The evolution of the incidence of type 1 diabetes (T1D) in all groups is controversial. Our objective is to study the incidence from 2009 to 2020, based on the Type 1 Diabetes Registry of Navarra, and to analyze the clinical presentation as diabetic ketoacidosis (DKA), and HbA1c at onset.
Materials and methodsDescriptive study of all cases diagnosed as T1D and included in the Population Registry of T1D of Navarra in the period 1/1/2009 to 12/31/2020. Data are obtained from primary and secondary sources with an ascertainment rate of 96%. Incidence rates are expressed per 100,000 person-years of risk, by age group and sex. Likewise, a descriptive analysis of the HbA1c and DKA at diagnosis of each patient is performed.
Results627 new cases are registered, which represents an incidence of 8.1 (10 in men, 6.3 in women), without differences throughout the analyzed period. The group with the highest incidence is the 10–14 years old children (27.8), followed by that of 5–9 years old (20.6). The incidence in people older than 15 years is 5.8. Twenty six percent of patients present DKA at onset. The global mean of HbA1c is 11.6%, without changes throughout the studied period.
ConclusionsThe population registry of T1D of Navarra shows a stabilization in the incidence of T1D at all ages in the 2009–2020 period. The percentage of presentation as severe forms is high, even in adulthood.
La evolución de la incidencia de diabetes tipo 1 (DM1) en todos los grupos de edad es controvertida. Nuestro objetivo es estudiar la incidencia del periodo 2009–2020 con base en el Registro de DM1 de Navarra, y analizar la presentación como cetoacidosis diabética (CAD) y la HbA1c al debut.
Materiales y MétodosEstudio descriptivo de todos los casos diagnosticados de DM1 incluidos en el registro poblacional de DM1 de Navarra en el periodo del 1 de enero del 2009 al 31 del diciembre del 2020. Los datos se obtienen de fuentes primarias y secundarias con una exhaustividad del registro de 96%. Se estiman las tasas de incidencia expresadas por 100.000 personas-año de riesgo, por grupos de edad y sexo. Asimismo se realiza un análisis descriptivo del nivel de HbA1c y situación de CAD de cada paciente al diagnóstico.
ResultadosSe registran 627 nuevos casos, lo que supone una incidencia de 8,1 (10 en hombres y 6,3 en mujeres), no observándose diferencias a lo largo del tiempo analizado. El grupo de mayor incidencia es el de 10 a 14 años (27,8), seguido por el de cinco a nueve años (20,6). La incidencia en mayores de 15 años es de 5,8. El 26,5% presentan CAD al debut. La HbA1c media global es 11,6%, sin cambios a lo largo del periodo estudiado.
ConclusionesEl registro poblacional de DM 1 de Navarra muestra una estabilización en la incidencia de DM1 en todas las edades en el periodo 2009–2020. El porcentaje de presentación en formas graves es elevado, incluso en edad adulta.
The onset of type 1 diabetes (T1DM) results from the interaction of predisposing genetic factors and environmental factors that act as triggers. Data on the incidence of the disease and incidence trends are important in order to identify these factors more quickly and design prevention strategies, as well as to estimate the resources necessary for its proper care.
Although the incidence is higher in childhood, T1DM is by no means exclusive to this age group, since it is believed that between one third1 and half2 of new cases of T1DM occur in people over 18 years of age. There is growing interest in this topic, with a 40% increase in the number of publications over the last decade. However, most of these publications provide incidence data referring to the paediatric age group. Even the global data provided biannually in the International Diabetes Federation (IDF) Diabetes Atlas3 are limited to childhood. The greatest difficulty in gathering epidemiological data on T1DM in adulthood is the problem posed by its differentiation with insulin-dependent type 2 diabetes when autoimmune parameters are not available, or with latent autoimmune diabetes in adults (LADA). This may explain why there are very few national or regional registries that include the incidence of the disease in all age groups.
The incidence is believed to have been increasing in recent decades, but this increase is greater in countries with a low incidence, with a tendency to stabilise in countries with higher incidences, such as in northern Europe.4 In Spain, incidence data is available from 0 to 15 years of age from almost all the autonomous communities, and in two of them patients up to 30 years5 and 40 years6 of age are included. However, these Spanish regional registries that included incidence in adults have not provided data in the last 10 years.
The most severe forms of presentation of the disease, with ketoacidosis (DKA), occur at younger ages in up to 25%–30% of children diagnosed in Europe7–9 and up to 80% in the United Arab Emirates.10 There are few published studies in adults in this regard, although the frequency of DKA at onset appears to be reduced by at least one third.2,10 Before 2020, the percentage of patients presenting with DKA at onset was on the decline11,12 or stabilised.13 However, coinciding with the COVID-19 pandemic, the risk of DKA at onset has increased14,15 and in some cases has more than doubled.9,16
The objective of this study was to provide data on the time trend of the incidence of T1DM in all age groups from 2009 to 2020, based on data from the Navarre Type 1 Diabetes Registry, as well as to study the clinical profile at onset and its possible age-related differences.
Patients and methodsWe conducted a descriptive study of the data collected in the Navarre Type 1 Diabetes Population Registry from 2009 to 2020. The registry was created by Regional Order 10/2010, of 21 January, and it prospectively collects all the cases diagnosed in Navarre of patients who have been residing in the community for at least six months. The primary sources of information are all the public and private hospitals in the community, and the secondary sources are the primary care centres (through the Servicio de Apoyo a la Gestión Clínica y Continuidad Asistencial [Clinical Management and Healthcare Continuity Support Service]) and the Asociación de Diabetes de Navarra [Diabetes Association of Navarre] (ANADI).
The registry completeness was assessed using the capture-recapture method and was 96% (81% for the primary source).
The registry data manager is responsible for contacting the collaborating researcher at each health centre, primary care and ANADI.
The following diagnostic criteria for type 1 diabetes have been considered: a) positive anti-GAD and/or anti-IA2 antibodies along with the persistent need for insulin therapy with onset less than six months after diagnosis; or b) when the antibodies were negative: low C-peptide levels, along with typical characteristics of its onset (clinical and analytical: ketosis or ketoacidosis) and the persistent need for insulin therapy with onset less than six months after diagnosis. Since all patients with T1DM are followed up in endocrinology clinics, if they suspend insulin therapy during its course and come to be considered patients with type 2 diabetes, they are withdrawn from the registry for all purposes. Patients with LADA are also excluded.
Statistical analysisFor incidence calculations, population data obtained from the census and electoral roll collected by the National Institute of Statistics were used, which provide total population data broken down by gender and age groups. The incidence is expressed per 100,000 person-years of risk of all the years studied.
The 95% confidence intervals (95% CI) were estimated using Poisson distribution. Incidences by age groups and gender were compared, and the incidence rate ratio was estimated using Poisson regression. The open source software for epidemiological statistics for public health OpenEpi version 3.01 (Rollins School of Public Health, Emory University, Atlanta, USA) was used.
Student's t test was used to compare the means between genders, and when there were more than two groups, single factor ANOVA with Tukey's post hoc a posteriori test were used, both with a level of statistical significance of P < .05. For these analyses, the Statistical Package for the Social Sciences (SPSS) software version 20 (IBM Corp., Armonk, NY, USA) was used.
This study was reviewed and approved by the Independent Ethics Committee of Navarre.
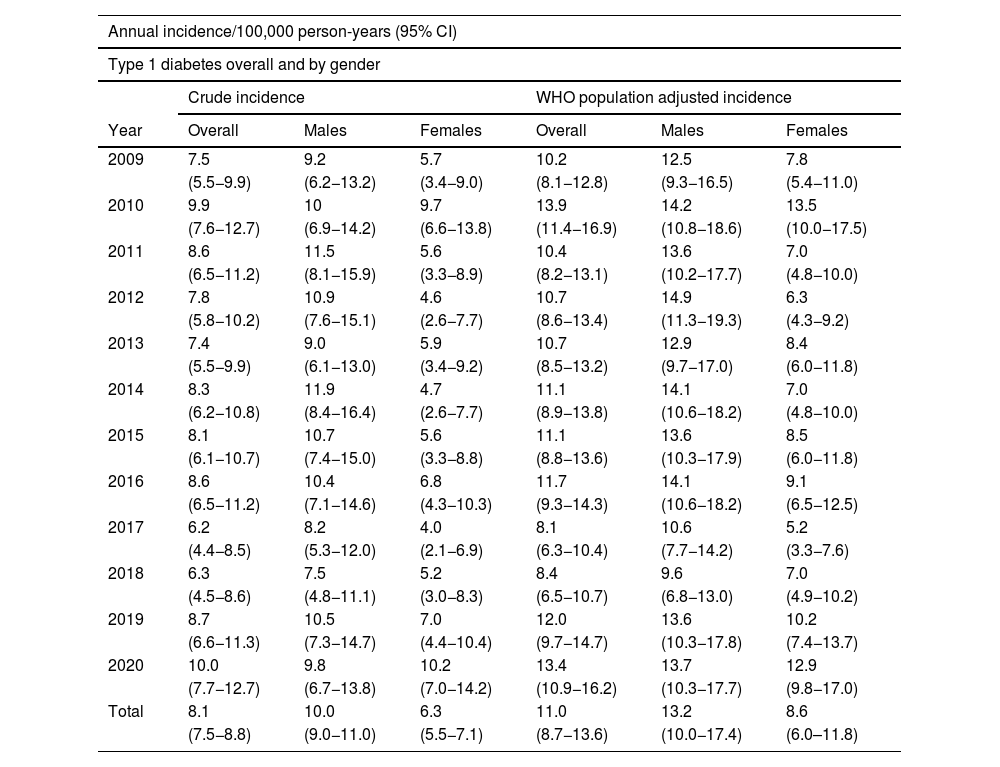
ResultsOverall, 627 new cases of T1DM (61.2% males) were recorded, representing an incidence of 8.1/100,000 person-years. Public centres contributed 602 cases and private centres 25 cases. The average annual population in the period 2009–2020 was 643,892 inhabitants per year (49.7% males).
Although the incidence has slight annual variations during the 12-year follow-up, no significant differences were observed in that period (Table 1).
Annual incidence of T1DM from 2009 to 2020, overall and by gender.
| Annual incidence/100,000 person-years (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Type 1 diabetes overall and by gender | ||||||
| Crude incidence | WHO population adjusted incidence | |||||
| Year | Overall | Males | Females | Overall | Males | Females |
| 2009 | 7.5 | 9.2 | 5.7 | 10.2 | 12.5 | 7.8 |
| (5.5−9.9) | (6.2−13.2) | (3.4−9.0) | (8.1−12.8) | (9.3−16.5) | (5.4−11.0) | |
| 2010 | 9.9 | 10 | 9.7 | 13.9 | 14.2 | 13.5 |
| (7.6−12.7) | (6.9−14.2) | (6.6−13.8) | (11.4−16.9) | (10.8−18.6) | (10.0−17.5) | |
| 2011 | 8.6 | 11.5 | 5.6 | 10.4 | 13.6 | 7.0 |
| (6.5−11.2) | (8.1−15.9) | (3.3−8.9) | (8.2−13.1) | (10.2−17.7) | (4.8−10.0) | |
| 2012 | 7.8 | 10.9 | 4.6 | 10.7 | 14.9 | 6.3 |
| (5.8−10.2) | (7.6−15.1) | (2.6−7.7) | (8.6−13.4) | (11.3−19.3) | (4.3−9.2) | |
| 2013 | 7.4 | 9.0 | 5.9 | 10.7 | 12.9 | 8.4 |
| (5.5−9.9) | (6.1−13.0) | (3.4−9.2) | (8.5−13.2) | (9.7−17.0) | (6.0−11.8) | |
| 2014 | 8.3 | 11.9 | 4.7 | 11.1 | 14.1 | 7.0 |
| (6.2−10.8) | (8.4−16.4) | (2.6−7.7) | (8.9−13.8) | (10.6−18.2) | (4.8−10.0) | |
| 2015 | 8.1 | 10.7 | 5.6 | 11.1 | 13.6 | 8.5 |
| (6.1−10.7) | (7.4−15.0) | (3.3−8.8) | (8.8−13.6) | (10.3−17.9) | (6.0−11.8) | |
| 2016 | 8.6 | 10.4 | 6.8 | 11.7 | 14.1 | 9.1 |
| (6.5−11.2) | (7.1−14.6) | (4.3−10.3) | (9.3−14.3) | (10.6−18.2) | (6.5−12.5) | |
| 2017 | 6.2 | 8.2 | 4.0 | 8.1 | 10.6 | 5.2 |
| (4.4−8.5) | (5.3−12.0) | (2.1−6.9) | (6.3−10.4) | (7.7−14.2) | (3.3−7.6) | |
| 2018 | 6.3 | 7.5 | 5.2 | 8.4 | 9.6 | 7.0 |
| (4.5−8.6) | (4.8−11.1) | (3.0−8.3) | (6.5−10.7) | (6.8−13.0) | (4.9−10.2) | |
| 2019 | 8.7 | 10.5 | 7.0 | 12.0 | 13.6 | 10.2 |
| (6.6−11.3) | (7.3−14.7) | (4.4−10.4) | (9.7−14.7) | (10.3−17.8) | (7.4−13.7) | |
| 2020 | 10.0 | 9.8 | 10.2 | 13.4 | 13.7 | 12.9 |
| (7.7−12.7) | (6.7−13.8) | (7.0−14.2) | (10.9−16.2) | (10.3−17.7) | (9.8−17.0) | |
| Total | 8.1 | 10.0 | 6.3 | 11.0 | 13.2 | 8.6 |
| (7.5−8.8) | (9.0−11.0) | (5.5−7.1) | (8.7−13.6) | (10.0−17.4) | (6.0–11.8) | |
95% CI: 95% confidence interval; WHO: World Health Organization.
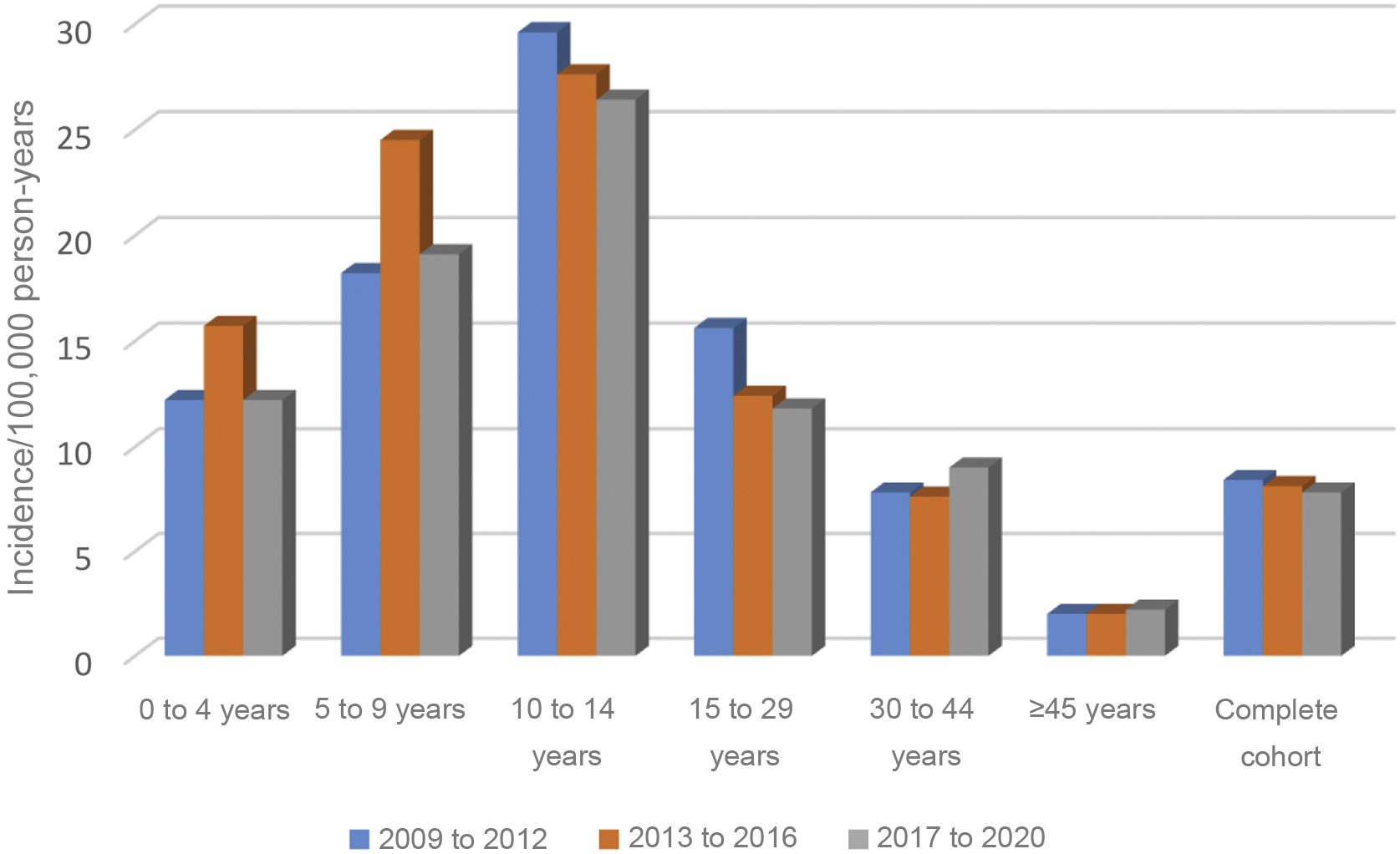
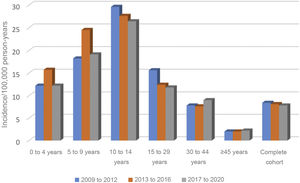
Nor were any significant differences found over these 12 years in the incidence by age group, grouped into four-year periods (Fig. 1).
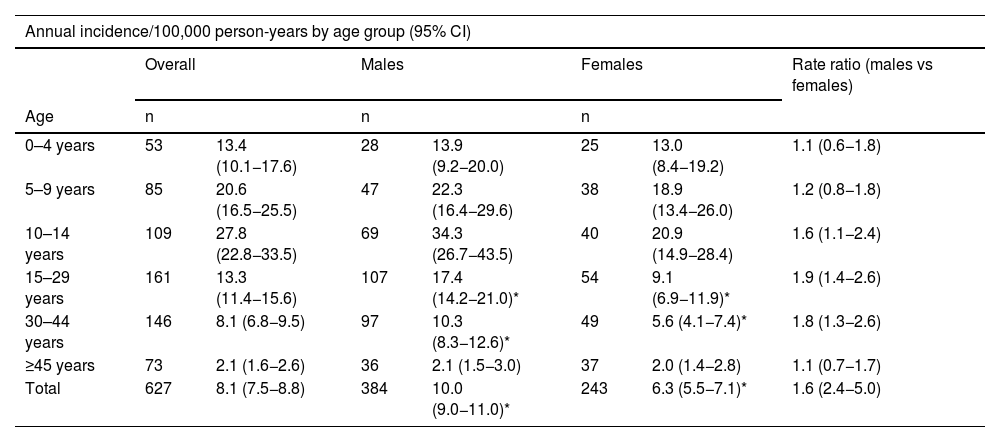
The distribution by gender was 384 males (61.2%) and 243 females (38.8%), with an incidence of 10 and 6.3, respectively (13.2 and 8.6, respectively, adjusted to world population according to the WHO). The rate ratio shows that males have a 60% higher risk of presenting with T1DM. By age, the incidence rate was higher in males, in the age groups 10–14 years, 15–29 years and 30–44 years, with no differences in the other age groups (Table 2).
Annual incidence of T1DM from 2009 to 2020, by age group and gender.
| Annual incidence/100,000 person-years by age group (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Overall | Males | Females | Rate ratio (males vs females) | ||||
| Age | n | n | n | ||||
| 0–4 years | 53 | 13.4 (10.1−17.6) | 28 | 13.9 (9.2−20.0) | 25 | 13.0 (8.4−19.2) | 1.1 (0.6−1.8) |
| 5–9 years | 85 | 20.6 (16.5−25.5) | 47 | 22.3 (16.4−29.6) | 38 | 18.9 (13.4−26.0) | 1.2 (0.8−1.8) |
| 10–14 years | 109 | 27.8 (22.8−33.5) | 69 | 34.3 (26.7−43.5) | 40 | 20.9 (14.9−28.4) | 1.6 (1.1−2.4) |
| 15–29 years | 161 | 13.3 (11.4−15.6) | 107 | 17.4 (14.2−21.0)* | 54 | 9.1 (6.9−11.9)* | 1.9 (1.4−2.6) |
| 30–44 years | 146 | 8.1 (6.8−9.5) | 97 | 10.3 (8.3−12.6)* | 49 | 5.6 (4.1−7.4)* | 1.8 (1.3−2.6) |
| ≥45 years | 73 | 2.1 (1.6−2.6) | 36 | 2.1 (1.5−3.0) | 37 | 2.0 (1.4−2.8) | 1.1 (0.7−1.7) |
| Total | 627 | 8.1 (7.5−8.8) | 384 | 10.0 (9.0−11.0)* | 243 | 6.3 (5.5−7.1)* | 1.6 (2.4−5.0) |
95% CI: 95% confidence interval; T1DM: type 1 diabetes mellitus.
In the analysis by age group, the group with the highest incidence was 10–14 years: 27.8 (22.8−33.5), followed by five to nine years: 20.6 (16.5–25.5). In the overall cohort and in males, the differences between the 10–14 age group and all other age groups except the five to nine age group were significant. However, in terms of females, no significant differences were found between those under 14 years of age, although there were between girls aged 10–14 years and those over this age (Table 2).
The incidence in children under 15 years of age is 20.6 (18.2–23.3), much higher than that observed in patients aged 15 years and over: 5.8 (5.3–6.4). This difference is observed both in males (23.5 [19.9–27.5] vs 7.4 [6.5–8.4]), and in females (17.6 [14.5–21.4] vs 4.2 [3.6–5.0]).
Regarding the clinical signs and symptoms at onset, 26.5% (23.0−29.9) of the patients presented with DKA, with no significant differences by gender. By age groups, those from zero to four years old (43.4%) and those from 10 to 14 years old (38.5%) presented higher figures than those from 30 to 44 years old (19.2%) and those from 45 years and older (12.3%) (P < .05) (Table 3).
Biochemical characteristics at onset.
| Characteristics at onset | ||
|---|---|---|
| Ketoacidosis, % (95% CI) | HbA1c, % (95% CI) | |
| Overall | 26.5 (23.0−29.9) | 11.6 (11.0−12.0) |
| Male | 26.6 (22.1−31.0) | 11.7 (11.4−11.9)∫ |
| Female | 26.3 (20.8−31.9) | 11.0 (10.7−11.3)∫ |
| Age | ||
| 0–4 years | 43.4 (29.6−57.2)* | 10.2 (9.7−10.7)‡ |
| 5–9 years | 25.9 (16.4−35.4) | 10.7 (10.3−11.2)¶ |
| 10–14 years | 38.5 (29.2−47.8)† | 11.6 (11.2−12.0)‡ |
| 15–29 years | 26.1 (19.2−32.9) | 11.8 (11.5−12.2)‡ ¶ |
| 30–44 years | 19.2 (12.7−25.6)* † | 11.5 (11.1−12.0)‡ |
| ≥45 years | 12.3 (4.6−20.1)* † | 11.7 (11.0−12.3)‡ |
95% CI: 95% confidence interval; HbA1c: glycosylated haemoglobin.
The mean level of glycosylated haemoglobin (HbA1c) at diagnosis was 11.6% (11.0−12.0). It was 11.7% (11.4−11.9) in males and 11% (10.7−11.3) in females, with this difference being significant (P < .05). By age groups, the group from zero to four years old had an HbA1c of 10.2% (9.7−10.7), significantly lower than all groups from 10 years old upwards. Differences were also detected between the five to nine year age group and the 15–29 year age group (Table 3).
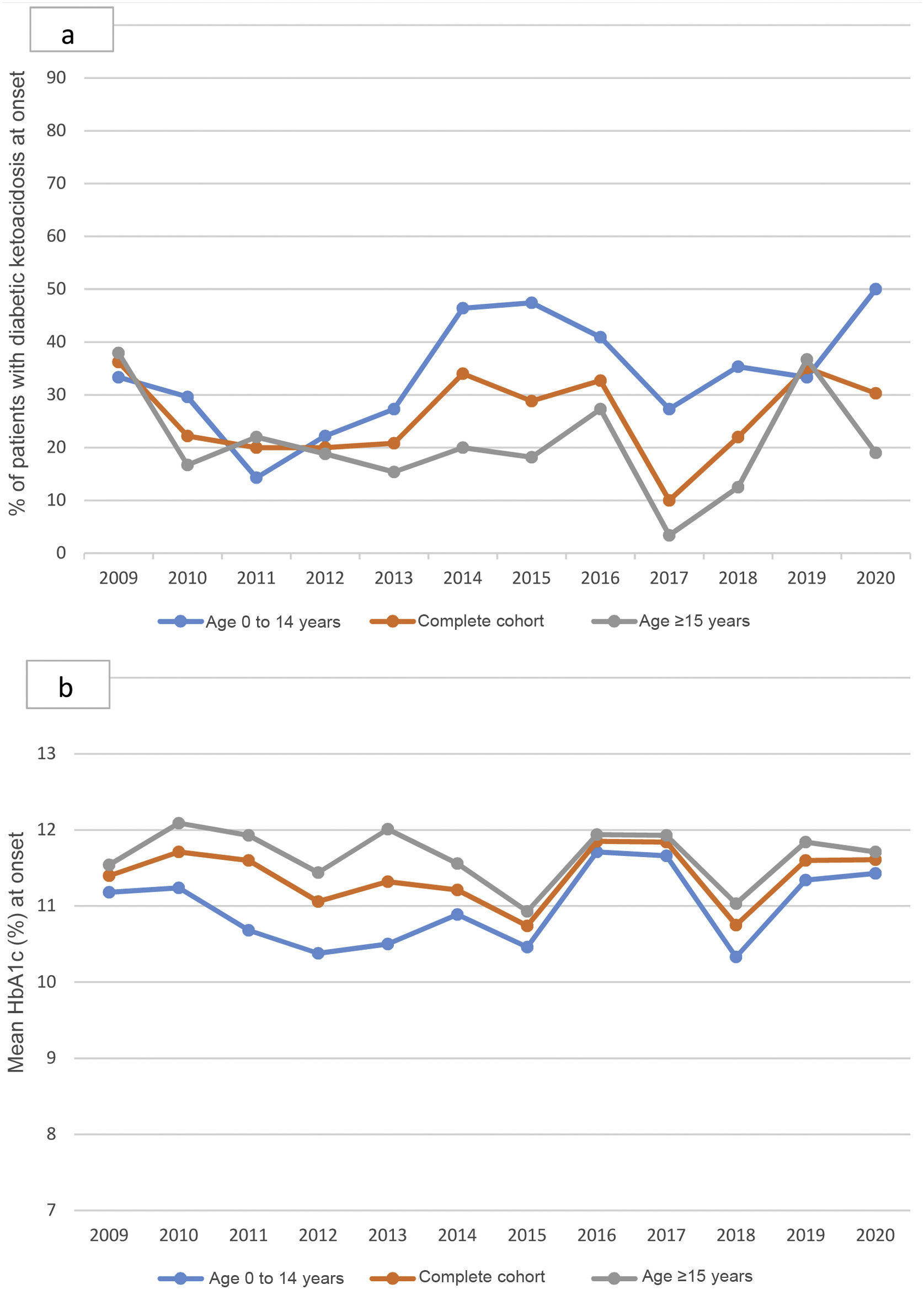
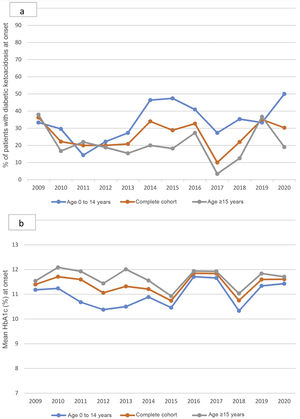
The percentage of patients with DKA at onset varied without significant differences throughout the follow-up, although it is worth noting the increase in 2020 in children under 15 years of age, the highest figure reached in the historical series: 50% (Fig. 2a). HbA1c at onset also did not change between 2009 and 2020 (Fig. 2b).
a; annual percentage of patients with ketoacidosis at the onset of T1DM from 2009 to 2020, in children and adults. Non-statistically significant differences throughout the period studied. b; Mean annual HbA1c at the onset of T1DM from 2009 to 2020, in children and adults. Non-statistically significant differences throughout the period studied.
Our data show a stabilisation of the incidence of T1DM in the Community of Navarre in the period 2009–2020. Annual differences were observed that were not statistically significant. This latest analysis completes and confirms what was previously reported for the 2009–2016 time period.17 Regarding the form of presentation, DKA continues to account for a high percentage, both in children and adults.
In the last edition (10.a) of the International Diabetes Federation Diabetes Atlas, which includes patients from zero to 19 years of age, data are available from 45% of all countries globally, with data provided by 74.6% of European countries.3 Worldwide, the lowest incidence is in South and Central America (12.3) and in Western Pacific countries (11.6). In Europe, an incidence rate of 31 has been calculated. Most of the publications are not recent, with only 28% of countries offering data after 2010.18 In the zero to 14 age group, Finland (52.2) and Sweden (44.1) have the highest rates, followed by Norway (33.6) and the United Kingdom (28.1). Spain is in the group of countries with a low incidence (10–20 cases).3 In Navarre, conversely, according to our data, the incidence in children under 15 years of age is high (20.6). Additionally, incidence data in the 20–40 year age group between 1973 and 2019 are available from 32 countries around the world. Spain is one of those providing data in this age range and, with an incidence of 9.9, is within the group of countries with low incidence (data from the period 1987–1990).3 Only eight studies have been published with incidence data in people over 60 years of age, among which are the results of the Type 1 Diabetes Registry of Navarre.1,13,19
Many recent articles have studied the trend over time. They include a total of 15 studies,1,13,20 among which there are also previous publications of our registry of Navarre.17 The trends are variable, with most showing a decline (Serbia, Sweden, Korea, Taiwan, and the USA)20 or stabilisation, as in our study, the United Kingdom, Iceland and Hong Kong1,20 and only two studies showing an increase, in Mali20 and China.13 These trends are similar across all age groups.
In other Spanish groups, the most recent publications show stabilisation (Asturias)6 or increase (Gran Canaria,21 Community of Madrid22). However, in the latter case, it is probably due more to a bias in the methodology than a true increase.
In our analysis, no significant differences were observed in the time trend of the incidence by age group. The peak incidence was maintained in the 10–14 age group, with a rate of 27.8, followed by the five to nine year age group (rate of 20.6). The predominance in these ages is constant throughout the follow-up. Our study confirmed that the incidence decreases with age, falling to 2.1 in the over 45 s. In Gran Canaria, with the highest incidence in Spain in the paediatric population (30.5), no significant differences were found by age group or gender.21 In contrast, in communities with a lower incidence, more similar to ours, such as Asturias or Madrid, there is also a global predominance in the 10–14 age group, although in Madrid, as in our previous publication,17 in girls, the highest incidence is in the five to nine years age group.22
The registry of Navarre confirms the higher incidence in males when analysing the total number of patients (rate ratio 1.6), primarily accounted for by the 10–14 years (rate ratio 1.6) and 15–29 years (rate ratio 1.8) age groups. This finding is in line with what has been reported in the literature, which shows a higher incidence in males in 81% of the published studies, although our rate is higher than that described in another review (rate ratio 1.47).19
On the other hand, in patients under 10 years of age and over 45 years of age in our cohort, the difference found between males and females was not significant, as described in other reviews.19
This confirmed predominance in males could be related to a possible hormonal influence on insulin resistance, different lifestyles and/or genetic predisposition.23 More studies on this aspect are needed to be able to determine its cause.
Age is a factor that influences the rate of decline in pancreatic beta function and, therefore, the rate of progression of the disease.13 It is known that its manifestation during the early years of life is due to greater genetic predisposition and a greater humoral response, and this could give rise to a more severe form of the disease, with a greater frequency of DKA2,9,10,13 and/or higher levels of HbA1c.2 We have analysed both variables in our registry. The global DKA rate is 26.5%, confirming that the lower the age at diagnosis, the higher the incidence of DKA. Nevertheless, the new-onset percentage in people older than 14 years continues to be significant.
These results highlight the need to insist on campaigns for its early detection, both aimed at the general population and also at healthcare professionals who care for patients in the earliest phases. This could prevent progression to severe forms of the disease such as DKA.
Regarding the progression of DKA at onset, in Navarre we observed wide fluctuations in the 12-year follow-up but, similar to China13 and unlike Croatia11 and Italy,12 without significant variations. In 2020, the year of the pandemic, our global figure was within the range of previous years. In this way our study differs from the literature.8,9,15,16 However, the increase in children under 15 years of age (DKA at onset in 2020 in 50% of cases) is striking, being the largest in the series. These data are consistent with that reported in the literature.8,9
On the other hand, our results show that the lowest HbA1c levels at onset occur in children in the age group from zero to four years. The apparent contradiction between lower HbA1c levels and a higher percentage of DKA is probably due to the lower reserve of insulin levels in children than in adults, which would cause DKA to occur even with similar HbA1c levels. From the age of four, HbA1c values increase until they reach figures similar to those described in several series.2,8 In the cohort as a whole, males show significantly higher HbA1c values than do females. We have not found references to similar studies with which to compare our results in the literature.
In the analysis of the evolution of HbA1c levels in our series between 2009 and 2020, we did not observe significant differences over the years, not even in 2020. In this our study data are consistent with those from Portugal8 and differ to findings in the United Kingdom.9
The limitations of this study are that it is a regional registry as well as the fact that follow-up was limited to 12 years. However, there are very few studies that provide evolutionary data on incidence, including populations of all ages.
Regarding its strengths, we highlight the completeness of the registry using the capture-recapture method, with follow-up of the included cases and diagnostic confirmation in this follow-up in doubtful cases. In addition, all diagnosed patients were included, with no age limit. Finally, all patients were diagnosed based on both clinical and analytical parameters, such as the determination of pancreatic antibodies and C-peptide level. Only 20% of articles reporting on the incidence of the disease in adults include these parameters as diagnostic criteria.20
ConclusionsThe type 1 diabetes population registry in Navarre shows that the incidence of T1DM in the period 2009–2020 stabilised at all ages. The highest incidence was recorded in the 10–14 years age group, followed by the five to nine years group, and predominance in males was confirmed. The percentages of severe forms of the disease were high, even in adulthood, without significant differences in HbA1c levels at diagnosis in ages over 10 years. This form of disease does not change over the years of follow-up of the registry.
We also provide data on the time trend of the incidence of the disease, especially in adulthood, where the information provided by the different registries and population studies on T1DM is more limited.
Authors' contributionsM.J. Goñi helped conceive and design the study, interpret the data and draft the article.
A. Brugos-Larumbe and F. Guillén-Grima carried out the statistical analysis of the data and drafted the results.
A. Sainz de los Terreros helped interpret the data and prepared the tables and figures of the article.
M. Chueca helped collect and interpret data in children.
L. Forga collected and helped interpret data in adults, and helped draft the article.
All authors critically reviewed the article and approved its final version.
FundingThis study was funded by the Instituto de Salud Carlos III (PI10/02715), the Government of Navarre (53/2008) and the Fundación CAN/La Caixa [CAN/La Caixa Foundation] (Pyto 28/2014). In addition, the unconditional collaboration of Sanofi-Aventis SA (2018) and Lilly SAU (2019) for this project should be noted. These funding sources were in no way involved in the preparation of this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank the other members of the Navarre Type 1 Diabetes Study Group, listed below, for their collaboration in this publication: Emma Anda, Marta García, Ana Iriarte, Francisco Javier Pineda, Juan Pablo Martínez de Esteban, Marta Toni, Patricia Munárriz, Francisco Javier Basterra, Ander Ernaga, Nerea Eguilaz (Department of Endocrinology and Nutrition of the Hospital Universitario de Navarra, Pamplona), Sara Berrade (Department of Paediatric Endocrinology of the Hospital Universitario de Navarra, Pamplona), María Dolores Ollero and Ana Irigaray (Endocrinology, Hospital García Orcoyen [García Orcoyen Hospital], Estella), José Jorge Ortez (Endocrinology, Hospital Reina Sofía [Reina Sofía Hospital], Tudela) Francisco Javier Escalada and Marta García (Department of Endocrinology and Nutrition, Clínica Universidad de Navarra), Darío Carrillo (Clínica San Miguel [San Miguel Clinic], Pamplona), Óscar Lecea (on behalf of the Servicio de Apoyo a la Gestión Clínica y Continuidad Asistencial of the Department of Health) and Juan José Remón (on behalf of ANADI).