Current treatment of type 1 diabetes mellitus (T1DM) does not always achieve metabolic control because, among other things, the ocurrence of hypoglycemic events associated to insulin use.
Material and methodsA descriptive real life study of 247 T1DM patients, 55.5% male, aged 46.53±16.23 years, and with a mean diabetes duration of 21.89±11.99 years, who were switched from basal insulin glargine U100 to glargine U300. The primary endpoints were changes in Hba1c and number of hypoglycemic events, while secondary endpoints included changes in weight and insulin dose after 6 and 12 months.
ResultsAfter one year, no changes were seen in HbA1c, but the proportion of patients with HbA1c values <7.5% increased at 6 months (33.5 vs. 40.5%; p<0.05) and remained stable during one year of follow-up. Hypoglycemic events significantly decreased after one year of treatment in patients with previous hypoglycemic events. No changes were seen in body weight.
Total insulin dose (U/kg) increased 7.24% at 6 months of treatment and by 8.69% at one year, mainly due to basal insulin. No changes were seen between the doses given at 6 and 12 months. These changes were similar in the different metabolic control groups and in patients with or without hypoglycemia. This increase was not related with prior basal insulin dose, baseline HbA1c level, number of hypoglycemic events or baseline weight.
DiscussionGlargine U300 is a good basal insulin alternative to treat T1DM, improving metabolic control in patients with HbA1c levels >7.5 and decreasing hypoglycemic events in patients with history of hypoglycemia without increasing body weight.
Los tratamientos insulínicos actuales para diabetes tipo 1 (DM1) no siempre consiguen los objetivos de control metabólico debido, entre otros aspectos, a la aparición de episodios de hipoglucemia asociados al uso de insulina.
Material y métodosEstudio descriptivo en la vida real con 247 pacientes DM1, el 55,5% varones, de 46,53 ± 16,23 años, con un tiempo de evolución de 21,89 ± 11,99 años, a los que se sustituyó su insulina basal, glargina U100, por glargina U300. Los objetivos primarios fueron los cambios en la HbA1c y en el número de hipoglucemias y los secundarios fueron los cambios en el peso y en la dosis de insulina trascurridos 6 y 12 meses.
ResultadosTras un año, no se observaron cambios en la HbA1c en el total de los pacientes, si bien se comprobó un descenso significativo en los pacientes mal controlados. Sin embargo, aumentó el porcentaje de pacientes con HbA1c < 7,5% a los 6 meses (33,5 vs. 40,5%; p < 0,05), que se mantuvo al año. El número de hipoglucemias leves se redujo tras los 12 meses de tratamiento en aquellos pacientes con antecedentes de hipoglucemias leves. En cuanto al peso, no observamos cambios.
La dosis total de insulina/kg se incrementó significativamente en un 7,24% a los 6 meses y en un 8,69% al año por el aumento de la insulina basal. Las diferencias en las dosis a los 6 meses y al año no fueron significativas. Este aumento fue similar entre los grupos según el control metabólico, la presencia de hipoglucemias y no se relacionó con la insulina basal inicial, la HbA1c inicial, el número de hipoglucemias leves ni con el peso inicial.
DiscusiónEn la vida real glargina U300 muestra un mejor control glucémico en pacientes mal controlados, al reducir las hipoglucemias en pacientes con antecedentes de hipoglucemias sin incrementar el peso corporal.
Type 1 diabetes mellitus (DM1) accounts for 10–15% of all cases of diabetes mellitus worldwide, and its incidence is increasing 3–5% annually.1 In Spain, DM1 has been estimated to affect 0.08–0.2% of the population,2 and recent studies have revealed an increase in the incidence of the disease mainly in the population between 0 and 4 years of age.3,4
Good glycemic control is essential in order to reduce the occurrence of diabetes-related complications. In effect, good glycemic control as measured by glycosylated hemoglobin (HbA1c) concentration has been shown to reduce complications in the form of cardiovascular and cerebral events, neuropathic damage and vision loss.5,6
However, the current insulin treatments do not always achieve the metabolic control targets. One of the main obstacles in this regard is the occurrence of hypoglycemic events associated with insulin use. It is estimated that every DM1 patient experiences 1.8 episodes of mild hypoglycemia a week and between 0.2 and 3.2 episodes of severe hypoglycemia a year in which the help of a third person is needed.7,8
The association between symptomatic hypoglycemic episodes and an increased risk of cardiovascular events and mortality is known. Therefore, we should select the treatment with the lowest risk of hypoglycemia, mainly in those patients at an increased risk of suffering cardiovascular events.9,10
This risk of hypoglycemic events can be reduced through adequate patient diabetes education and the use of new treatment schemes based on insulin analogs.11
In early 2016, insulin glargine U300 (Gla-300) was approved in Europe for use in adults with DM1 and type 2 diabetes mellitus (DM2). This new insulin analog has pharmacological features that ensure a more sustained release, with a more level and lasting effect than glargine U100 (Gla-100). In clinical practice, this results in more stable glycemic control beyond 24h, lesser glycemic variability and a lower risk of hypoglycemia compared to Gla-100.12,13
The present study comprised patients with DM1 receiving Gla-100 who were switched to Gla-300, with an evaluation of its effectiveness and safety in a real-life setting.
Material and methodsA retrospective descriptive study was designed involving patients diagnosed with DM1 and subjected to follow-up at the endocrinology and nutrition outpatient clinic of our hospital. The included patients had been on insulin therapy for at least one year. Pregnant women were excluded, as were patients with secondary diabetes, individuals with any other disease, and those receiving any intercurrent medication capable of altering glycemic control.
A total of 247 patients with DM1 were selected, of which 55.5% were males. The mean age was 46.53±16.23 years (range 13–84), with a disease duration of 21.89±11.99 years (range 1–58). The patients were subjected to treatment with bolus-basal insulin, with the use of Gla-100 as basal insulin. In all cases the basal insulin analog (Gla-100) was switched to Gla-300 between March 2016 and February 2017. The main reasons for switching were glycemic variability, the presence of hypoglycemia, the effect of Gla-100 ending after less than 24h, or the convenience of the device.
The timing of Gla-300 administration was the same as with the previous basal analog (11.8% in the morning; 48.8% at midday; 39% at night, and 0.4% [equivalent to a single patient] in 2 doses). Furthermore, the dose was not modified at the time of switching from one drug to the other. The basal insulin dose adjustments were made by the patient on an ambulatory basis according to capillary blood glucose control with the aim of securing a target of 80–130mg/dl, using the same scale as before (i.e., increasing the basal dose by 2 units if mean baseline glycemia over a 3-day period exceeded 130mg/dl). Preprandial insulin adjustments were made by the patient as required to achieve postprandial figures <180mg/dl 2h after meals.
The primary endpoints were the changes in HbA1c and the number of hypoglycemic episodes 6 and 12 months after the switch in treatment. Hypoglycemic episodes were determined in accordance with the recommendations of the American Diabetes Association (ADA).14 Thus, mild hypoglycemia was defined as when capillary blood glucose was <70mg/dl, with or without symptoms, while serious hypoglycemia was defined as when the patient required the help of another person or hospital admission. The number of mild hypoglycemic episodes was reported by the patient and counted as episodes per week, while the severe hypoglycemic episodes were counted as those reported by the patient and recorded in the electronic medical records.
We subdivided our sample into two patient groups according to previous metabolic control: good control (HbA1c before switching treatment≤7.5%) or poor control (HbA1c before switching>7.5%). In addition, we analyzed the sample, based on the presence or absence of mild hypoglycemia (i.e., the presence of one or more hypoglycemic events a week before switching treatment versus no such events over the previous 6 months).
The secondary endpoints were the changes in body weight and insulin dose per kilogram, the changes in basal insulin units, and the changes in the amount of prandial insulin at 6 and 12 months.
The data were analyzed using the SPSS version 15.0 statistical package for MS Windows (SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was applied for repeated quantitative variables, with Bonferroni correction as a post hoc analysis. Qualitative variables were analyzed using the chi-squared test or Fisher exact test where needed, and correlations were established by means of the Pearson test. Statistical significance was considered for p<0.05 in two-tailed testing, and the data were reported as the mean±standard deviation (SD), unless otherwise indicated.
ResultsAfter switching to Gla-300 in 247 patients, 6 discontinued treatment (2 due to the start of continuous subcutaneous insulin infusion [CSII], and 4 because of worsened glycemic control); 8 were lost to follow-up; and 13 had not yet completed their evaluation after 12 months. We present the data corresponding to 216/220* patients that completed two evaluations at 6 and 12 months of follow-up.
* 247 – 6 – 8 – 13=220
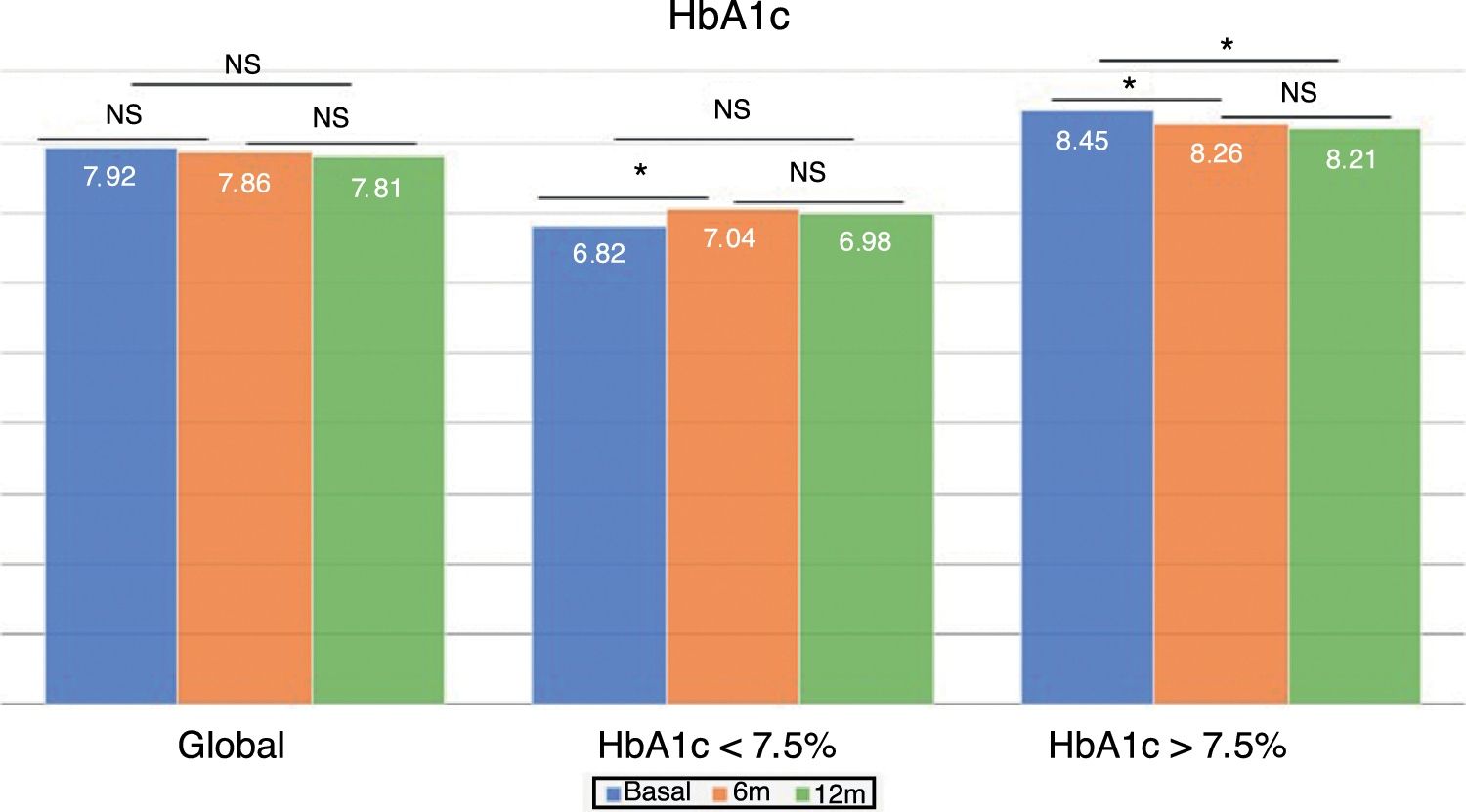
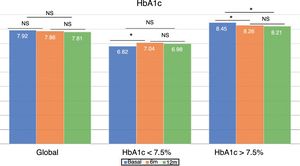
Changes in glycemic control and hypoglycemiaThe global patient sample showed no differences in HbA1c concentration at either 6 or 12 months after the switch in treatment (Fig. 1). However, the percentage of patients with HbA1c<7.5% increased significantly at 6 months (33.5% vs. 40.5%; p<0.05) and one year (33.5% vs. 40.7%; p<0.05). The same applied to the proportion of patients with HbA1c<8%, which increased significantly at 6 months (52.7% vs. 61.0%; p<0.05) and one year of follow-up (52.7% vs. 64.4%; p<0.05).
The group with previous good glycemic control showed a significant increase in HbA1c at 6 months (6.82±0.52% vs. 7.04±0.74%; p<0.05), which was no longer observed at 12 months (6.82±0.52% vs. 6.98±0.73%; p=NS). However, patients with previous poor glycemic control showed improved control at 6 months (8.45±0.68% vs. 8.26±0.96%; p<0.05), which persisted after one year of follow-up (8.45±0.68% vs. 8.21±0.92%; p<0.05) (Fig. 1).
In the 6 months before the switch in insulin, 6 patients suffered severe hypoglycemia (7 episodes). Six months after the switch in treatment, only four episodes of severe hypoglycemia were reported in four patients versus two episodes in two patients one year after the switch in treatment (one of them had moreover suffered severe hypoglycemia at 6 months).
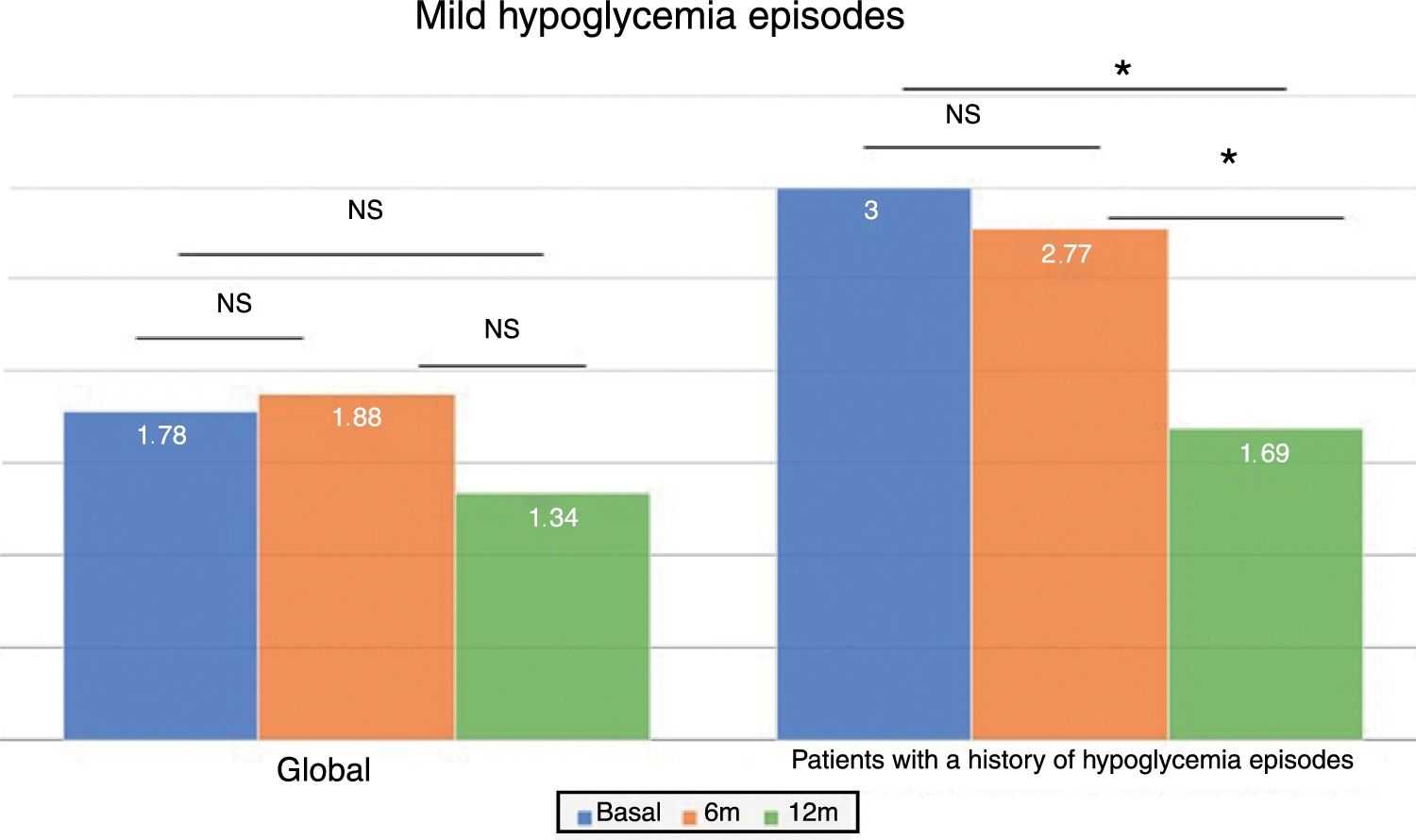
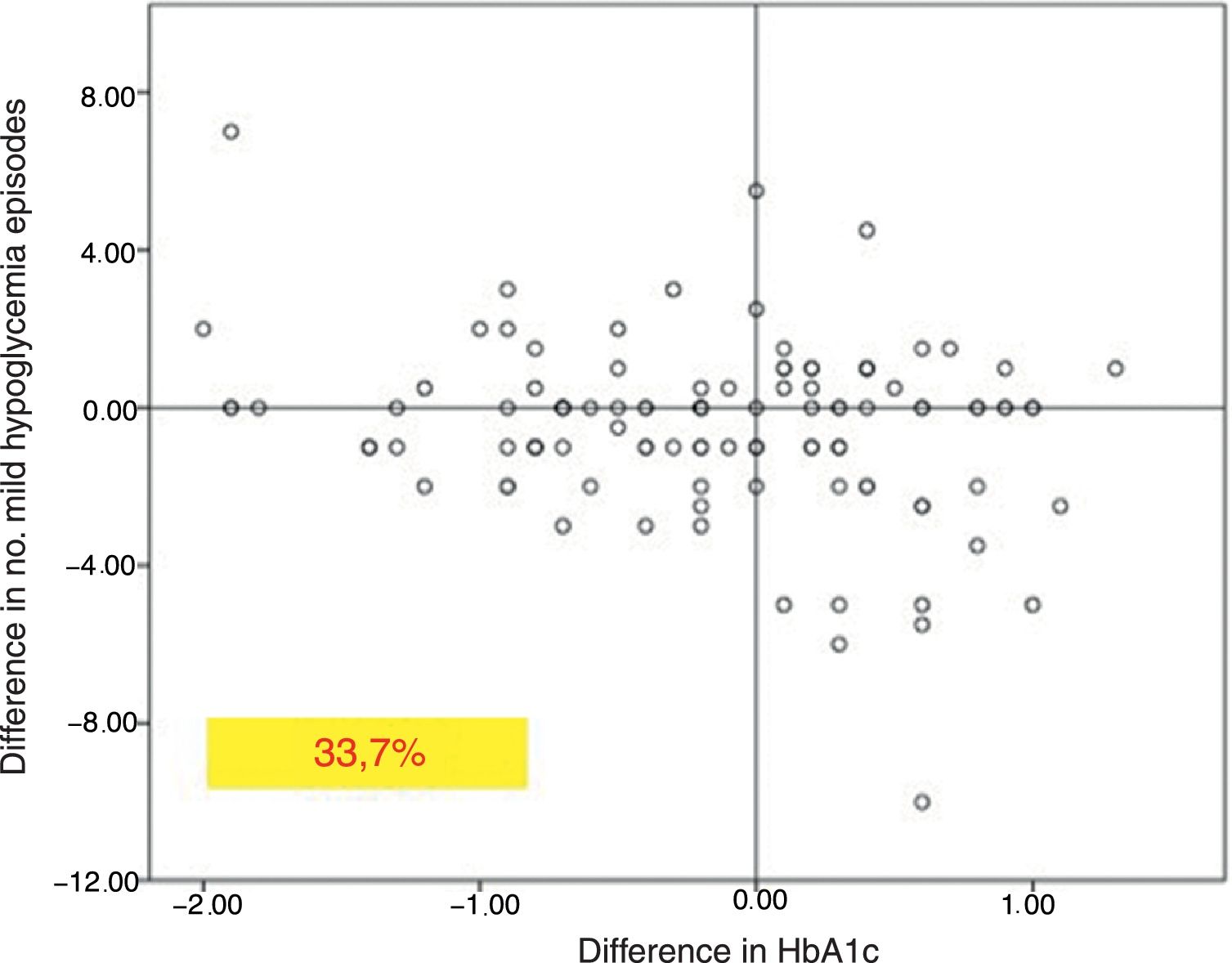
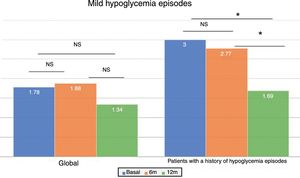
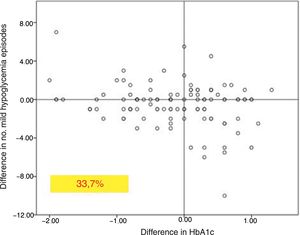
In the global patients, the mean number of mild hypoglycemic episodes a week before the switch in insulin was 1.64±2.20, and this figure did not show changes over time (Fig. 2). However, in the 101 patients with a history of mild hypoglycemia (40.5%), a significant decrease was observed after 12 months of treatment, from 3.06±2.72 to 1.70±2.17 episodes/week (p<0.05) (Fig. 2). No significant differences were observed at 6 months. A total of 83.8% of these patients with a history of mild hypoglycemic episodes showed a decrease in the number of events per week after one year of Gla-300 treatment, and 33.7% showed a decrease in both HbA1c and the number of hypoglycemic events (Fig. 3).
Based on glycemic control, the patients with initial HbA1c<7.5% (n=82) showed a nonsignificant decrease in hypoglycemic events 12 months after the switch in treatment (2.11±2.64 vs. 1.89±2.86 episodes/week at 6 months and 1.24±1.75 episodes/week at 12 months; p=NS). This effect was not observed in those with previous poor glycemic control. The reduction in hypoglycemic episodes was particularly significant 12 months after the switch in treatment among the patients with HbA1c<7.5% and with a history of hypoglycemic episodes (n=43) (3.47±3.04 episodes/week at baseline vs. 1.55±2.11 episodes/week at 12 months; p<0.05).
Changes in weight and amount of insulinIn terms of body weight, we observed a decrease at 6 months after the switch in treatment (74.16±14.90 vs. 73.59±14.99kg; p<0.05). However, after one year there were no changes in weight versus baseline (74.16±14.90 vs. 74.10±15.10kg; p=NS).
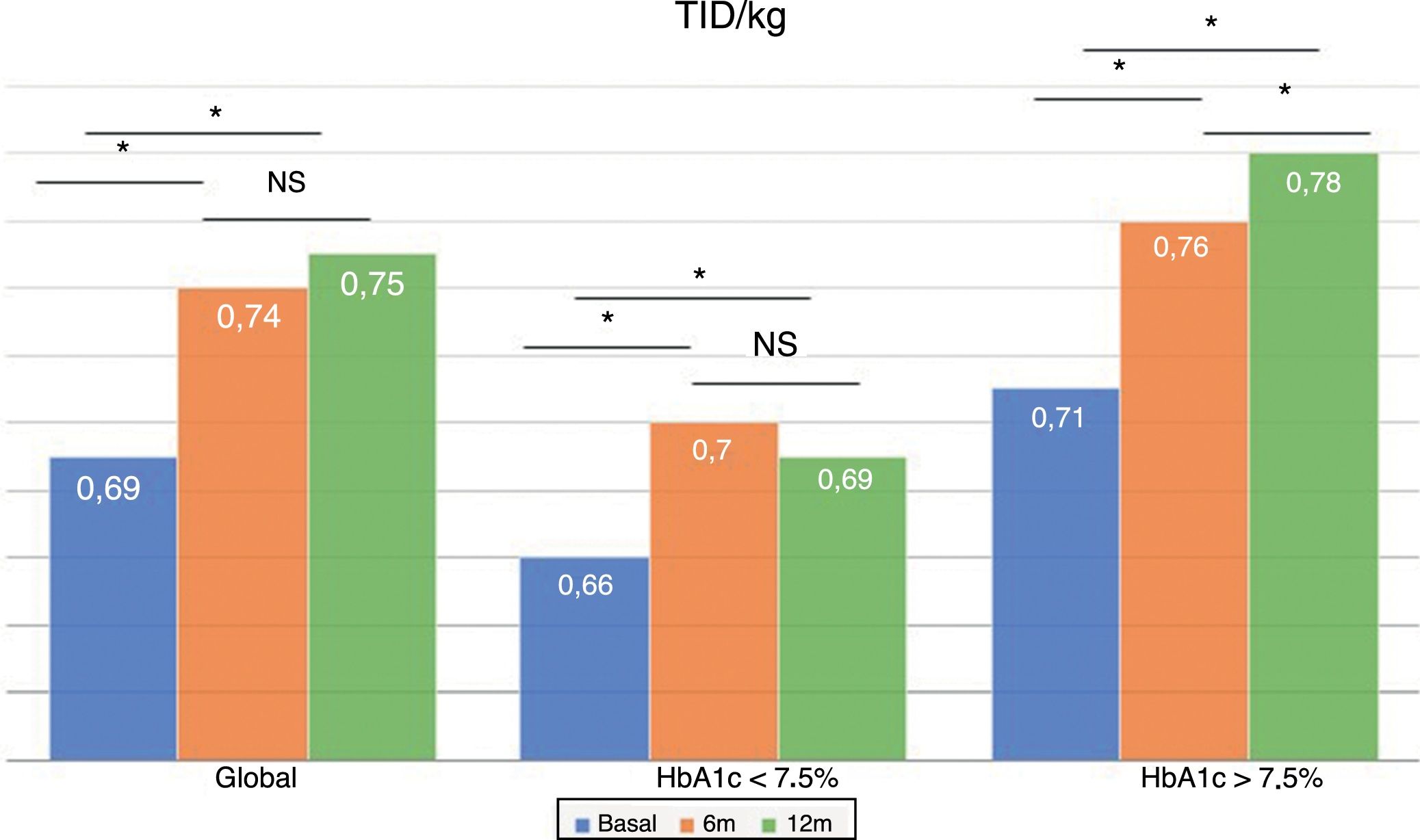
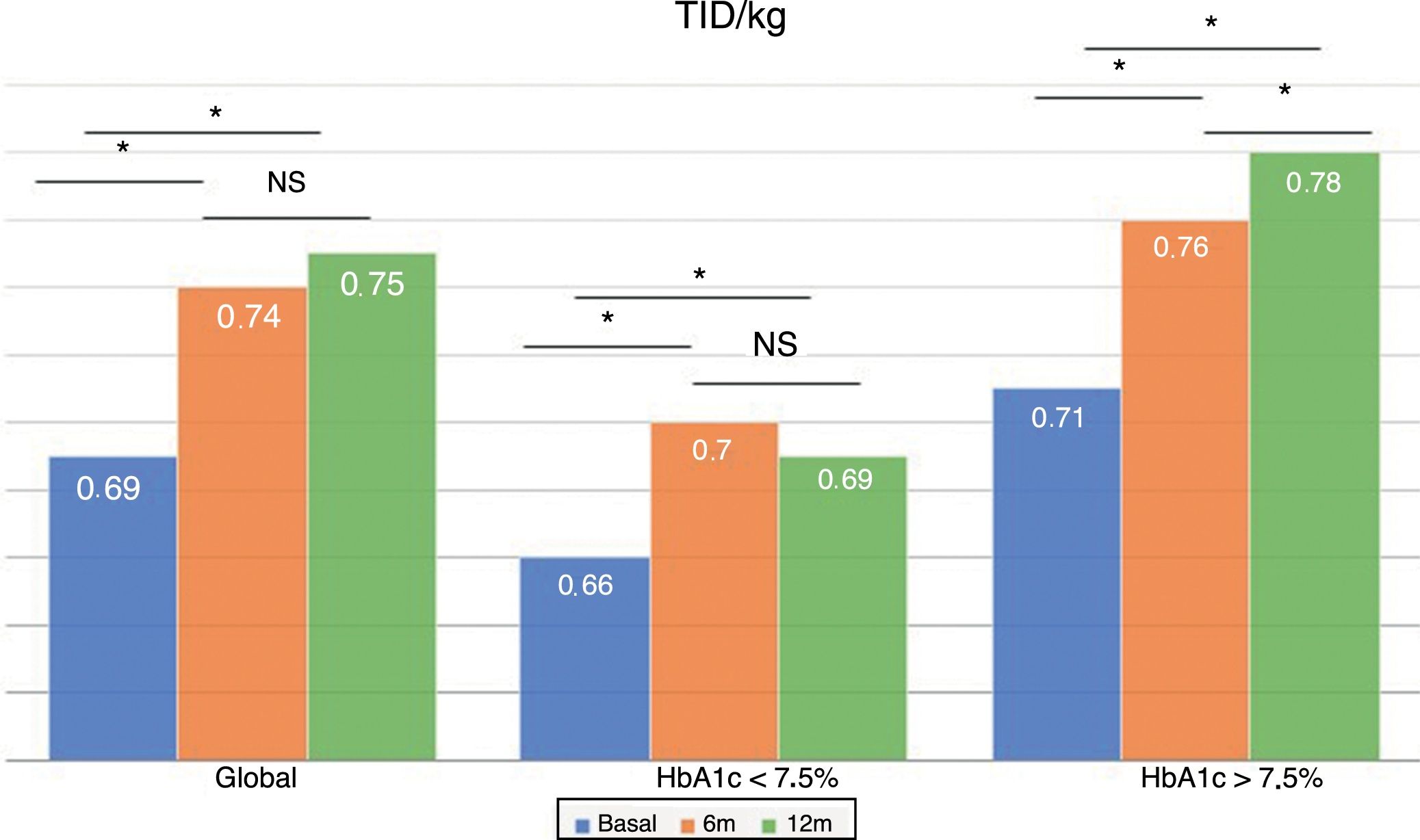
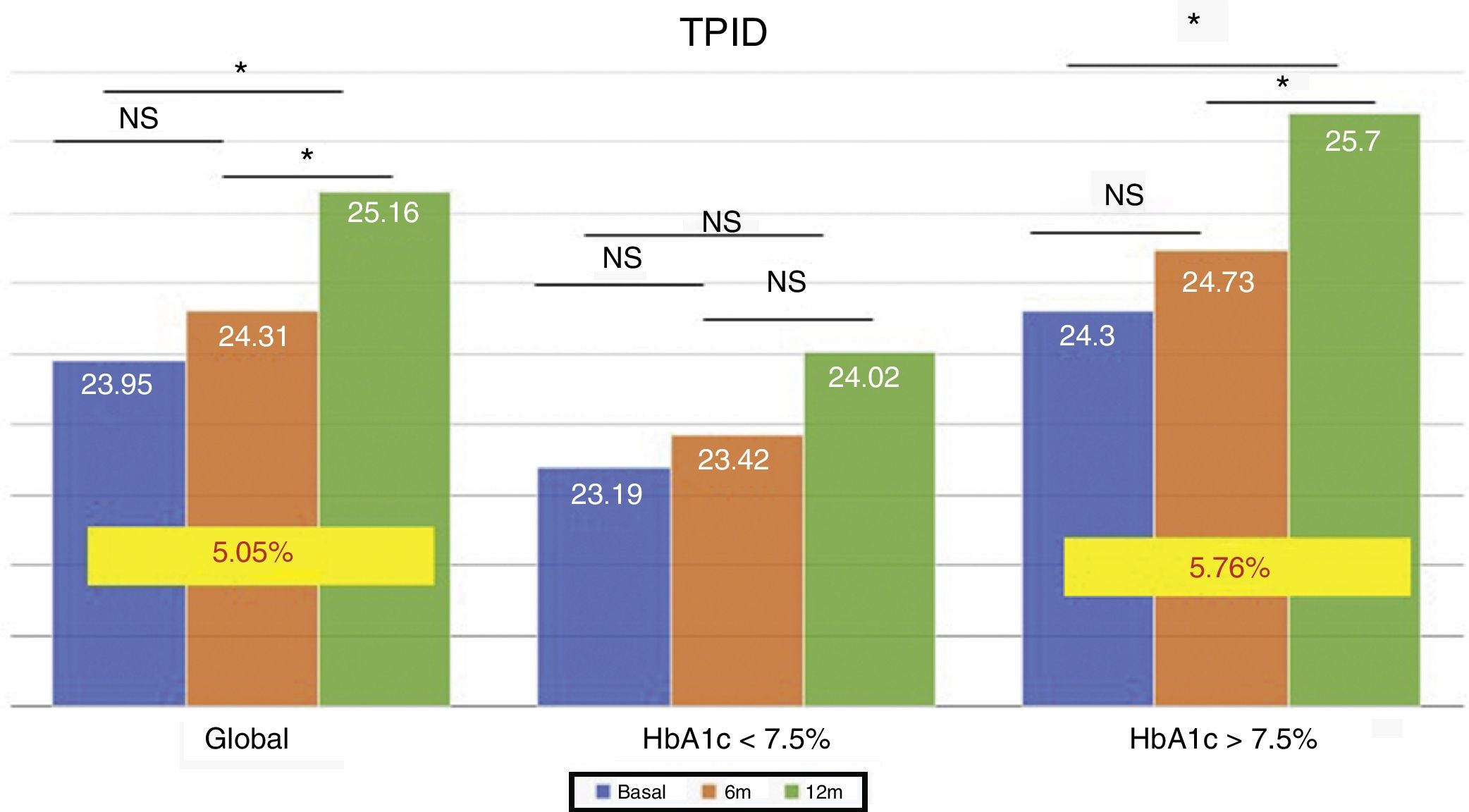
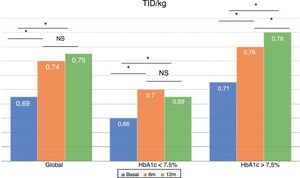
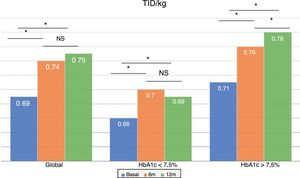
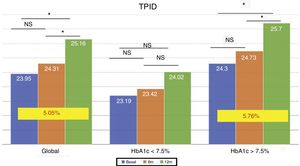
The total dose of insulin/kg increased significantly in the global patients by 7.25% at 6 months (0.69±0.23 vs. 0.74±0.23IU/day/kg; p<0.05) and at one year by 10.14% (0.69±0.23 vs. 0.76±0.24IU/day/kg; p<0.05) (Fig. 4), though there were no differences in dose between 6 months and one year. This increase was mainly due to the rise in basal insulin in the first 6 months (the mean rise being 9.46% at 6 months and 11.06% at one year) (Fig. 5). The increase in basal insulin after one year was similar in the metabolic control groups and in the groups based on whether or not they experienced mild hypoglycemic episodes before the switch in insulin therapy. In addition, the change in basal insulin dose was not related to the initial amount of basal insulin, initial HbA1c concentration, the number of mild hypoglycemic episodes, or initial body weight. Patients with good glycemic control increased their basal insulin dose significantly by 8.43% at 6 months and 7.80% at one year (Fig. 5), but did not show significant changes in the prandial insulin dose. Patients with previous poor glycemic control increased their basal insulin dose significantly by 9.87% at 6 months and 12.38% at one year, with no significant differences versus the patients with good control (Fig. 5). However, they increased the amount of fast insulin by 5.76% after one year of treatment (24.30±12.73 vs. 25.70±13.08IU; p<0.05) (Fig. 6).
Our study demonstrates the efficacy and safety of Gla-300 in patients with DM1 in a real-life setting. Real-life studies of patients with DM2 in which basal insulin was switched to Gla-300 have reported a decrease in HbA1c similar to that observed with other slow insulin analogs, but with a significant decrease in hypoglycemia and its associated consequences.15
The EDITION program, involving patients with DM1, demonstrated that Gla-300 achieves sustained glycemic control similar to that obtained with Gla-100 at 12 months, with a comparative reduction in hypoglycemia time.16 However, real-life studies such as our own have reported a decrease in HbA1c. In this regard, Gradiser et al.17 conducted a study in 18 patients presenting DM1 with suboptimal control who were switched from Gla-100 to Gla-300. After 6 months of follow-up, these authors observed a significant decrease in HbA1c, with fewer hypoglycemia episodes. These data are consistent with our own findings, in which poorly controlled patients showed a decrease in HbA1c at 6 months, and a significant increase was noted in the percentage of patients with HbA1c<7.5%. We also found this acute effect to persist for at least a year. The study of Gradiser et al.17 did not include patients with HbA1c<7.5%. In our study, this subgroup of patients showed a slight increase in HbA1c in the first 6 months, but there were no significant differences versus baseline after one year. Further studies are therefore needed involving patients with well-controlled DM1 and taking into account other aspects (glycemic variability, quality of life) in order to confirm that this subgroup will benefit from the switch of Gla-100 to Gla-300.
With regard to hypoglycemia, we found that patients with a history of mild hypoglycemic episodes showed a significant decrease in the number of such episodes one year after the switch in treatment. It should be noted that 33.7% of the patients with a history of mild hypoglycemic episodes were able to reduce such events, as well as lower their HbA1c concentration. These data differ from those of other studies, in which mild hypoglycemic episodes decreased in the first weeks of treatment18,19 mainly at the expense of nocturnal hypoglycemia, but without the observation of differences between Gla-100 and Gla-300 after one year of follow-up. These differences observed with respect to our sample could be explained by the low number of hypoglycemic episodes per week observed in our patients or by the fact that such episodes were not documented as in other studies that recorded the mean number of hypoglycemic episodes per patient.17 A study specifically designed to assess this issue, based on continuous glycemia measurements, could help clarify the acute benefits of Gla-300 in relation to mild hypoglycemia in DM1. In addition, the decrease in hypoglycemic episodes could be related to the longer duration of the effect of Gla-300, with a more level release profile and the effect extending beyond 24h.12
In terms of body weight, we noted no change after switching to Gla-300 in the course of the one-year follow-up period, despite an increase in the total insulin dose. This differs from other previous studies, in which discrete weight increments were recorded with Gla-300,20 though of lesser magnitude than with Gla-100.16
The total insulin dose per kg weight increased significantly in all patients at 6 months and one year, mainly at the expense of basal insulin, with no significant differences on comparing the groups according to glycemic control or the presence of hypoglycemic episodes. This demonstrates that the dose increment is related to the pharmacokinetic properties of Gla-300. Different studies have shown this same effect,16 attributing this need for dose elevation to an increased subcutaneous deposit.21 As was observed in the EDITION JP1 study,19 this increase in basal insulin occurs in the first 6 months and is maintained during the year of follow-up. On the other hand, the real-life study conducted by Gradiser et al.17 recorded no changes in the basal insulin dose after 6 months of follow-up. Patient selection, the initial HbA1c concentration, or the fact that all selected patients presented hypoglycemia episodes could explain these differences.
In conclusion, this real-life study of patients with DM1 shows insulin Gla-300 to be a safe and effective alternative in the management of these patients. This new basal insulin analog can afford better glycemic control in poorly controlled patients and reduce the appearance of mild hypoglycemic episodes in patients with a history of mild hypoglycemia, without increasing body weight. It remains to be clarified what benefits it may offer in patients with good metabolic control and no history of mild hypoglycemic episodes.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pujante Alarcón P, Rodríguez Escobedo R, García Urruzola F, Ares J, Manjón L, Sanchez Ragnarson C, et al. Experiencia tras el cambio de insulina glargina U100 a glargina U300 en pacientes con diabetes tipo 1. Estudio tras un año de tratamiento en vida real. Endocrinol Diabetes Nutr. 2019;66:210–216.