series and small exploratory (non-randomized) studies have evaluated the efficacy and safety of RHO in the prevention and treatment of various clinical conditions such as radiotherapy-induced epithelitis, hidradenitis, venous ulcers, postoperative hypertrophic or torpidly evolving scars, and scars secondary to traffic accidents or cryosurgery or electrosurgery, with positive results.10–19 A team of investigators applied RHO twice daily, morning and evening, for three months in a group of 10 adult women subjected to mastectomy, following suture removal. The scars were found to be less manifest, with no skin thickening, and both skin elasticity and colour had improved.19 Another study applied 26% RHO in a group of 10 patients with varicose ulcers and postsurgery wounds. When compared to the control group, the oil was seen to have exerted a favourable effect on epithelization (mean epithelization time 24.1 days versus 52.2 days), with no side effects.13 A study evaluated the effect of pure rosehip seed oil on postsurgery scars and defects. Over a two-year period, this pure oil was used in different dermatological processes requiring epithelization or the improvement of scar characteristics. The results showed RHO to be of great help in epithelization or healing, mainly in relation to the aesthetic improvement of hypertrophic or atrophic scars.11
The primary objective of the present study was to assess the clinical improvement of lesions using RHO. The findings indicate that the application of RHO does not result in a statistically significant improvement of the lesions in terms of any of the primary endpoints. Moreover, in the global assessment variable, a statistically significant improvement was observed only in the control group. This leads us to postulate that either the application of RHO exerted no effect upon these dermatological lesions; that it had not been applied for a sufficient period of time for any beneficial effects to be observed; or that there may have been methodological errors in the study design.
The methodological limitations of the study are as follows: (1) its exploratory nature, given the lack of other similar studies in the literature; (2) the fact that the previously calculated sample size was not reached, which altered the size of the two groups due to the randomization process; (3) the fact that the dermatological lesions were very mild in both groups at baseline, which considerably reduced the potential margin for improvement; (4) the fact that the patients did not follow the usual rotation routine but were instructed to perform systematic rotation of all fingers of the hand. At least 7 daily capillary blood glucose controls were performed, which when multiplied by 12 days represented at least 82 capillary blood glucose tests during the study period. The patients performed a systematic rotation of the 5 digits of each hand, with replacement of the lancets at each puncture. Each digit was thus used 8 times on average. All this implied a rest for the usual puncture sites. Resting of the usual puncture sites in both the intervention group and the control group may be a very useful strategy in terms of lesion prevention, though in this study protocol it could have acted as a confounding factor; (5) the study was probably not long enough to show significant dermatological changes associated with these lesions, since in standard clinical practice skin lesions are not usually seen in the fingertips in the first months of T1DM; and (6) the outcome variables exhibited a non-normal distribution, requiring the use of nonparametric tests, which resulted in a loss of statistical power.
The secondary study endpoints were patient satisfaction with the cosmetic properties of the product, the perceived usefulness of RHO, and its safety. The score referring to satisfaction with the cosmetic properties and the perceived usefulness of the product were positive, and no side effects were recorded during the study. This facilitates the application of the oil in children and adolescents.
ConclusionsThis exploratory study has demonstrated a high frequency of skin lesions secondary to finger capillary puncture testing in children and adolescents with T1DM subjected to intensive treatment, though most such lesions were mild. The application of RHO was found to be safe, but did not result in any improvement regarding skin lesions. This study opens a door to future research needed to define the magnitude of the problem posed by these skin lesions and their appropriate management.
Authorship/collaborationsABAR and FGV participated in the study design, data compilation, data analysis and interpretation, and the writing of the manuscript, and contributed equally to the study.
ECM, DVT and PCD participated in the study design and dermatological photographic analysis.
All the authors reviewed and approved the final manuscript.
Conflicts of interestNone declared.
Thanks are due to the administrative staff and volunteers (monitors and healthcare staff) of the Diabetes Madrid Association, for facilitating the conducting of the study during the summer camp.
Please cite this article as: Aguirre-Romero AB, Galeano-Valle F, Conde-Montero E, Velázquez-Tarjuelo D, de-la-Cueva-Dobao P. Eficacia y seguridad del aceite de rosa mosqueta en las lesiones de los dedos provocadas por las punciones capilares para el control glucémico en niños con diabetes tipo 1; un ensayo clínico aleatorizado, abierto, controlado. Endocrinol Diabetes Nutr. 2020;67:186–193.