An epidemiological study conducted between 1988 and 1992 showed iodine deficiency and endemic goiter in the schoolchildren of the autonomous community of the Basque Country.
Objectives(1) To ascertain the iodine nutrition status of schoolchildren aged 6–7 years, and (2) to estimate the prevalence of abnormal TSH levels in capillary blood.
Population and methodsThe study was conducted on 497 schoolchildren selected by random sampling. Median urinary iodine concentration (mUIC) was used to assess iodine nutritional status, and the reference interval derived from the study population was used to estimate the prevalence of abnormal TSH levels.
ResultsThe mUIC (P25–P75) was 140 (82–217) μg/L. A higher value was found in those who used iodized salt at home than in those who did not (146 [85–222] versus 126μg/L [73–198], p<0.05). It was also higher in those who consumed 2 or more daily servings of milk and yogurt than in those taking less than 2 servings (146 [87–225] versus 110μg/L [66–160], p<0.0001). Abnormal TSH levels were found in 2% of children. There was no correlation between TSH levels in capillary blood and urinary iodine concentrations (R=0.082; p=0.076).
Discussion and conclusionsSchoolchildren aged 6–7 years of the autonomous community of the Basque Country have an adequate iodine nutrition status. Use of iodized salt at home and daily consumption of milk and yogurt were associated to the highest UICs.
Un estudio epidemiológico realizado entre 1988 y 1992 puso de manifiesto la existencia de deficiencia de yodo y bocio endémico en la población escolar de la comunidad autónoma del País Vasco.
Objetivos1) Conocer el estado de nutrición de yodo de los escolares de 6-7 años de edad y 2) estimar la prevalencia de concentraciones anormales de TSH en sangre capilar.
Población y métodosFueron estudiados 497 escolares seleccionados mediante muestreo aleatorizado. Para evaluar el estado de nutrición de yodo se utilizó la mediana de las concentraciones urinarias de yodo (mCUY). Para estimar la prevalencia de concentraciones anormales de TSH se utilizó el intervalo de referencia derivado de la población estudiada.
ResultadosLa mCUY (P25-P75) fue de 140μg/L (82-217). Fue mayor en los que utilizaban sal yodada en sus domicilios que en los que no lo hacían (146 [85-222] frente a 126μg/L [73-198]; p<0,05). También fue mayor en los que consumían 2 o más raciones diarias de leche y yogur que en los que consumían menos de 2 raciones (146 [87-225] vs. 110μg/L [66-160]; p<0,0001). La prevalencia de concentraciones anormales de TSH fue del 2%. No hubo correlación entre las concentraciones de TSH en sangre capilar y las CUY (R=0,082; p=0,076).
Discusión y conclusionesLos escolares de 6-7 años de la comunidad autónoma del País Vasco tienen un estado de nutrición de yodo adecuado. La utilización de sal yodada en el domicilio y el consumo diario de leche y yogur se asociaron con las mayores CUY.
Iodine, a key nutrient for animal species, is an essential substrate in thyroid hormone synthesis.1 In humans, iodine deficiency (ID) leads to a broad range of adverse effects affecting growth and development, and health that are collectively known as “conditions caused by iodine deficiency”.2 The most visible and known consequence of ID is goiter, though the most serious effects are found in the developing central nervous system, with the risk of irreversible brain damage and psychomotor disorders of variable severity ranging from subtle neurological and cognitive deficits to cretinism as the most extreme situation.2 In addition, ID adversely affects reproductive functions and increases the frequency of miscarriage and congenital anomalies, as well as of stillbirths and perinatal mortality.2,3 Disorders resulting from ID are avoidable thanks to the availability of effective and low cost iodization methods. Salt iodization is the most practical and manageable way of supplying iodine to population groups that need it to cover their physiological needs.4,5 Due to its safety and efficacy, iodine supplementing of all food-grade salt used in homes and in the food industry is the strategy proposed by the World Health Organization (WHO) for the prevention and control of ID disorders worldwide.5
An epidemiological study conducted between 1988 and 1992 revealed the existence of ID and endemic goiter in the school population aged 6–14 years in the Autonomous Community of the Basque Country (Spain).6 Following these findings, a number of initiatives were developed to promote the use of iodized salt (IS) rather than salt without iodine supplementation among the population segments that benefit most from iodine prophylaxis, i.e., children and adolescents, women of childbearing age, pregnant women and nursing mothers.
The public health authorities of the Basque Government adopted several actions to evaluate the results of these health interventions.7–9 The most recent data can be found in the “Tirokid Study. A study of iodization and thyroid function in the Spanish pediatric population”, sponsored by the Spanish Society of Endocrinology and Nutrition, and funded by Merck-Serono. The schoolchildren screening phase in the “Tirokid Study” (involving children aged 6 and 7 years in the first year of primary school) was carried out during 2010 and 2011, and the results were published in 2016.10
In accordance with the methodology of the “Tirokid Study”, a random selection was made in two of the three provinces of the Basque Country (Álava and Guipúzcoa), with two schools being selected in each province.10 Further to joining this initiative and participating in its development, the public health authorities of the Basque Government designed an epidemiological study entitled “Iodine nutritional status and the prevalence of abnormal TSH levels in the school population aged 6–7 years in the Basque Country”, in which the geographical setting was extended to all three of the provinces comprising the Community (Álava, Biscay and Guipúzcoa).
The objectives were: (1) to determine the iodine nutritional status of schoolchildren aged 6–7 years in the Basque Country and the associated dietary factors; and (2) to estimate the prevalence of abnormal TSH levels in capillary blood.
Population and methodsPopulationThe population of this cross-sectional observational study consisted of minors receiving primary education in schools in 2010–2011, in the Basque Country (n=20,580 pupils: 10,057 girls and 10,523 boys). Of the 536 school centers in the Basque Country where primary education was provided, 12 were randomly selected (2.2%) (4 in each province: 2 of them in the provincial capital and the other 2 elsewhere in the province).
Sample sizeThe WHO and different international bodies and experts in the field recommend the use of median urinary iodine concentration (mUIC) as a surrogate biological marker of recent iodine intake in population studies.4,11,12 Estimating iodine intake in a group of subjects with a precision range of ±10% within the 95% confidence interval requires approximately 125 casual urine samples, while approximately 500 samples are required to secure a precision range of ±5%.13
The 700 boys and girls aged 6–7 years enrolled in the 12 selected school centers were invited to participate (177 in Álava [25.3%], 236 in Biscay [33.7%] and 287 in Guipúzcoa [41%]). Of these children, 561 received permission from their parents or guardians to participate in the study (80.1%), and returned the administered questionnaire completed or partially completed by their parents or guardians. Casual urine samples were collected from 497 of the schoolchildren with the corresponding authorization (88.6%). In accordance with the primary objective of the study, these were the children finally included in the study: 261 boys (52.5%) and 236 girls (47.5%).
MethodsThe study variables were obtained using a questionnaire completed by the parents or guardians who authorized the inclusion of the schoolchildren in the study, and through the analysis of two biological samples from the children: one capillary blood sample and one urine sample. The participants were informed that fasting was not necessary on the day of the collection of the biological samples.
The questionnaire, which was the same as that used in the “Tirokid Study”,10 yielded information on the personal history of the child with reference to thyroid disorders, active drug treatments, the use of iodinated compounds for wound disinfection during the month prior to study participation and for surgery in the previous 6 months, the use of IS in the home, and the intake of iodine-rich foods (milk, yogurt and other dairy products, eggs, seafood and algae). The amount and frequency of consumption of these foods was taken into account. In order to standardize the intake of iodine-rich foods, we used as reference the serving definitions and consumption frequencies recommended by the Spanish Society of Community Nutrition (SENC) for the pediatric population.14
In addition, a drop of capillary blood was obtained in school from each participant through finger puncture, the sample being deposited on Whatman 903 filter paper, and allowed to dry at room temperature. The capillary blood samples were kept refrigerated at 4°C until delivered to the laboratory. In parallel, a 20ml casual urine sample from each participant was stored in a polypropylene tube. The urine samples were kept refrigerated between 2 and 8°C until delivery to the laboratory, where they were frozen and stored at a temperature of −20°C until analysis.
Capillary blood TSH measurements were performed by fluoroimmunometry (AutoDelfia™ Neonatal hTSH test kit; Perkin-Elmer, Inc.) at the Neonatal Screening Laboratory of Hospital Clínic de Barcelona (within- and between-assay coefficient of variation [CV]: 1.82% and 3.67%, respectively). Estimation of the capillary blood TSH reference range (0.26; 95%CI: 0.21–0.33mU/l and 2.44; 95%CI: 2.25–2.73mU/l) was based on the TSH levels of the schoolchildren participating in the study. In order to obtain the reference limits and their confidence intervals, the nonparametric rank-based estimates method of the RefVal Program tool (http://sourceforge.net/projects/refval), developed and updated by the International Federation of Clinical Chemistry was used.15
The determination of urinary iodine concentration (UIC) was carried out in the Public Health Standard Laboratory of the Basque Government in Derio (Biscay) using ion-pair reversed-phase liquid chromatography with electrochemical detection and a silver electrode (Waters Chromatography, Milford, MA, USA). Detailed information on the procedure and validation of the method and its within- and between-serial precision is available in another publication.16 This laboratory is accredited under ISO standard 15189 by the Spanish National Accreditation Entity and participates in two external quality evaluation programs: that organized by the Centers for Disease Control and Prevention (CDC, Atlanta, USA), Ensuring the Quality of Urinary Iodine Procedures; and that organized by the Spanish Neonatal Screening Society. The evaluation of nutritional iodine status was based on the criteria established by the WHO and by other international organizations (collective mUIC: <100μg/l, iodine deficiency; 100–199μg/l, adequate iodine intake; 200–299μg/l, iodine intake higher than recommended; and ≥300μg/l, excess iodine intake).4,11
Statistical analysisVariables were expressed as measures of central tendency and dispersion in the case of quantitative variables, while absolute and relative frequencies (percentages, with their respective confidence intervals) were used for qualitative or categorical variables.
Urinary iodine concentration values usually do not follow a normal distribution.4,11,12 The median and interquartile range (IQR) were therefore used as measures of central tendency and dispersion, respectively, for UIC. The analysis of differences in mUIC was based on the use of nonparametric tests, with the Mann–Whitney U-test for the comparison of two medians, and the Kruskal–Wallis test for the comparison of more than two medians. The comparison of frequencies of categorical variables was based on the Pearson chi-squared test. The Fisher exact test was applied where required. The Spearman correlation coefficient was used to analyze the direction and magnitude of association between the two quantitative variables not meeting normal distribution criteria, i.e., UIC and TSH in capillary blood.
Statistical significance was considered for p<0.05. The SPSS version 21 statistical package for MS Windows (SPSS Inc., Chicago, IL, USA) was used throughout.
The “Iodine nutrition status and prevalence of abnormal TSH levels in schoolchildren aged 6–7 years in the Autonomous Community of the Basque Country (Spain)” study protocol was approved by the Clinical Research Ethics Committee of the Basque Country. The field work was carried out in March 2011.
ResultsIodine-rich food questionnaireThe proportion of IS use in the home was 69.2% (95%CI: 65–73%), and there were no gender differences (p=0.923).
The median (P25–P75) daily servings of milk and yoghurt were 2.5 (2–3). There were no significant differences between boys and girls (p=0.097). The median (P25–P75) weekly frequency of cheese consumption was 2 (1–3), with no significant differences in weekly consumption according to gender (p=0.394).
The median (P25–P75) frequency of seafood consumption was twice a week (2–3), with no significant differences according to gender (p=0.166). The median (P25–P75) egg consumption was 2 units a week (2–3), with no significant differences according to gender (p=0.064).
Urinary iodine concentrationThe UIC values (minimum 21μg/l and maximum 766μg/l) did not follow a normal distribution (p<0.0001). The mUIC (P25–P75) was 140μg/l (82–217). The value was significantly higher in boys than in girls (150 versus 122μg/l, respectively; p<0.01).
The mUIC (P25–P75) among the schoolchildren that consumed IS at home was higher than in those who did not consume iodine-supplemented salt: 146 (85–222) versus 126μg/l (73–198) (p<0.05).
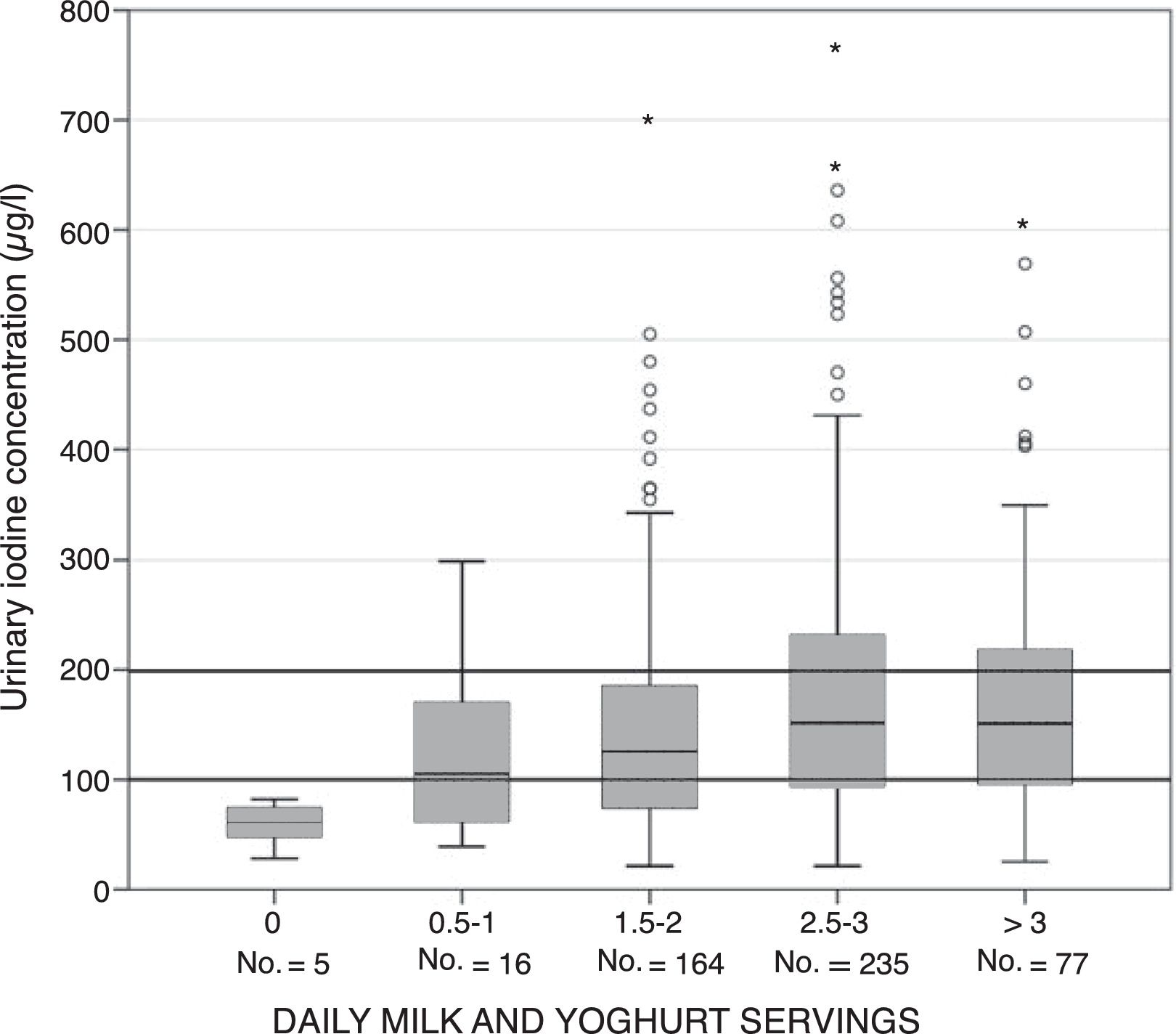
The mUIC in schoolchildren who consumed less than two daily servings of milk and yoghurt was significantly lower than in those who received two or more daily servings: 110 (66–160) versus 146μg/l (87–225) (p<0.0001). As can be seen in Fig. 1, there was an association between daily milk and yoghurt intake and UIC. The relationship between the two variables proved linear and increasing up to intakes equivalent to three daily servings of milk and yoghurt, followed by stabilization at higher consumption levels: 0 servings, 61μg/l; 0.5–1 serving, 105μg/l; 1.5–2 servings, 125μg/l; 2.5–3 servings, 152μg/l; and 3.5 or more servings, 151μg/l (p=0.001).
Urinary iodine concentration according to daily servings of milk and yoghurt. * Urinary iodine concentration intervals according to the reference cut-off points of the World Health Organization (WHO), the United Nations Children's Fund (UNICEF) and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD).
In the group of schoolchildren who consumed IS at home, the mUIC among consumers of less than two servings of milk and yoghurt a day was significantly lower than in those who consumed two or more servings: 124 (69–177) versus 154μg/l (91–231) (p<0.05). Likewise, in the group of schoolchildren who did not consume IS at home, the mUIC among consumers of less than two servings of milk and yoghurt a day was significantly lower than in those who consumed two or more servings: 102 (60–141) versus 137 (77–208) μg/l (p<0.05).
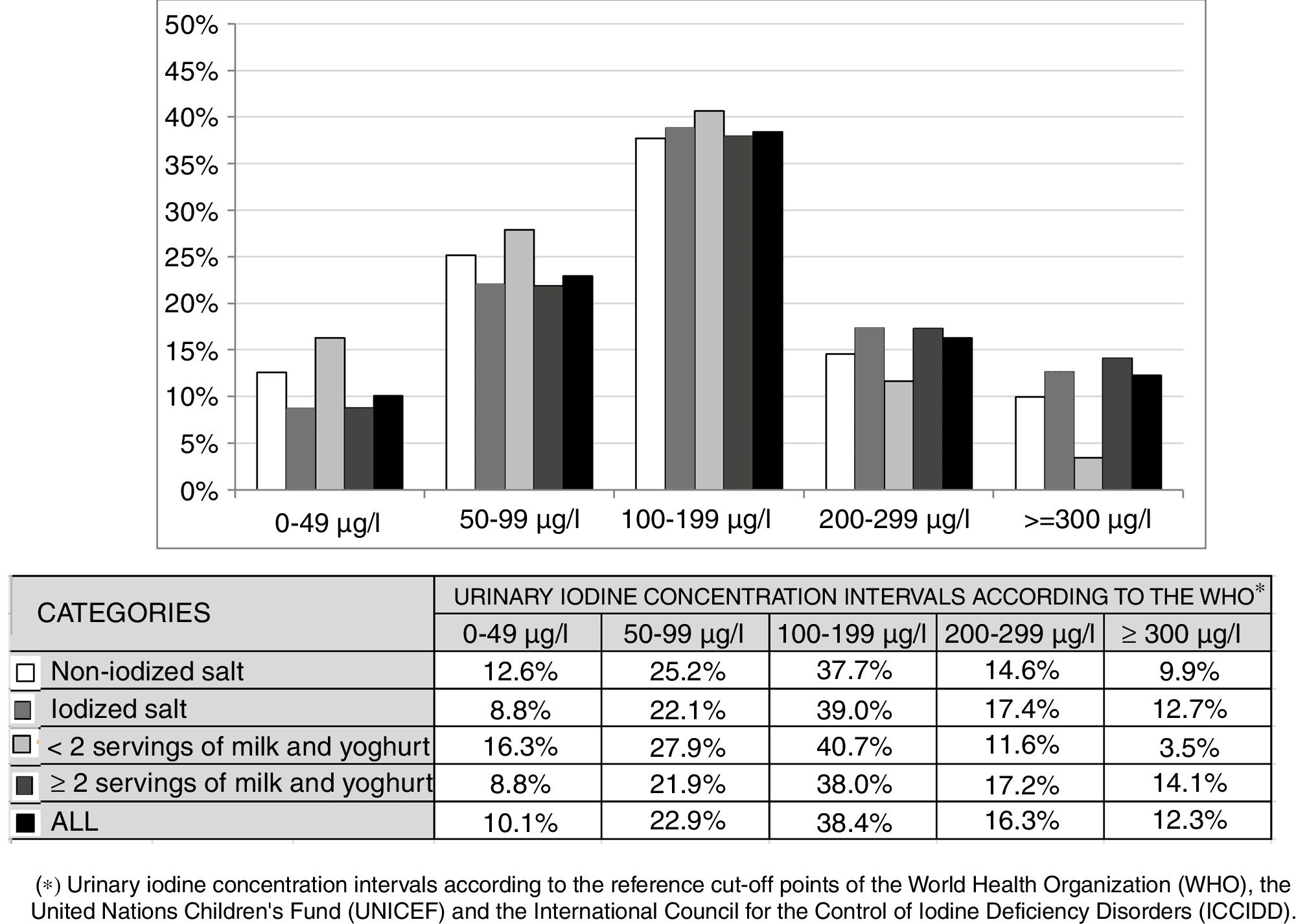
Fig. 2 shows the distribution of UIC frequencies in each of the intervals proposed by the WHO according to the use or not of IS at home and the consumption of less than two daily servings or more than two daily servings of milk and yoghurt. There were no significant differences between the UIC frequencies in any of the four scenarios. Likewise, no significant differences were observed on analyzing the combined use of salt and the daily consumption of milk and yoghurt, except when the two most extreme situations were compared: the combination of IS use at home and the consumption of two or more daily servings of milk and yoghurt versus the combination of non-supplemented salt at home and the consumption of less than two daily servings of milk and yoghurt (p<0.05).
Distribution of urinary iodine concentration frequencies according to the type of salt used at home and the number of daily servings of milk and yoghurt. * Urinary iodine concentration intervals according to the reference cut-off points of the World Health Organization (WHO), the United Nations Children's Fund (UNICEF) and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD).
Neither cheese consumption with a weekly frequency above the overall median, nor egg consumption in weekly amounts equal to or higher than that recommended by the Spanish Society of Community Nutrition (SENC), nor the consumption of seafood with a weekly frequency equal to or greater than that recommended by the SENC, was associated with greater UIC. Among algae consumers (n=17), the only child that consumed them on a daily basis had an UIC>300μg/l (319μg/L). The other children consumed algae 1–4 times a week, and their UIC ranged from 41 to 223μg/l.
The UIC did not exceed 300μg/l in any of the 8 schoolchildren who underwent surgery in the 6 months prior to the study, though it did do so in two of the 46 children treated with iodinated antiseptics in the month prior to the study. In one of them UIC was 315μg/l, versus 608μg/l in the other.
Prevalence of abnormal TSH values in capillary bloodAccording to the questionnaire completed by the parents or guardians, no children were diagnosed with or treated for hypothyroidism or hyperthyroidism.
The capillary blood TSH values showed a non-normal distribution (p=0.000). The median (P25–P75) TSH concentration in capillary blood was 1.0mU/ml (0.72–1.35). There were no significant gender differences in TSH values (p=0.119).
The percentage of subjects with TSH levels below the lower limit of the 95%CI of P2.5 (0.21mU/l) was 1%, and that of subjects with TSH levels above the upper limit of the 95%CI of P97.5 (2.73mU/l) was likewise 1%.
There was no correlation between the capillary blood TSH levels and UIC (R=0.082; p=0.076).
DiscussionThe United States Institute of Medicine recommends the daily intake of 90μg of iodine in children under 4–8 years of age,1 while the WHO recommends 120μg of iodine a day for 6–12 year-olds.4 The European Food Safety Authority estimates adequate iodine intake for children between 4 and 10 years of age to correspond to 90μg/day.17
The iodine content in seafood and shellfish is highly variable, with a mean concentration of the micronutrient ranging from 46 to 116μg/100g edible portion.18 As a result of iodine prophylaxis to protect health and to reduce the economic costs caused by ID in animals raised as food for human consumption, an increase has been observed in iodine content that is much higher in eggs and milk than in poultry or livestock, because the oligoelement is concentrated through active transport in egg yolk and mammary gland tissue during the breastfeeding period.19,20 One glass of regular cow's milk (200–250ml) available in the Basque Country contains 50μg iodine,21 and this amount suffices to cover 55% of the adequate iodine intake in children between 4 and 10 years of age. Eggs show important variation in iodine content, with concentrations ranging from 17 to 162.5μg/100g edible portion.18 These differences most likely reflect very diverse dietary and veterinary practices.19
An epidemiological study conducted between 1988 and 1992 demonstrated the existence of ID and endemic goiter among schoolchildren in the Basque Country.6 The use of IS and the consumption of iodine-enriched foods allowed for the rapid normalization of iodine intake in the school population between 1992 and 1998.7,9 The mUIC in schoolchildren aged 6–14 years increased from 65μg/l in 1992 to 147μg/l in 2005.9 An important part of the phenomenon was probably due to the increased iodine concentration in milk resulting from the progressive incorporation of iodine prophylaxis measures in cattle, as also happened in Denmark, Germany, Switzerland, France, Italy, the Czech Republic and Poland in the late 1980s and especially during the 1990s.9
Iodine supplementation of salt is the prophylactic strategy recommended by the WHO and other international agencies for the prevention of ID and the control of its disorders in geographical settings characterized by iodine-poor soils and fresh water.4,5 According to these agencies, access to IS should be made possible in virtually the entire territory of the affected geographical setting and moreover should be maintained, in order to ensure that IS can be used uninterruptedly in over 90% of all households.4 Legislation regulating salt supplementation with iodine in Spain has established that the finished product should contain 60mg of iodine per kg of salt (60ppm of iodine), with a tolerance margin of ±15%.22
The percentage use of IS in the homes of schoolchildren aged 6–7 years in the Basque Country was 69.2%, which is very close to the figure of 69.8% of schoolchildren of the same age participating in the “Tirokid Study” conducted throughout Spain, though in this case there were important variations among the participating Autonomous Communities: Madrid 59.6%; Navarre 60.8%; Castilla-León 61.1%; the Balearic Islands 65.5%; Extremadura 67.7%; the Basque Country 71.4%; Andalusia 75%; Catalonia 76%; Asturias 77.3%; Castilla-La Mancha 77.8%; and Aragón 80.4%.10 The percentage use of IS in schoolchildren aged 6–7 years of age in the Basque Country was markedly higher than that found in the nutrition survey conducted in the Basque Country in 2005 among children and adolescents, where the percentage use of IS in households of the school population between 6 and 14 years of age was seen to be 53%.9 In order to prevent the irreversible damage ID can cause to the central nervous system of the fetus and young child, the health authorities should seek to extend the use of IS to virtually all homes with women of childbearing age, pregnant women and nursing mothers. The sustained intake of IS among women of childbearing age allows for the optimization of their thyroid iodine stores. In the event of pregnancy, these large amounts of the oligoelement stored in the thyroid gland, together with the daily amounts of iodine supplied through the diet, are able to adequately cover the increased iodine needs that characterize this physiological condition.23,24
The mUIC in schoolchildren between 6 and 7 years of age in the Basque country (140μg/l) lies within the interval 100–199μg/l corresponding to adequate iodine nutritional status in children aged 6–12 years.4,11 Of note is the fact that not only did children consuming IS at home have an adequate nutritional iodine status (mUIC 146μg/l), but also those in homes without iodine-supplemented salt (mUIC 126μg/l). The normal iodine nutritional status found in children without iodine-supplemented salt in the home suggests that there are important sources of this micronutrient other than IS in these children.
The daily consumption of milk and yoghurt was the dietary factor associated with the highest UIC values in the schoolchildren of the Basque Country, together with the consumption of IS at home. An increasing linear relationship was recorded between the number of daily servings of milk and yoghurt and UIC up to intakes equivalent to three daily servings, followed by stabilization at higher intakes, a phenomenon very similar to that seen in the “Tirokid Study”.10 In a study conducted among the school population in Málaga (Spain), the only food group correlated to UIC consisted of dairy products (milk, yoghurt and milkshakes), the observed association being linear and dependent upon the amount consumed.25,26 The most recent studies in preschool and school populations also describe a positive association between milk consumption and UIC, which confirms that dairy products are an important dietary source of iodine.27,28
The mUIC was significantly higher in boys than in girls, this being a common finding in studies conducted in school children. Part of this phenomenon may be attributed to the greater food consumption in boys as compared to girls. The “enKid Study” conducted in children and adolescents in Spain found males to consume greater amounts of foods from all groups, except vegetables, where females showed slightly higher intakes, and sugars and cocoa, where intake was similar in both genders.29
The TSH reference ranges may vary considerably depending on the population involved and the analytical method used.30 The information available in this regard in the pediatric population between 6 and 7 years of age is very limited, and the reference limits calculated from the TSH levels of schoolchildren participating in the study have been used to estimate the prevalence of abnormal capillary TSH values. Regarding the significance of the observed prevalence of abnormal TSH values, the study design contemplated referring schoolchildren with TSH values outside the reference range to the primary care physician. However, follow-up for a definitive diagnosis of thyroid function based on the pertinent clinical studies in their primary or specialized care centers was not considered. In a cohort of 121,052 Israeli children and adolescents aged 0.5–16 years subjected to serum TSH testing, 1126 subjects finally needed medical treatment for thyroid dysfunction (1.01%), 49 for hyperthyroidism (0.04%) and 1177 for hypothyroidism (0.97%).31
ConclusionsSchoolchildren between 6 and 7 years of age from the Basque Country have adequate iodine nutritional status. The consumption of IS at home and the daily consumption of milk and yoghurt were associated with higher UIC values.
Financial supportMerck-Serono provided logistic support and covered the costs of the field work and blood tests. The costs of the urine tests were covered by the Department of Health of the Basque Government.
Conflicts of interestNone.
Thanks are due to the participating children and their parents and guardians, as well as to the management boards of the participating schools. The authors also acknowledge the invaluable collaboration of Mercedes Estébanez-Carrillo (Director of Public Health of the Department of Health and Consumer Affairs of the Basque Government, Vitoria-Gasteiz); Cándido Hernández-Garduño (Director of Educational Innovation of the Department of Education of the Basque Government, Vitoria-Gasteiz); and Manuel Lansac-Aquelue (Department of Education of the Basque Government, Vitoria-Gasteiz). Thanks are likewise due to Marcos Orellana for logistic support of the “Tirokid Study” (Medical Department, Merck-Serono, Madrid); and to José María Salazar for logistic support of the “Tirokid Study” in the Basque Country (Merck-Serono, Vitoria-Gasteiz).
Please cite this article as: Arrizabalaga JJ, Jalón M, Espada M, Cañas M, Arena JM, Vila L. Estado de nutrición de yodo y prevalencia de concentraciones anormales de TSH en la población escolar de 6-7 años de la comunidad del País Vasco. Endocrinol Diabetes Nutr. 2018;65:247–254.