Type 1 diabetes mellitus (DM1) is a chronic disease with important socio-health repercussions that requires epidemiological information for proper health management. The aim of this study was to determine the incidence of DM1 in Asturias between 2011–2020.
MethodsDescriptive study which included diagnoses of DM1 in Asturias between 2011–2020 captured as a primary source by reviewing the register of pancreatic autoimmunity analysis. Incidence rates were estimated, expressed per 100,000 population-years of risk by age group, sex, and health area.
ResultsA total of 815 patients were diagnosed, 53.13% men. The mean age was 34.32±22.07 years; 9.85±4.46 in children under 19 years of age (10.48±4.45 in males and 9.00±4.36 in females). Of the diagnoses, 55.34% occurred at an age over 30 years. The incidence was 7.82 (7.29–8.37); 19.65 (17.17–22.39) in under 15s and 12.84 (11.73–14.03) in under 40s. The maximum incidence peak was between 10−14 years, both in males 31.16 (23.89−39.95) and in females 21.72 (15.59−29.47). There was no significant increase in incidence over the years studied.
ConclusionsAsturias has a high incidence of DM1. In our study no earlier age at diagnosis was observed or an increase in incidence. Compared to previous studies, the increase in incidence is most likely due to an improvement in data capture, not to a real increase in incidence. A high percentage of diagnoses occur in adulthood.
La diabetes mellitus tipo 1 (DM1) es una enfermedad crónica con importante repercusión sociosanitaria ante la que se requiere de información epidemiológica para una correcta gestión sanitaria. El objetivo del estudio es conocer la incidencia de DM1 en Asturias entre 2011 y 2020.
Material y métodosEstudio descriptivo en el que se han incluido los diagnósticos de DM1 en Asturias entre los años 2011 y 2020, captados como fuente primaria mediante revisión del registro de análisis de autoinmunidad pancreática. Se han estimado las tasas de incidencia (TI), expresadas por 100.000 habitantes-año de riesgo por grupos de edad, sexo y área sanitaria.
ResultadosFueron diagnosticado 815 pacientes; el 53,13% eran hombres. La edad media fue de 34,32±22,07 años; 9,85±4,46 en menores de 19 años (10,48±4,45 en varones y 9,00±4,36 en mujeres). El 55,34% de los diagnósticos se produjo a edad superior a los 30 años. La TI fue de 7,82 (7,29–8,37); de 19,65 (17,17–22,39) en menores de 15 años y de 12,84 (11,73–14,03) en menores de 40. El pico máximo de TI se produjo entre los 10 y los 14 años, tanto en hombres (31,16; 23,89–39,95) como en mujeres (21,72; 15,59–29,47). No se apreció aumento significativo de la incidencia en los años estudiados.
ConclusionesAsturias presenta una incidencia alta de DM1. En nuestro estudio no se aprecia adelanto en la edad al diagnóstico ni aumento de la TI. Con respecto a estudios previos, la TI aumenta debido, con alta probabilidad, a una mejora en la captura de datos, no a un aumento real de la incidencia. Un alto porcentaje de los diagnósticos se producen en la edad adulta.
Type 1 diabetes mellitus (T1DM) is a disease based on hyperglycaemia, a consequence of the destruction of pancreatic β cells by an autoimmune attack.1 It is a chronic disease with significant health and socioeconomic repercussions, so it is important to have up-to-date epidemiological information to help plan the healthcare resources allocated to addressing it.
There are multiple studies around the world on the incidence of T1DM in childhood and adolescence, but information in adults is much scarcer.2 This is due to the complexity of distinguishing it from type 2 diabetes mellitus (T2DM), but, more so, to the fact that the peak incidence of T1DM is between 10 and 14 years of age,3 which has caused it to be traditionally considered a disease with onset at a young age. However, T1DM can appear at any age.4,5
Epidemiological studies carried out at the end of the 20th and the beginning of the 21st centuries showed an increased incidence of T1DM6 and it was predicted that this increase would be maintained over time.7 However, this trend is currently under discussion and changes in incidence have occurred unevenly in the different regions of the world. In those areas with a high incidence, both increases8 and stability have been reported.9,10 However, it is in areas with lower incidence where increases have been reported more consistently.11 Furthermore, it has been speculated that the change in incidence could consist of an advance in the age of diagnosis.4,12
The incidence of T1DM is highly variable in different regions of the world.13 Based on data from the 10th edition of the IDF Diabetes Atlas published in 2021,14 Europe is the global leader in children and adolescents under 19 years of age, with an incidence of 31 cases per 100,000 inhabitants/year. The countries with the highest incidence are in northern Europe, such as Finland (52.2) and Sweden (44.1).
According to this report,14 the incidence in Spain in children under 14 years of age is below the European average, between 10 and 20 cases per 100,000 inhabitants/year. In the last Spanish national review in children under 15 years of age,15 published in 2013, an incidence of 17.69 cases per 100,000 inhabitants/year was calculated. This puts Spain in a high-incidence category, according to the World Health Organization classification. In this publication,15 an unequal incidence is reported in the different communities of Spain, with an inverse north-south gradient to that mentioned in Europe; that is, with a higher incidence in the southern communities than in the north.
Regarding the incidence in adults, the IDF14 identifies Eritrea as the world leader in the 20–40 years age range, with 46.2 per 100,000 inhabitants/year, followed by Sweden (30.6), Ireland (30.6) and Finland (24.0). Spain ranks tenth in the world, with an incidence of 9.9 cases per 100,000 inhabitants/year, according to studies from 1987 to 1990. In fact, there are practically no recent Spanish studies in adults, with the exception of the registry carried out in Navarre, which indicated an incidence of 14.4 in 15−29 year-olds and 7.6 in 30−45 year-olds.16
In Asturias, the last T1DM epidemiology study was published in 2017.17 This paper analysed the incidence of T1DM between 2002 and 2011 in people under 40 years of age. In their results, the incidence rate in those under 40 years of age was 9.45 per 100,000 inhabitants/year, 10.82 in those under 30 years of age and 15.60 in those under 15 years of age. In the above-mentioned national review,15 Asturias was ranked as the community with the lowest incidence of T1DM in Spain, according to data from 1991 to 1995, in children under 15 years of age, with an incidence of 11.50.
The objective of this study was to update the T1DM incidence registry in Asturias. It was proposed without an age limit to contribute to our understanding of the incidence in adults. In addition, it sought to determine if it is being diagnosed at a later age and if incidence is increasing. We understand that the results are not only useful to better understand the disease, but also have practical applications in healthcare for these patients, since greater epidemiological knowledge about T1DM encourages the adaptation of the necessary health resources to offer quality care.
Material and methodsAn observational and descriptive study was carried out in which new diagnoses of T1DM were included between the years 2011 and 2020, inclusive. As a primary source, the pancreatic autoimmunity registry (anti-GAD, anti-IA2 and anti-Zn) of the Hospital Universitario Central de Asturias was obtained, which centralises this analysis for all the health districts of Asturias. The medical records of all patients who tested positive for any of the aforementioned antibodies were reviewed. As secondary sources, the Principality of Asturias Health Service registry of hospital admissions for T1DM, and the Hospital Universitario Central de Asturias T1DM registry of the Endocrinology and Nutrition Department and the Paediatric Department were obtained.
Only those patients residing in Asturias who were diagnosed with T1DM during the study period were included. Patients meeting the criteria for the diagnosis of DM established by the American Diabetes Association, along with positivity for pancreatic antibodies and the need to start insulin use in the first six months after diagnosis, were deemed to have T1DM. Where antibody levels could not be determined or were negative, clinical characteristics such as age at onset of diabetes, body mass index or C-peptide were considered, in addition to the opinion of the healthcare professional responsible for each case. In the event that there had been a diagnostic reclassification from T2DM to T1DM, the age at the time of reclassification was considered.
To calculate the incidence rate, population data were obtained from the Spanish National Institute of Statistics. The incidence rate is expressed per 100,000 inhabitants/year of risk, according to age groups, sex and health district. The incidence in different age groups was analysed with the aim of enabling comparisons with other studies and facilitating the inclusion of the data in future national or international reviews. The statistical analysis program used was Epidat 4.2. Confidence intervals were estimated at 95%, following the Poisson distribution. For the statistical calculation of the incidence rate ratio, Poisson regression models were constructed, applying a 95% confidence interval. For this calculation, the statistical analysis program used was the R program (R Development Core Team), version 4.1.3.
The study was approved by the Independent Ethics Committee of the Principality of Asturias, project number 2020.323.
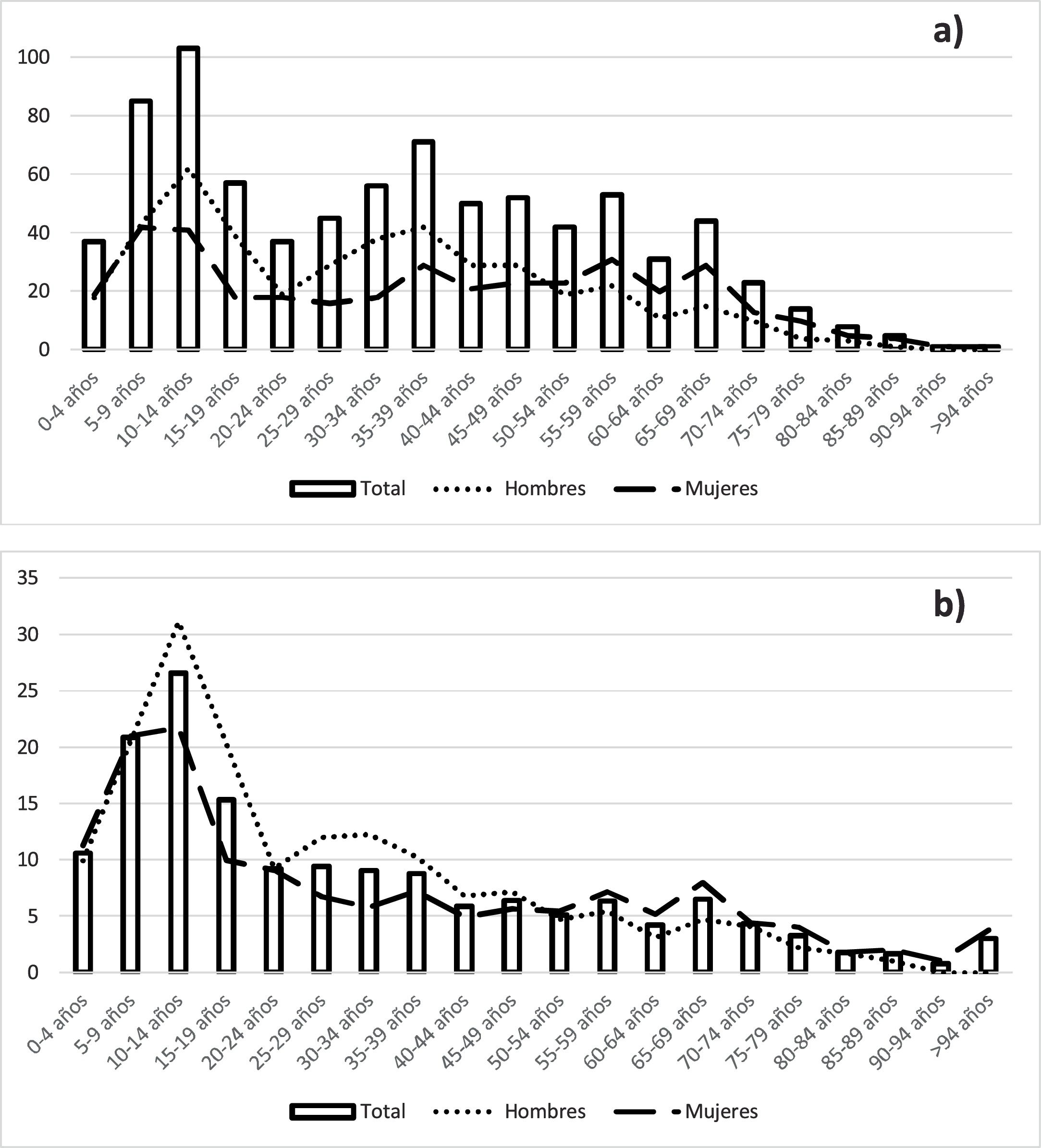
ResultsBetween 2011 and 2020, 815 new cases of T1DM were diagnosed in Asturias; 53.13% of which were male. In total, 451 patients were 30 years old or over at diagnosis, accounting for 55.34% of the total, while 27.61% were under 15 years of age. The mean age at diagnosis was 34.32±22.07 years, with a range between one and 96 years. The mean age in children under 19 years of age was 9.85±4.46 years; 10.48±4.45 years in males and 9.00±4.36 years in females. The distribution of the number of diagnoses by age can be seen in Fig. 1a.
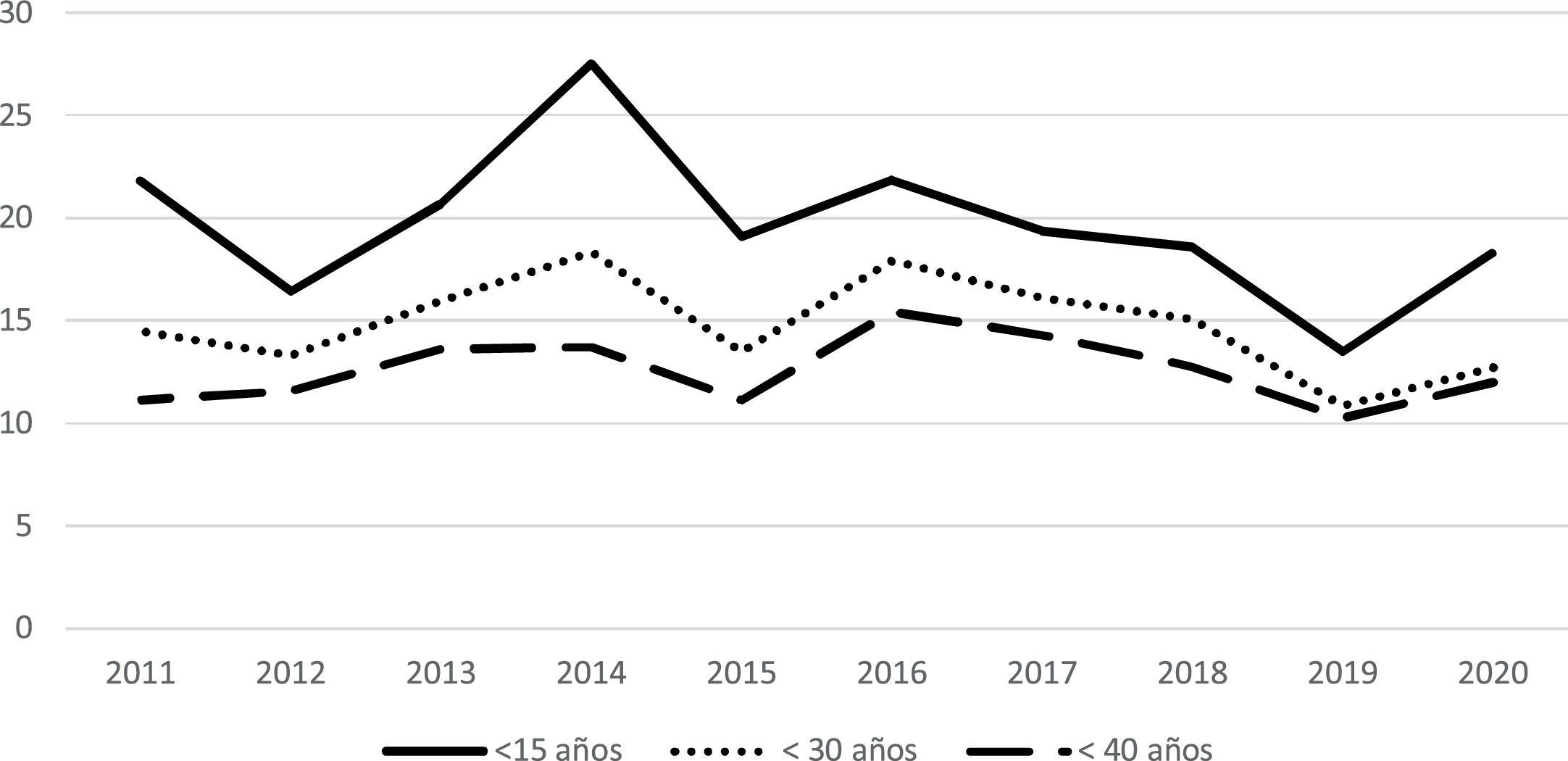
The incidence rate of T1DM in Asturias during this period was 7.82 (7.29–8.37); 8.70 (7.90–9.56) in males and 7.01 (6.33–7.75) in females. In those under 40 years of age, the incidence rate was 12.84 (11.73–14.03); 14.92 (13.25–16.74) in males and 10.69 (9.26–12.28) in females. In those under 30 it was 15.19 (13.67–16.83); 17.14 (14.90–19.62) in males and 13.15 (11.16–15.40) in females. In children under 15 it was 19.65 (17.17–22.39); 20.92 (17.38–24.96) in males and 18.32 (14.93–22.23) in females. The highest incidence rate by age group was in the 10−14 year-old group, which was 26.57 (21.69−32.22); 31.16 (23.89−39.95) in males and 21.72 (15.59−29.47) in females. The second incidence peak was in the five-to-nine-year-old group (20.84; 16.64−25.76). The incidence rate by age group and sex is shown in Fig. 1b and in Table 1.
Incidence rate of T1DM/100,000 inhabitants/year according to age group and sex.
| n | IR | CI | |
|---|---|---|---|
| 0−4 years | 37 | 10.59 | 7.46−14.60 |
| Male | 18 | 9.95 | 5.90−15.72 |
| Female | 19 | 11.29 | 6.80−17.63 |
| 5−9 years | 85 | 20.84 | 16.64−25.76 |
| Male | 43 | 20.66 | 14.95−27.83 |
| Female | 42 | 21.02 | 15.15−28.41 |
| 10−14 years | 103 | 26.57 | 21.69−32.22 |
| Male | 62 | 31.16 | 23.89−39.95 |
| Female | 41 | 21.72 | 15.59−29.47 |
| 15−29 years | 139 | 11.11 | 9.34−13.12 |
| Male | 87 | 13.65 | 10.94−16.84 |
| Female | 52 | 8.47 | 6.32−11.10 |
| 30−39 years | 127 | 8.90 | 7.42−10.59 |
| Male | 80 | 11.14 | 8.83−13.87 |
| Female | 47 | 6.63 | 4.87−8.81 |
| 0−20 years | 282 | 18.60 | 16.49−20.90 |
| Male | 162 | 20.79 | 17.71−24.25 |
| Female | 120 | 16.28 | 13.50−19.47 |
| 20−40 years | 209 | 9.06 | 7.88−10.37 |
| Male | 128 | 11.00 | 9.17−13.07 |
| Female | 81 | 7.09 | 5.63−8.81 |
| 40−60 years | 197 | 5.94 | 5.14−6.82 |
| Male | 99 | 6.07 | 4.93−7.39 |
| Female | 98 | 5.81 | 4.72−7.08 |
| >60 years | 127 | 3.87 | 3.23−4.60 |
| Male | 44 | 3.14 | 2.28−4.21 |
| Female | 83 | 4.42 | 3.52−5.47 |
CI: confidence interval; IR: incidence rate.
The analysis by year revealed that, in people under 40 years of age, the minimum incidence rate occurred in 2019 (10.27; 7.19−14.22), while the maximum occurred in 2016 (15.43; 11.75−19.90). In children under 15 years of age, the minimum incidence rate occurred in 2019 (13.46; 7.53−22.19), while the maximum was in 2014 (27.52; 18.82−38.85). Statistical analysis demonstrated an incidence rate ratio of –2.55 (–6.92−2.01; p=0.269) in children under 15 years of age, of –1.46 (4.93−2.12; p=0.419) in those under 30 years of age and –0.51 (–0.04−3.12; p=0.781) in those under 40 years of age. The incidence rates according to year by age group are shown in Table 2 and Fig. 2.
Incidence rate of T1DM/100,000 inhabitants/year according to age group and year.
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|---|---|---|---|
| <40 years | ||||||||||
| n | 49 | 50 | 57 | 56 | 44 | 59 | 53 | 46 | 36 | 41 |
| IR | 11.14 | 11.60 | 13.61 | 13.72 | 11.15 | 15.43 | 14.30 | 12.78 | 10.27 | 11.97 |
| CI | 8.24−14.73 | 8.61−15.29 | 10.31−17.63 | 10.36−17.81 | 8.10−14.97 | 11.75−19.90 | 10.71−18.70 | 9.35−17.04 | 7.19−14.22 | 8.59−16.24 |
| 30 years | ||||||||||
| n | 39 | 35 | 41 | 46 | 33 | 43 | 38 | 35 | 25 | 29 |
| IR | 14.49 | 13.29 | 15.99 | 18.34 | 13.50 | 17.94 | 16.12 | 15.06 | 10.88 | 12.72 |
| CI | 10.30−19.81 | 9.26−18.49 | 11.47−21.69 | 13.42−24.46 | 9.29−18.96 | 12.99−24.17 | 11.41−22.13 | 10.49−20.95 | 7.04−16.06 | 8.52−18.27 |
| <15 years | ||||||||||
| n | 25 | 19 | 24 | 32 | 22 | 25 | 22 | 21 | 15 | 20 |
| IR | 21.78 | 16.40 | 20.67 | 27.52 | 19.08 | 21.83 | 19.35 | 18.60 | 13.46 | 18.26 |
| CI | 14.09−32.15 | 9.87−25.61 | 13.25−30.76 | 18.82−38.85 | 11.96−28.89 | 14.13−32.23 | 12.13−29.29 | 11.51−28.43 | 7.53−22.19 | 11.15−28.20 |
CI: confidence interval; IR: incidence rate.
In the analysis of the incidence according to health district, the lowest rate can be seen in the Cangas del Narcea district (4.86; 2.51–8.48) and the highest in the Mieres district (11.04; 8.50–14.10). In those under 30 years of age, the lowest incidence rate was in Cangas del Narcea (12.58; 4.62–27.38), while the highest occurred in Jarrio (19.87; 11.57–31.81). The highest incidence rate in children under 15 years of age occurred in the Arriondas district (25.42; 13.14–44.41), while the lowest was in Mieres (18.17; 8.31–34.49). The analysis of the incidence rate by health district is shown in Table 3.
Incidence rate of T1DM/100,000 inhabitants/year according to health district and age group.
| <15 years | <30 years | <40 years | Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | IR | n | IR | n | IR | n | IR | |
| District 1 Jarrio | 8 | 20.26 | 17 | 19.87 | 24 | 18.48 | 37 | 8.50 |
| District 2 Cangas del Narcea | 4 | 19.61 | 6 | 12.58 | 7 | 9.54 | 12 | 4.86 |
| District 3 Avilés | 31 | 19.98 | 48 | 14.81 | 71 | 14.95 | 114 | 7.87 |
| District 4 Oviedo | 83 | 22.09 | 132 | 16.89 | 170 | 14.77 | 288 | 8.77 |
| District 5 Gijón | 64 | 20.36 | 104 | 16.39 | 133 | 13.74 | 213 | 7.24 |
| District 6 Arriondas | 12 | 25.42 | 18 | 17.75 | 22 | 14.42 | 29 | 5.94 |
| District 7 Mieres | 9 | 18.17 | 19 | 17.21 | 34 | 19.73 | 64 | 11.04 |
| District 8 Langreo | 14 | 20.99 | 20 | 14.04 | 30 | 13.84 | 58 | 8.36 |
IR: incidence rate.
The incidence rate of T1DM in Asturias between 2011 and 2020 was 7.82 cases per 100,000 inhabitants/year: 19.65 in children under 15 years of age and 12.84 in people under 40 years of age.
The peak incidence of T1DM occurred between 10 and 14 years of age, similar to most publications16, although studies such as the one carried out in Vizcaya put it in the five-to-nine-years range18. In our sample, the peak incidence in females also occurs at 10−14 years of age. However, the difference between five-to-nine years and 10−14 years is much more marked in males (20.66 vs 31.16) than in females (21.02 vs 21.72). Other studies have reported peak incidence in females from five-to-nine years of age,16 as also occurred in the previous study carried out in Asturias (16.47 at five-to-nine years vs 16.27 at 10−14 years).17 In our sample, the average age in females is lower than that of males, which, in conjunction with that previously mentioned, confirms that it is diagnosed at a younger age in females than in males. However, despite the fact that a temporal evolution towards an onset of T1DM at earlier ages had been predicted, our data indicate that this advance has not occurred, consistent with other studies in Spain16 and also in other regions of the world.19
We saw a marked drop in incidence from the age of 20, with a gradual decline thereafter. An increased incidence at advanced ages has recently been published.13 However, this increase was not observed in our study. A differential element of our study is that, from the age of 50, the incidence is higher in females than in males. In fact, in people over 60 years of age, the incidence in women is 4.42 vs 3.14 in men. We found studies in the literature in which a similar trend can be seen,20 contrary to the majority of studies, which show a maintained predominance of incidence in males.2 In any case, the overall incidence rate is higher in males than in females.
As has been mentioned, T1DM has traditionally been considered a disease with onset at a young age. Our study demonstrates that it can appear at any age, as has already been clearly established. This is especially relevant if we take into account the demographic structure of our society, which means that, despite a higher incidence at younger ages, a large number of diagnoses do not occur in this age group.13 An example of this fact is our study, which demonstrates that more than half of new diagnoses occurred after 30 years of age. This indicates that we must maintain a high level of suspicion for T1DM on diagnosis of DM at any age.
According to the World Health Organization classification, our study puts Asturias in the category of high incidence of T1DM in children under 15 years of age, although it is very close to the category of very high incidence. According to this study, Asturias exceeds the national average incidence of T1DM in children under 15 years of age, calculated in 2013 at 17.69.15 Combining our data and the aforementioned registry, Asturias would no longer occupy last place but would become the community with the eighth highest incidence in Spain, if other updates are not taken into account, compared to the findings published in 2013. In any case, it would be useful to have new studies in the different communities and to collect the data in a new national publication. Our data are consistent with the north-south gradient mentioned for Spain. Asturias remains closer to Galicia (17.2) and Cantabria (13.8) than to Castile-La Mancha (27.6) or the Canary Islands (23.2).
Regarding adults, the incidence between 20 and 40 years of age in our study is 9.06 cases per 100,000 inhabitants/year. This result is very similar to that provided in the data from the IDF,14 which puts Spain as the country with the tenth highest incidence (9.9, with data from 1987 to 1990). The most recent study in Spain is from Navarre.16 Although the age groups used in this study do not match ours exactly, the results are nonetheless very similar. The incidence in the 30−45 years group is 7.6, while in ours it is 8.90 in the 30−39 years group: a minimal difference that can be justified by the age ranges used. It is currently thought that the incidence of T1DM in adults behaves in a similar way to that of children in the different regions of the world; that is, a higher incidence in adults in regions with higher incidence in children and vice versa.2,21 In this sense, the incidence of T1DM in adults in Asturias is much lower than that reported in countries such as Sweden, Ireland or Finland, where the incidence is very high in both children and adults.14
In the years included in our study, no significant changes were observed in the incidence rate. However, if we compare our results with those of the previous study carried out in Asturias,17 a clear increase in incidence can be seen in all age groups, though we understand that this finding can be explained by an improvement in data capture. In fact, both studies included data from 2011 and the incidence rate in both those under 15 years of age (17.42 vs 21.78) and those under 40 years of age (7.96 vs 11.14) is higher in our study, based on a greater number of new diagnoses found. A similar phenomenon is mentioned in the study carried out in the Community of Madrid, where a change in data capture methodology led to a marked increase in incidence rates.22 As also discussed in that study, we believe that our data reflect reality more reliably than previous data. To analyse the behaviour of other regions, data are available from Navarre,16 where the incidence is stable, and Gran Canaria,23 where an annual increase of 1.39% is reported, which is not statistically significant. Taking into account our results and the review of the literature, we believe that the actual incidence of T1DM in Spain has not increased. Rather, improvements made to the study methodology are sometimes leading to an increase in detected cases and, with it, an increase in the published incidence rate.
Lastly, in our study no major differences were observed in the incidence of T1DM between the different healthcare districts of Asturias, and those differences found are not clearly attributable to the healthcare district setting; that is, there are no differences in the incidence between predominantly rural areas and other more urban areas. Comparing our data with the previous study of this region,17 a practically generalised increase in the incidence can be seen, except in children under 15 years of age in the districts of Jarrio and Cangas del Narcea, probably due to the sample size. Therefore, there are no differences between predominantly rural areas and predominantly urban areas.
The main limitation of this study is the lack of a unified registry of T1DM diagnoses in Asturias and the fact that it has not been possible to ascertain the completeness of the data using the capture-recapture method. However, we believe that the sources used allow us to obtain a very real approximation of the diagnoses. Another limitation is the use of the criterion of need for insulin use in the first six months after diagnosis, since it can exclude patients who have T1DM but do not have that requirement initially. A strength of the study lies in the fact that the medical records of all the patients were reviewed to ensure that they met the inclusion criteria in the study, a fact of special relevance in elderly patients, given the complexity in distinguishing between T1DM and T2DM.
In conclusion, our study represents an update to the T1DM registry in Asturias and shows a high incidence. The incidence found is higher than that of previous studies, most probably due to an improvement in data collection and not to a real increase in incidence. No data were found to support an advance in the age of onset of T1DM. Our study also shows that a significant percentage of new diagnoses occur after childhood and adolescence. We believe that it is important to create a registry of T1DM diagnoses both regionally and nationally, and in both children and adults, in order to ensure the correct epidemiological assessment of the disease and, with it, improve patient care.
Conflicts of interestThe authors declare that they have no conflicts of interest.